Abstract
Objective
Overexpression of bcl-2 is a mechanism of drug resistance in cervical cancer. Agents that downregulate bcl-2 may decrease tumor cell threshold and sensitize tumor cells to chemotherapy. The objective of this multi-institutional phase II trial was to evaluate the efficacy and toxicity of paclitaxel and bcl-2 modulators (13-cis retinoic acid and interferon alfa-2b) in patients with advanced-stage or recurrent cervical cancer.
Methods/materials
Patients had biopsy-proven metastatic, first relapse, or persistent cervical cancer with no prior chemotherapy except for chemosensitizing agents. The treatment consisted of oral 13-cis retinoic acid 1 mg/kg and subcutaneous interferon alfa-2b 6 mU/m2, days 1-4, and intravenous paclitaxel 175 mg/m2, day 4 until disease progression or adverse events prohibited treatment. The primary endpoint was overall response rate.
Results
Thirty-three patients were enrolled between March 2001 and June 2009. Thirty-one patients were eligible for evaluation of treatment response. Twenty-seven patients (82%) received prior concurrent chemoradiation or radiotherapy alone before study enrollment. The overall response rate was 30% (six complete responses, four partial responses). Furthermore, seven patients (21%) had stable disease. Grade 3 or 4 adverse events included neutropenia (n=16, 48%), febrile neutropenia (n=1, 3%), and anemia (n=1, 3%). There were no treatment-related deaths. The median progression-free survival was 3.4 months (95% CI, 2.0-7.4 months), and overall survival was 11.2 months (95% CI, 7.5-26.2 months). Of six patients with complete responses, five survived more than two years.
Conclusions
Combination therapