Hepatocellular Carcinoma (HCC) in the United States: Current Epidemiological Trends
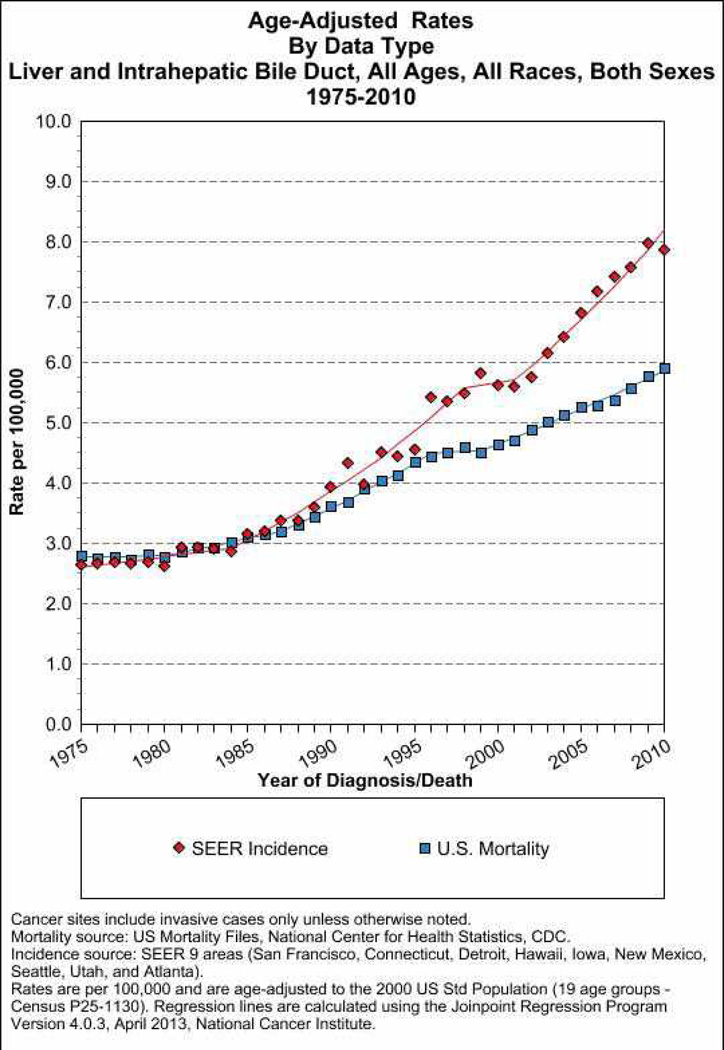
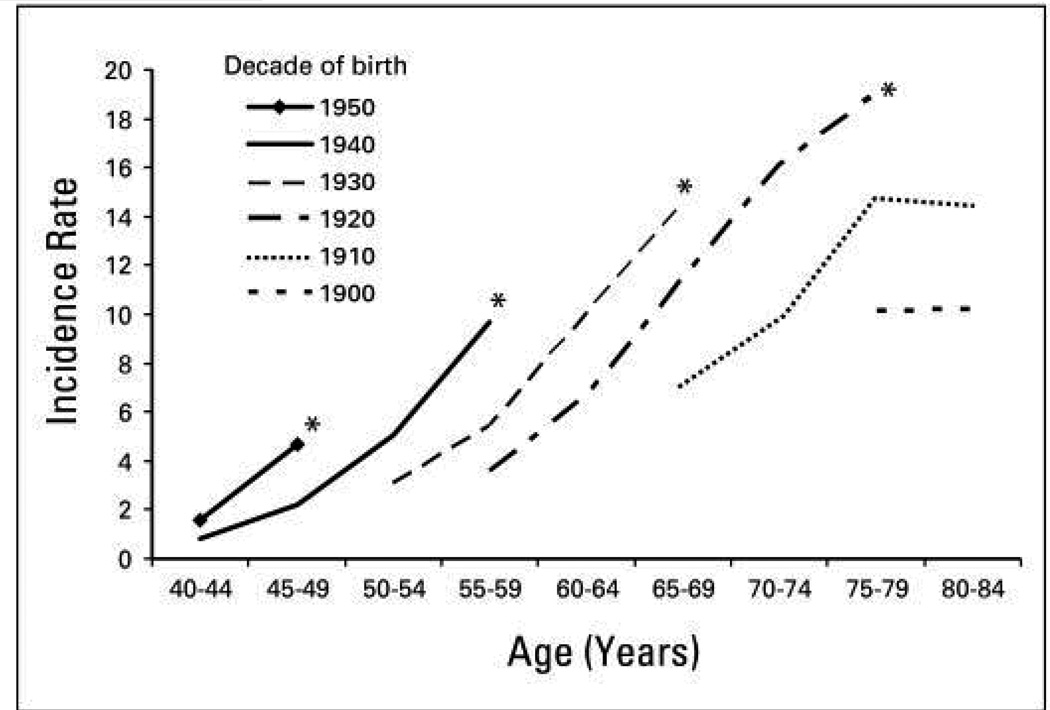
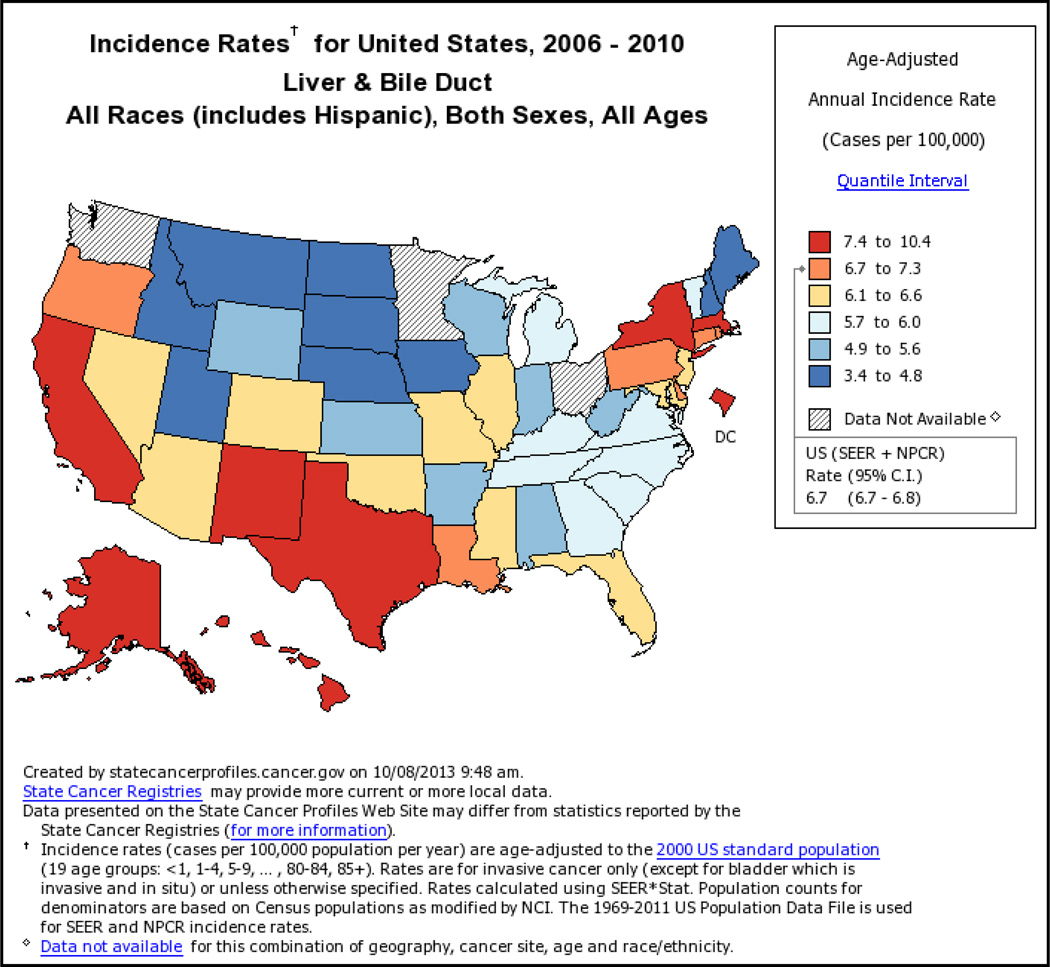
The incidence of HCC has almost tripled since the early 1980s in the United States where it is the fastest rising cause of cancer-related deaths1. According to population based Surveillance Epidemiology and End Results registry data, the overall HCC age adjusted incidence rates for liver and intrahepatic ducts cancer is as high as 8 per 100,000 underling population in 2010 (Fig. 1) of which at least 6 per 100,000 related to HCC. Men are at approximately three times higher risk than women. Asian men (i.e., Chinese, Korean, Filipino, and Japanese) have the highest age-adjusted incidence rates. However, the largest proportional increases have occurred among Hispanics followed by blacks and non-Hispanic whites, whereas the lowest proportional increases have occurred among Asians. In contrast to Asians/Pacific Islanders, HCC incidence rates are reported to be higher among Hispanics born in the United States than among foreign-born Hispanics2. HCC incidence rates have increased in each successive birth cohort born between 1900 and 19593 (Fig. 2). In addition, the age distribution of HCC patients has shifted to younger ages, with the greatest proportional increases among individuals 45–60 years old (Fig. 2). There is a south to north gradient in the incidence and mortality of HCC; Southern states including Texas, Louisiana, and Mississippi have some of the highest HCC incidence rates in the nation (Fig. 3). In one study, Texas Latino and especially South Texas Latinos had the highest age-adjusted HCC incidence rates (as high as 10.6/100,000)4.
Figure 1.
The time trends (1975–2010) of population-based age-adjusted incidence and mortality rates for liver and intrahepatic bile ducts cancer in the United States. Both sexes and all races are included.
Figure 2.
State-specific population-based age-adjusted incidence rates for liver and intrahepatic bile ducts cancer. Both sexes and all races are included.
Figure 3.
The time-trends of HCC by age groups and birth cohorts in the United States. Source: Altekruse SF, McGlynn KA, Reichman ME. Hepatocellular carcinoma incidence, mortality, and survival trends in the United States from 1975 to 2005. J Clin Oncol 2009;27:1485–1491.
Risk factors for HCC
Most HCC risk factors (chronic infection with hepatitis B (HBV) and/or C virus (HCV) and alcoholic liver disease) operate by promoting the development of cirrhosis. Exceptions are rare in HCV-related HCC and mostly represent cases with at least bridging hepatic fibrosis5. While most cases of HBV-related HCC also occur in the background of cirrhosis (as high as 85% in some studies)6, HBV can cause HCC in the absence of advanced fibrosis or cirrhosis. Although there are several mechanisms for non-alcoholic fatty liver disease-non-alcoholic steatohepatitis (NAFLD-NASH) related HCC conceivably in the presence of mild or no fibrosis7, there are no systematic studies to confirm or quantify this contention8. Indirect measures of severity of hepatic fibrosis such as degree of liver stiffness using elastography is associated with risk of HCC9.
The risk of developing HCC in cirrhosis patients varies with the underlying condition. The highest 5-year cumulative risks are seen in HCV cirrhosis (17% in the West and 30% in Japan), hemochromatosis (21%), HBV cirrhosis (10% in the West and 15% in Asia), alcoholic cirrhosis (8%–12%), and biliary cirrhosis (4%)10,11.
HBV
Most large studies and consistent data about the clinical course of HBV infection come from Asian countries, where HBV is endemic and vertical transmission is frequent. This pattern is different in areas that have low incidence of HCC (such as the U.S) where HBV infection is mostly acquired in adulthood either through sexual or parenteral routes. There have been only a few adequate studies in Europe and North America that determined the incidence of HCC in hepatitis B surface antigen (HBsAg)-positive individuals—most included only small numbers of HBsAg-positive patients. Nevertheless, the summary HCC incidence rate in these regions is approximately 0.02 per 100 person-years in inactive carriers (HBsAg-positive but with normal levels of alanine aminotransferase, ALT), 0.3 in subjects with chronic HBV without cirrhosis, and 2.2 in subjects with HBV related compensated cirrhosis. On the other hand, cohort studies estimated the incidence rates of HCC among subjects with chronic HBV infection in East Asian countries to be 0.2 per 100 person-years in inactive carriers, 0.6 per 100 person-years for those with chronic HBV infection without cirrhosis, and 3.7 per 100 person-years for those with compensated cirrhosis.
Several demographic (male sex, older age, Asian or African ancestry, family history of HCC), viral (higher levels of HBV replication; HBV genotype; longer duration of infection; co-infection with HCV, HIV, or HDV), and environmental factors (exposure to aflatoxin, heavy intake of alcohol or tobacco) increase HCC risk among individuals with chronic HBV2. HBV viral load appears to be an important determinant of HCC risk. The incidence of cirrhosis and HCC increased in proportion to the serum level of HBV DNA12 in Asian countries as well as Alaska13. HBV genotypes also seem to affect clinical outcomes. In North America and Western Europe, individuals with genotype D had a higher incidence of HCC than those with genotype A. Some data associate genotype B HBV with the development of HCC in young carriers without cirrhosis14;15. Mutations in the region of the HBV genome that encode the basal core promoter16, have been associated with increased HCC risk, whereas those in the precore region have been associated with decreased HCC risk17.
Aflatoxin causes a mutation at serine 249 in the tumor suppressor p53 that was detected in 30%–60% of HCC tumor samples collected from individuals in aflatoxin-endemic areas, most of whom also had HBV infections. Aflatoxin associated mutation is rarely detected in HCC cases diagnosed in the US.
HCV
HCV infection is associated with a 15- to 20-fold increase in risk for HCC compared with HCV-negative subjects. The rate of HCC in cohort studies of HCV-infected persons ranges from 1% to 3% over 30 years of chronic infection. Once HCV-related cirrhosis is established, HCC develops at an annual rate of 1%–8% (average 3.5%). Risk factors for HCC in HCV-infected individuals include male sex, co-infection with HBV or HIV, diabetes, obesity, and high level of alcohol consumption.
HCV viremia of any level is a strong risk factor for HCC compared to no viremia, however, while few studies reported a correlation between HCV viral load levels and risk of progression to cirrhosis18 or HCC, most studies did not find such an association18. Reports of the association between HCV genotype and HCC risk are inconsistent but suggest a slightly greater risk of developing HCC in patients with HCV genotype 1b, and possibly genotype 3, than patients with other HCV genotypes19.
Meta-analyses of observational studies from various countries20 report additive effects of HBV and HCV on risk for HCC (35- to 165-fold increase), although a sub-additive effect has been suggested based on more recent studies, cohort studies, and studies conducted in areas in which HBV and HCV infection were not common21. Persons co-infected with HCV (and to a lesser extent HBV) and HIV have faster progression to cirrhosis and decompensated liver disease, especially during immunosuppression, than patients with monoinfection.
For both HBV and HCV, men have 2–4 times greater risk of HCC across almost all liver disease etiologies than women. Gender-based differences in behavior and environmental exposures such as alcohol use might explain some of this difference. However, male and female sex hormones may also play a role. Nested case-control studies in China reported that baseline testosterone levels were higher in HBsAg positive males compared to age-matched controls22. There is a functional polymorphism, a trinucleotide polyglutamine (CAG) short tandem repeat, in exon 1 of the AR gene, where increasing number of CAG repeats results in decreased androgen receptor (AR) signaling. Carriage of the high risk AR allele (i.e., fewer CAGs) conveyed greater risk of HBV-related HCC in Taiwanese males22;23, whereas increased AR CAG repeats were associated with greater HCC risk in females24. Molecular data indicate that androgens contribute to the HCC development by acting as tumor promoters via induction of DNA damage and oxidative stress while estrogens may act as general suppressors of HCC through reduction in the proinflammatory effects of MyD88-mediated secretion of IL625.
Metabolic Syndrome
Patients with metabolic syndrome or with various components of metabolic syndrome (e.g., diabetes, obesity) also have higher incidence of HCC than those without metabolic syndrome. There are also emerging reports of HCC in the setting of metabolic syndrome arising in the absence of cirrhosis however the true extent of this condition or its risk factors are unclear27;28.
Diabetes
Meta-analyses of observational studies report pooled odds ratios of approximately 2.5 for the association between diabetes and HCC independent of viral hepatitis or alcohol use29;30. Cirrhosis causes glucose intolerance and type 2 diabetes, and also leads to HCC, making it difficult to interpret the association between HCC and diabetes especially in case-control and cross-sectional studies. However, this bias is less likely to be present in longitudinal studies; several cohort studies also showed a similar association between diabetes and HCC. This association is less consistent in areas with a high incidence of HBV infection than in other regions. Factors that change HCC risk among patients with diabetes are not clear, but it has been suggested that long diabetes duration and high HbA1C increase the risk, while metformin treatment decreases HCC risk31.
Obesity
A meta-analysis of 26 prospective cohort studies, including 25,337 primary liver cancer cases demonstrated that a BMI ≥ 25 kg/m2 as well as a BMI ≥ 30 kg/m2 were associated with an increased risk of primary liver cancer. The summary relative risk for a 5-unit increment in BMI was 1.39 kg/m2 (95% CI: 1.25–1.55) with the most pronounced increase in risk among persons with a BMI >32 kg/m2. The association between BMI and liver cancer was independent of geographic location, alcohol consumption, or history of diabetes. However, obese males had a higher risk of primary liver cancer than obese females. Furthermore, the association between increasing BMI and HCC was much stronger in individuals with concomitant HCV infection than in persons with HBV infection.
Nonalcoholic fatty-liver disease (NAFLD) is the hepatic manifestation of metabolic syndrome, and it affects about a third of the US adult population. Epidemiologic studies support at least a modest association between NAFLD or NASH and HCC, but this association seems to be predominantly limited to those who develop cirrhosis.
The few population-based cohort studies of patients with NAFLD are limited by low number of HCC cases and inability to identify high risk sub-groups (such as cirrhosis, obesity, diabetes8). Several cross-sectional and case-control studies have evaluated this association indirectly by concomitantly examining the prevalence of diabetes and obesity in NAFLD/NASH -related HCC cases. Diabetes and obesity prevalence was greater in the NAFLD/NASH-related HCC cases compared to their respective controls with other chronic liver diseases. Prevalence of cirrhosis among HCC cases attributed to NAFLD/NASH ranged between 36% and 90%, with the majority of studies reporting cirrhosis rates ≥70%. These studies are limited due to the difficulty in ascertaining the exposure (histopathological features for confirmed NAFLD/NASH diagnosis) once cirrhosis is established. Furthermore, cirrhosis causes glucose intolerance and type 2 diabetes, and also leads to HCC, rendering it difficult to interpret the association between HCC and diabetes and to exclude reverse causality.
Recently, a genetic polymorphism of the patatin-like phospholipase domain-containing (PNPLA3) protein has been shown to be associated with an increase in hepatic fat deposition as well as an increase in the risk of HCC development32. This and other genetic markers may serve as a part of predictive algorithms to identify high risk groups for surveillance or chemoprevention.
Alcohol
Heavy alcohol intake, defined as ingestion of >50–70 g/day for prolonged periods, is a well-established HCC risk factor. However, even moderate alcohol consumption may increase the risk of HCC in women33. There is evidence for a synergistic effect between heavy ingestion of alcohol and HCV infection and, to a lesser extent, HBV infection on HCC risk; similar synergism may be present with diabetes. These factors presumably operate together to promote cirrhosis and further increase the risk in individuals with cirrhosis34;35.
Cigarette Smoking
The association between cigarette smoking and HCC has been inconsistent with few studies finding a positive association and others finding no associations. Among studies reporting positive associations, several found that effects were limited to only those with HBV or HCV infection36.
Burden of HCC related to the major risk factors in the US
Estimates of relative risk such as risk ratios or odds ratios for any of the individual HCC risk factors do not describe their contribution to HCC burden, which is also driven by the prevalence of the risk factor in the general population. Population attributable fraction (PAF) accounts for both an estimate of relative risk as well as prevalence of a given risk factor in the population, and it describes the proportional reduction in disease that would occur if exposure to a risk factor were to be eliminated. Metabolic syndrome is likely to have the greatest PAF (Table 1). A population based study of SEER-Medicare diagnosed HCC cases reported the highest PAF was for diabetes or obesity (36.6%), followed by alcohol-related disease (23.5%), HCV (22.4%), and HBV (6.3%). Diabetes/obesity had the greatest PAF among whites and Hispanics (38.9% and 38.1%), and HCV had the greatest PAF among Asians and blacks (35.4% and 34.9%). The second greatest PAF were alcohol-related disorders in whites, Hispanics, and blacks (25.6%, 30.1%, and 18.5%) and HBV in Asians (28.5%)37. Therefore despite having the lowest relative risk among the risk factors examined, the high prevalence of diabetes/obesity translate into a high attributable fraction.
Table 1.
Burden of the main risk factors for HCC in United States.
| Prevalence in general US population |
Risk estimate of HCC* |
Current prevalence in HCC cases |
Population attributable fraction |
|
|---|---|---|---|---|
| HBV | 0.5–1% | 20–25 | 10–15% | 5–10% |
| HCV | 1–2% | 20–25 | 30–60% | 20–25% |
| Alcoholic liver disease | 10–15% | 2–3 | 20–30% | 20–30% |
| Metabolic syndrome | 30–40% | 1.5–2.5 | 20–50% | 30–40% |
relative to controls without the risk factor
HBV: hepatitis B virus; HCV: hepatitis C virus
Among patients with HCC currently diagnosed in the US, 50%–60% are infected with HCV, 10%–15% are infected with HBV, and 20–25% have alcoholic liver disease. Approximately 20%–30% of HCC cases do not have any of the previously mentioned factors but have some features of the metabolic syndrome. Several studies examined time trends of risk factors among patients with HCC in the US; HCV-related HCC had the largest proportional increases during the 1990s and early 2000, whereas the proportion of HCC associated with HBV infection and alcoholic liver disease remained relatively stable. However, based on the PAF arguments, these proportions may change in the next 2–3 decades with declining HCV-related HCC and increasing metabolic syndrome related cases.
Prevention of HCC
Prevention of HCC focuses on preventing these risk factors from ever developing (primary prevention) or, once developed, treating them at an early enough stage (secondary prevention) in order to reduce the risk of HCC. On the other hand surveillance in patients at risk for HCC is intended to detect small, treatable cancer (tertiary prevention). The section below focuses on primary and secondary prevention.
HBV Vaccination Program
National HBV vaccination programs have been the most successful prevention strategy in reducing the incidence of HCC in HBV-endemic areas. These programs have dramatically reduced the prevalence of HBV (16% to 1.4% in China, 9.8% to 1.3% in Taiwan, and 9.3% to 0.9% in Spain) with a concomitant decrease in the incidence of HCC. Since the implementation of the universal vaccination program for newborns in Taiwan in 1986 to early 1990’s, the average annual incidence of HCC in children between 6 and 14 years of age has fallen by 65 to 75%38.
In these regions, HBV is the most important risk factor for HCC, and universal vaccination programs, with almost 100% penetration, have been successful in decreasing the rate of chronic HBV in the general population. In other regions (e.g., Spain) where HBV is mainly spread by different parenteral routes, a decline in HBV likely resulted from screening of blood products and use of disposable syringes and needles. However, there are still ~400 million individuals chronically infected with HBV in the world—a group that will not benefit from immunization and remains at increased risk for HCC. Of these, an estimated ~800,000 – 1.4 million live in the U.S.
Antiviral Treatment
There is moderately strong evidence that successful antiviral therapy for HBV or HCV substantially reduces but does not eliminate the risk of HCC in patients with viral hepatitis who already developed cirrhosis; the residual annual HCC risk in those patients may exceed the 1.5% threshold required by some cost-effectiveness models for HCC surveillance.
HBV Treatment
One randomized controlled clinical trial evaluated efficacy of antiviral treatment on long-term clinical outcomes in patients with HBV. In a large Taiwanese study, patients with chronic HBV infection who also had cirrhosis or advanced fibrosis were randomly assigned to receive 100 mg of lamivudine per day or placebo for up to 5 years; the incidence of HCC was significantly reduced in the lamivudine group as compared with the placebo group (3.9% vs. 7.4%; hazard ratio, 0.49; P=0.047)39; this trial was terminated early (after a median duration of treatment of 32.4 months) because of a significant difference between treatment groups in the number of end points reached.
Papatheodoridis et al. systematically reviewed data on HCC incidence from 21 studies including 2881 patients with HBV who were treated with medium-term nucleos(t)ide analogues (lamivudine was the initial treatment in 19, emtricitabine in one, and adefovir in one study)40. Only three of the 21 studies included untreated HBV patients followed for at least 24 months (one from the only randomized controlled trial on this topic as described above). A total of 168 (4.3%) patients receiving nucleos(t)ide analogue therapy were diagnosed to have HCC during a mean/median follow-up of 40 (24–102) months. In the three studies with untreated controls, which were all of high quality and large, the rate of HCC was significantly lower in the treated (22/779, 2.8%) than in the untreated patients (34/534, 6.4%, p = 0.003). Of note, most of these data on the effect of HBV treatment on the risk of HCC come from studies that used lamivudine. Newer treatments with greater potency and better resistance profiles—such as entecavir and tenofovir—are expected to further reduce the incidence of HCC, though these data have just started to emerge41.
A multinational European cohort study reported clinical outcomes in patients treated with entecavir for a median of 20 months42. Patients with a virologic response (defined as serum HBV DNA levels <80 IU/ml) had a significantly lower probability of developing hepatic decompensation, HCC, or death (HR 0.29, 95% CI 0.08 to 1.00, p=0.05). This effect was significant among patients with cirrhosis (HR 0.22, 95% CI 0.05 to 0.99, p=0.04) but not in those without cirrhosis (HR 0.24, 95% CI 0.02 to 3.76, p=0.27). Of the 372 patients in the trial, only three developed HCC. Although encouraging, this study is limited by a relatively small number of clinical events and a short follow up. A larger study following patients for a longer duration will be required to confirm a preventive effect of entecavir treatment in patients with HBV. In the interim, the existing data show that medium-term nucleos(t)ide analogue therapy significantly reduces but does not eliminate the risk of HCC, particularly in patients with cirrhosis. The use of all oral anti-viral agents in patients with cirrhosis is associated with improved virological, biochemical and clinical parameters at 1-year with tenofovir and entecavir being the most efficacious43.
The effect of interferon on HCC incidence in patients with chronic HBV has also been evaluated in several studies and meta-analyses44–46. Most of the data suggest that interferon treatment decreases overall HCC incidence in sustained responders.
HCV Treatment
A recent systematic review of observational studies examined the risk of HCC among HCV-infected adults who had been treated and either achieved a sustained virologic response (SVR) or did not respond to therapy47. SVR was associated with a reduction in the relative risk for HCC for patients at all stages of liver disease (hazard ratio, 0.24, 95% CI = 0.18 to 0.31, P < 0.001). Approximately 1.5% of patients responding to treatment develop HCC, compared with 6.2% of those who did not respond. SVR was associated with a similar reduction in the risk for HCC (hazard ratio, 0.23, 95% CI= 0.16 to 0.35, P < 0.001) in patients with advanced liver disease or cirrhosis; approximately 4.2% of patients with SVR developed HCC compared to 17.8% of those without SVR. Most studies included in this systematic review were from Asia. Given the fact that rates of HCC are higher in Asian populations than in European or U.S. populations, the absolute benefit of SVR on HCC risk may have been overestimated. Collectively however, these data show a moderate protective effect of treatment-related SVR on the development of HCC among HCV-infected persons. However, the absolute risk of HCC does not revert to baseline levels among those with cirrhosis, older age, high α-fetoprotein levels, low platelet counts, high fibrotic stage and diabetes48;49. Furthermore, HCC incidence in nonresponders to initial antiviral therapy is not reduced by maintenance IFN therapy. Newer and more effective therapies may further stem the risk of HCC in HCV infected persons.
Treatment of Non-alcoholic Fatty Liver Disease
There is currently no direct evidence to show that treatment for NAFLD/NASH by any modality (including bariatric surgery) can reduce this risk. Several agents may have potential chemo-preventive effects in reducing the risk of HCC in patients at high risk for NAFLD (section below).
Chemoprevention of HCC
Diabetes Treatment
Several studies29 indicate that the use of insulin-sensitizing agents (such as metformin) in diabetes may reduce the risk of HCC. In a meta-analysis50 of these observational studies, metformin use was associated with a 70% reduction in the odds of HCC in patients with diabetes (odds ratio =0.30, 95% CI =0.17, 0.52; P<0.001). The proportion of patients with NAFLD/NASH was not reported. However, given that all patients had established diabetes, one expects NAFLD to be disproportionately high in these studies. Therefore, although the extent to which metformin (or similar insulin sensitizing agents) may reduce HCC risk in individuals with NAFLD/NASH remains unknown.
Statins
Epidemiological data also suggest a potential chemo-preventive effect of statins specific to HCC. In a large cohort of diabetics, we found that statin use was associated with a 54% reduction in the odds of HCC (odds ratio=0.46, 95% CI = 0.40–0.517). A recent study from Taiwan found a strong dose-response relationship between statin use and the risk of HCC in individuals with HBV [hazard ratio 0.66 (95% CI, 0.44 to 0.99), 0.41 (95% CI, 0.27 to 0.61), and 0.34 (95% CI, 0.18 to 0.67) for statin use of 28 to 90, 91 to 365, and > 365 days]. A meta-analysis of observational studies and randomized trials evaluated 4,298 cases of HCC in 1,459,417 patients, found a 41% overall reduction in HCC risk with the use of statins51. This finding was driven entirely by observational studies that are subjected to confounding by indication and healthy patient bias. There was no benefit seen in the randomized trials, however, none of the trials were powered to detect differences in HCC.
Coffee
Several studies suggested an inverse relation between coffee drinking and risk of HCC. A meta-analysis combined the results from 16 studies, 8 of which were case-cohort studies and the other 8 of which were case-control studies52. The summary relative risk of developing HCC with coffee consumption was 0.60 (95% CI: 0.50–0.71). The calculated summary RR was 0.72 (95% CI: 0.61–0.84) for low versus no coffee consumption, 0.80 (95% CI: 0.77–0.84) for each extra cup of coffee consumed daily. Consumption of caffeine from sources other than coffee or of decaffeinated coffee is generally not associated with reduced levels of liver enzymes, fibrosis or HCC.
Biomarkers such as high alpha-fetoprotein, AST and/or ALT, and low platelet counts; clinical features such as esophageal varices and type 2 diabetes have been used in various algorithms to identify and enroll patients at high HCC risk in HCC surveillance programs26. A similar approach (targeting patients at high risk for HCC) may improve the effectiveness and cost-effectiveness of chemoprevention efforts in HCC.
Future Burden of HCC
In the US, the incidence of HBV-related HCC is likely to remain steady. Though vaccination against HBV could prevent HCC, it does not prevent cancer in persons with chronic infections. A larger proportion is chronically infected with HCV—most of them are unaware of their infection status53. Testing persons at high risk for infection, educating patients, and administering effective therapies for treating HBV and HCV is therefore an important component of prevention against HCC.
A recent mathematical model based on the prevalence and natural history of HCV in the U.S. general population of HCV-infected individuals estimated that the number of HCC cases increased from 37,697 between 1990 and 1999 to 86,765 (+130%) between 2000 and 2009, with a projected increase to 130,366 (+50%) cases between 2010 and 201954. We found similar time trends in the prevalence of HCC in a national cohort of Veterans with HCV55. Among HCV infected Veterans who visited the VA in a given calendar year, the prevalence of HCC increased 19-fold from 0.07% (95% CI, 0.04% to 1.0%) to 1.3% (95% CI, 1.23% to 1.35%) from 1996 to 2006 (p<0.0001). Collectively, these data show that number of individuals with HCV related HCC has continued to rise and is projected to peak in 2019.
Although the incidence of HCV-related HCC is expected decline after 2020, this may not translate into a parallel decrease in the number of overall HCC cases. NASH-related cirrhosis is rapidly becoming an important cause of HCC. Given the very high prevalence of the metabolic syndrome in the U.S., even small increases in risk related to NASH could translate into a large number of cases of HCC.
The recent breakthrough in the management of HCV may indeed change the trajectory of HCC in the U.S. However, data show that the benefit of antiviral treatment is primarily limited to patients with successful eradication of the virus. Recent data show that approximately 45%–70% of individuals with HCV in the United States remain unaware of their infection STATUS53. Based on the mathematical model by Davis et al.54, assuming an SVR rate of ~80%, antiviral treatment will decrease cases of cirrhosis by a mere 5% in 2020. However, extending the treatment of half of infected persons would reduce HCC by 30.2%; treatment of all infected individuals would reduce the risk by 60.4% after just 10 years. Thus, a reduction in the incidence of HCC may not be achieved unless an increasing number of patients are diagnosed and treated.
The Centers for Disease Control and Prevention (CDC) recently recommended to test all persons in the United States born between 1945 and 1965 with the hope that this will identify 400,000 new persons with HCV56. However, issues related to patient consent, buy-in, and reimbursement may reduce its overall effectiveness. Furthermore, the degree to which increased identification will result in treatment with new, highly potent yet expensive treatment remains to be seen57. The success of the CDC’s plan will likely be contingent upon the use of efficient and effective means of entering and retaining individuals in HCV care—steps that will need more focused efforts in the near-term.
Acknowledgments
Grant Support: This work is funded in part by the Houston VA HSR&D Center of Excellence (HFP90-020), and the Texas Digestive Disease Center NIH DK58338. Dr. El-Serag is also supported by NIDDK K24-04-107.
Footnotes
Disclosures: There are no competing interests for this publication.
Publisher's Disclaimer: Disclaimer: The views expressed in this article are those of the authors and do not necessarily reflect the position or policy of the Department of Veterans Affairs or the United States government.
Reference List
- 1.El-Serag HB. Hepatocellular carcinoma. N Engl J Med. 2011;365:1118–1127. doi: 10.1056/NEJMra1001683. [DOI] [PubMed] [Google Scholar]
- 2.El-Serag HB, Lau M, Eschbach K, Davila J, Goodwin J. Epidemiology of hepatocellular carcinoma in Hispanics in the United States. Arch Intern Med. 2007;167:1983–1989. doi: 10.1001/archinte.167.18.1983. [DOI] [PubMed] [Google Scholar]
- 3.Altekruse SF, McGlynn KA, Reichman ME. Hepatocellular carcinoma incidence, mortality, and survival trends in the United States from 1975 to 2005. J Clin Oncol. 2009;27:1485–1491. doi: 10.1200/JCO.2008.20.7753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ramirez AG, Weiss NS, Holden AE, et al. Incidence and risk factors for hepatocellular carcinoma in Texas Latinos: implications for prevention research. PLoS One. 2012;7:e35573. doi: 10.1371/journal.pone.0035573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lok AS, Seeff LB, Morgan TR, et al. Incidence of hepatocellular carcinoma and associated risk factors in hepatitis C-related advanced liver disease. Gastroenterology. 2009;136:138–148. doi: 10.1053/j.gastro.2008.09.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Yang JD, Kim WR, Coelho R, et al. Cirrhosis is present in most patients with hepatitis B and hepatocellular carcinoma. Clin Gastroenterol Hepatol. 2011;9:64–70. doi: 10.1016/j.cgh.2010.08.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Farrell G. Insulin Resistance, Obesity, and Liver Cancer. Clin Gastroenterol Hepatol. 2013 doi: 10.1016/j.cgh.2013.07.040. [DOI] [PubMed] [Google Scholar]
- 8.White DL, Kanwal F, El-Serag HB. Association between nonalcoholic fatty liver disease and risk for hepatocellular cancer, based on systematic review. Clin Gastroenterol Hepatol. 2012;10:1342–1359. doi: 10.1016/j.cgh.2012.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Singh S, Fujii LL, Murad MH, et al. Liver Stiffness Is Associated With Risk of Decompensation, Liver Cancer, and Death in Patients With Chronic Liver Diseases: A Systematic Review and Meta-analysis. Clin Gastroenterol Hepatol. 2013 doi: 10.1016/j.cgh.2013.07.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fattovich G, Stroffolini T, Zagni I, Donato F. Hepatocellular carcinoma in cirrhosis: incidence and risk factors. Gastroenterology. 2004;127:S35–S50. doi: 10.1053/j.gastro.2004.09.014. [DOI] [PubMed] [Google Scholar]
- 11.Mancebo A, Gonzalez-Dieguez ML, Cadahia V, et al. Annual incidence of hepatocellular carcinoma among patients with alcoholic cirrhosis and identification of risk groups. Clin Gastroenterol Hepatol. 2013;11:95–101. doi: 10.1016/j.cgh.2012.09.007. [DOI] [PubMed] [Google Scholar]
- 12.Chen CJ, Yang HI, Iloeje UH. Hepatitis B virus DNA levels and outcomes in chronic hepatitis B. Hepatology. 2009;49:S72–S84. doi: 10.1002/hep.22884. [DOI] [PubMed] [Google Scholar]
- 13.McMahon BJ, Bulkow L, Simons B, et al. Relationship Between Level of HBV DNA and Liver Disease - a Population-Based Study of Hepatitits B e Antigen-Negative Persons with Hepatitis B. Clin Gastroenterol Hepatol. 2013 doi: 10.1016/j.cgh.2013.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kao JH, Chen PJ, Lai MY, Chen DS. Basal core promoter mutations of hepatitis B virus increase the risk of hepatocellular carcinoma in hepatitis B carriers. Gastroenterology. 2003;124:327–334. doi: 10.1053/gast.2003.50053. [DOI] [PubMed] [Google Scholar]
- 15.Ni YH, Chang MH, Wang KJ, et al. Clinical relevance of hepatitis B virus genotype in children with chronic infection and hepatocellular carcinoma. Gastroenterology. 2004;127:1733–1738. doi: 10.1053/j.gastro.2004.09.048. [DOI] [PubMed] [Google Scholar]
- 16.Liu CJ, Chen BF, Chen PJ, et al. Role of hepatitis B viral load and basal core promoter mutation in hepatocellular carcinoma in hepatitis B carriers. J Infect Dis. 2006;193:1258–1265. doi: 10.1086/502978. [DOI] [PubMed] [Google Scholar]
- 17.Yang HI, Yeh SH, Chen PJ, et al. Associations between hepatitis B virus genotype and mutants and the risk of hepatocellular carcinoma. J Natl Cancer Inst. 2008;100:1134–1143. doi: 10.1093/jnci/djn243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kirk GD, Mehta SH, Astemborski J, et al. HIV, age, and the severity of hepatitis C virus-related liver disease: a cohort study. Ann Intern Med. 2013;158:658–666. doi: 10.7326/0003-4819-158-9-201305070-00604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Raimondi S, Bruno S, Mondelli MU, Maisonneuve P. Hepatitis C virus genotype 1b as a risk factor for hepatocellular carcinoma development: a meta-analysis. J Hepatol. 2009;50:1142–1154. doi: 10.1016/j.jhep.2009.01.019. [DOI] [PubMed] [Google Scholar]
- 20.Shi J, Zhu L, Liu S, Xie WF. A meta-analysis of case-control studies on the combined effect of hepatitis B and C virus infections in causing hepatocellular carcinoma in China. Br J Cancer. 2005;92:607–612. doi: 10.1038/sj.bjc.6602333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cho LY, Yang JJ, Ko KP, et al. Coinfection of hepatitis B and C viruses and risk of hepatocellular carcinoma: systematic review and meta-analysis. Int J Cancer. 2011;128:176–184. doi: 10.1002/ijc.25321. [DOI] [PubMed] [Google Scholar]
- 22.Yu MW, Yang YC, Yang SY, et al. Hormonal markers and hepatitis B virus-related hepatocellular carcinoma risk: a nested case-control study among men. J Natl Cancer Inst. 2001;93:1644–1651. doi: 10.1093/jnci/93.21.1644. [DOI] [PubMed] [Google Scholar]
- 23.Yu MW, Cheng SW, Lin MW, et al. Androgen-receptor gene CAG repeats, plasma testosterone levels, and risk of hepatitis B-related hepatocellular carcinoma. J Natl Cancer Inst. 2000;92:2023–2028. doi: 10.1093/jnci/92.24.2023. [DOI] [PubMed] [Google Scholar]
- 24.Yeh SH, Chang CF, Shau WY, et al. Dominance of functional androgen receptor allele with longer CAG repeat in hepatitis B virus-related female hepatocarcinogenesis. Cancer Res. 2002;62:4346–4351. [PubMed] [Google Scholar]
- 25.Li Z, Tuteja G, Schug J, Kaestner KH. Foxa1 and Foxa2 are essential for sexual dimorphism in liver cancer. Cell. 2012;148:72–83. doi: 10.1016/j.cell.2011.11.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yeh YP, Hu TH, Cho PY, et al. Evaluation of abdominal ultrasonography mass screening for hepatocellular carcinoma in Taiwan. Hepatology. 2013 doi: 10.1002/hep.26703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Paradis V, Zalinski S, Chelbi E, et al. Hepatocellular carcinomas in patients with metabolic syndrome often develop without significant liver fibrosis: a pathological analysis. Hepatology. 2009;49:851–859. doi: 10.1002/hep.22734. [DOI] [PubMed] [Google Scholar]
- 28.Yasui K, Hashimoto E, Komorizono Y, et al. Characteristics of patients with nonalcoholic steatohepatitis who develop hepatocellular carcinoma. Clin Gastroenterol Hepatol. 2011;9:428–433. doi: 10.1016/j.cgh.2011.01.023. [DOI] [PubMed] [Google Scholar]
- 29.El-Serag HB, Hampel H, Javadi F. The association between diabetes and hepatocellular carcinoma: a systematic review of epidemiologic evidence. Clin Gastroenterol Hepatol. 2006;4:369–380. doi: 10.1016/j.cgh.2005.12.007. [DOI] [PubMed] [Google Scholar]
- 30.Wang P, Kang D, Cao W, Wang Y, Liu Z. Diabetes mellitus and risk of hepatocellular carcinoma: a systematic review and meta-analysis. Diabetes Metab Res Rev. 2012;28:109–122. doi: 10.1002/dmrr.1291. [DOI] [PubMed] [Google Scholar]
- 31.Donadon V, Balbi M, Valent F, Avogaro A. Glycated hemoglobin and antidiabetic strategies as risk factors for hepatocellular carcinoma. World J Gastroenterol. 2010;16:3025–3032. doi: 10.3748/wjg.v16.i24.3025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hassan MM, Kaseb A, Etzel CJ, et al. Genetic variation in the PNPLA3 gene and hepatocellular carcinoma in USA: Risk and prognosis prediction. Mol Carcinog. 2013 doi: 10.1002/mc.22057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Allen NE, Beral V, Casabonne D, et al. Moderate alcohol intake and cancer incidence in women. J Natl Cancer Inst. 2009;101:296–305. doi: 10.1093/jnci/djn514. [DOI] [PubMed] [Google Scholar]
- 34.Donato F, Tagger A, Gelatti U, et al. Alcohol and hepatocellular carcinoma: the effect of lifetime intake and hepatitis virus infections in men and women. Am J Epidemiol. 2002;155:323–331. doi: 10.1093/aje/155.4.323. [DOI] [PubMed] [Google Scholar]
- 35.Hutchinson SJ, Bird SM, Goldberg DJ. Influence of alcohol on the progression of hepatitis C virus infection: a meta-analysis. Clin Gastroenterol Hepatol. 2005;3:1150–1159. doi: 10.1016/s1542-3565(05)00407-6. [DOI] [PubMed] [Google Scholar]
- 36.Chuang SC, Lee YC, Hashibe M, Dai M, Zheng T, Boffetta P. Interaction between cigarette smoking and hepatitis B and C virus infection on the risk of liver cancer: a meta-analysis. Cancer Epidemiol Biomarkers Prev. 2010;19:1261–1268. doi: 10.1158/1055-9965.EPI-09-1297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Welzel TM, Graubard BI, Quraishi S, et al. Population-attributable fractions of risk factors for hepatocellular carcinoma in the United States. Am J Gastroenterol. 2013;108:1314–1321. doi: 10.1038/ajg.2013.160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Chang MH, Chen CJ, Lai MS, et al. Universal hepatitis B vaccination in Taiwan and the incidence of hepatocellular carcinoma in children. Taiwan Childhood Hepatoma Study Group. N Engl J Med. 1997;336:1855–1859. doi: 10.1056/NEJM199706263362602. [DOI] [PubMed] [Google Scholar]
- 39.Liaw YF, Sung JJ, Chow WC, et al. Lamivudine for patients with chronic hepatitis B and advanced liver disease. N Engl J Med. 2004;351:1521–1531. doi: 10.1056/NEJMoa033364. [DOI] [PubMed] [Google Scholar]
- 40.Papatheodoridis GV, Lampertico P, Manolakopoulos S, Lok A. Incidence of hepatocellular carcinoma in chronic hepatitis B patients receiving nucleos(t)ide therapy: a systematic review. J Hepatol. 2010;53:348–356. doi: 10.1016/j.jhep.2010.02.035. [DOI] [PubMed] [Google Scholar]
- 41.Gordon SC, Lamerato LE, Rupp LB, et al. Antiviral Therapy for Chronic HBV Infection and Development of Hepatocellular Carcinoma in a U.S. Population. Clin Gastroenterol Hepatol. 2013 doi: 10.1016/j.cgh.2013.09.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Zoutendijk R, Reijnders JG, Zoulim F, et al. Virological response to entecavir is associated with a better clinical outcome in chronic hepatitis B patients with cirrhosis. Gut. 2013;62:760–765. doi: 10.1136/gutjnl-2012-302024. [DOI] [PubMed] [Google Scholar]
- 43.Singal AK, Fontana RJ. Meta-analysis: oral anti-viral agents in adults with decompensated hepatitis B virus cirrhosis. Aliment Pharmacol Ther. 2012;35:674–689. doi: 10.1111/j.1365-2036.2011.04990.x. [DOI] [PubMed] [Google Scholar]
- 44.Miyake Y, Kobashi H, Yamamoto K. Meta-analysis: the effect of interferon on development of hepatocellular carcinoma in patients with chronic hepatitis B virus infection. J Gastroenterol. 2009;44:470–475. doi: 10.1007/s00535-009-0024-z. [DOI] [PubMed] [Google Scholar]
- 45.Yang YF, Zhao W, Zhong YD, Xia HM, Shen L, Zhang N. Interferon therapy in chronic hepatitis B reduces progression to cirrhosis and hepatocellular carcinoma: a meta-analysis. J Viral Hepat. 2009;16:265–271. doi: 10.1111/j.1365-2893.2009.01070.x. [DOI] [PubMed] [Google Scholar]
- 46.Sung JJ, Tsoi KK, Wong VW, Li KC, Chan HL. Meta-analysis: Treatment of hepatitis B infection reduces risk of hepatocellular carcinoma. Aliment Pharmacol Ther. 2008;28:1067–1077. doi: 10.1111/j.1365-2036.2008.03816.x. [DOI] [PubMed] [Google Scholar]
- 47.Morgan RL, Baack B, Smith BD, Yartel A, Pitasi M, Falck-Ytter Y. Eradication of hepatitis C virus infection and the development of hepatocellular carcinoma: a meta-analysis of observational studies. Ann Intern Med. 2013;158:329–337. doi: 10.7326/0003-4819-158-5-201303050-00005. [DOI] [PubMed] [Google Scholar]
- 48.Arase Y, Kobayashi M, Suzuki F, et al. Effect of type 2 diabetes on risk for malignancies includes hepatocellular carcinoma in chronic hepatitis C. Hepatology. 2013;57:964–973. doi: 10.1002/hep.26087. [DOI] [PubMed] [Google Scholar]
- 49.Chang KC, Hung CH, Lu SN, et al. A novel predictive score for hepatocellular carcinoma development in patients with chronic hepatitis C after sustained response to pegylated interferon and ribavirin combination therapy. J Antimicrob Chemother. 2012;67:2766–2772. doi: 10.1093/jac/dks269. [DOI] [PubMed] [Google Scholar]
- 50.Zhang ZJ, Zheng ZJ, Shi R, Su Q, Jiang Q, Kip KE. Metformin for liver cancer prevention in patients with type 2 diabetes: a systematic review and meta-analysis. J Clin Endocrinol Metab. 2012;97:2347–2353. doi: 10.1210/jc.2012-1267. [DOI] [PubMed] [Google Scholar]
- 51.Singh S, Singh PP, Singh AG, Murad MH, Sanchez W. Statins are associated with a reduced risk of hepatocellular cancer: a systematic review and meta-analysis. Gastroenterology. 2013;144:323–332. doi: 10.1053/j.gastro.2012.10.005. [DOI] [PubMed] [Google Scholar]
- 52.Bravi F, Bosetti C, Tavani A, Gallus S, La VC. Coffee Reduces Risk for Hepatocellular Carcinoma: An Updated Meta-analysis. Clin Gastroenterol Hepatol. 2013 doi: 10.1016/j.cgh.2013.04.039. [DOI] [PubMed] [Google Scholar]
- 53.Volk ML, Tocco R, Saini S, Lok AS. Public health impact of antiviral therapy for hepatitis C in the United States. Hepatology. 2009;50:1750–1755. doi: 10.1002/hep.23220. [DOI] [PubMed] [Google Scholar]
- 54.Davis GL, Alter MJ, El-Serag H, Poynard T, Jennings LW. Aging of hepatitis C virus (HCV)-infected persons in the United States: a multiple cohort model of HCV prevalence and disease progression. Gastroenterology. 2010;138:513–521. 521. doi: 10.1053/j.gastro.2009.09.067. [DOI] [PubMed] [Google Scholar]
- 55.Kanwal F, Hoang T, Kramer JR, et al. Increasing prevalence of HCC and cirrhosis in patients with chronic hepatitis C virus infection. Gastroenterology. 2011;140:1182–1188. doi: 10.1053/j.gastro.2010.12.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Smith BD, Morgan RL, Beckett GA, Falck-Ytter Y, Holtzman D, Ward JW. Hepatitis C virus testing of persons born during 1945–1965: recommendations from the Centers for Disease Control and Prevention. Ann Intern Med. 2012;157:817–822. doi: 10.7326/0003-4819-157-9-201211060-00529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Kanwal F, Lok AS, El-Serag HB. CDC and USPSTF 2012 recommendations for screening for hepatitis C virus infection: overview and take-home messages. Clin Gastroenterol Hepatol. 2013;11:200–203. doi: 10.1016/j.cgh.2013.01.007. [DOI] [PubMed] [Google Scholar]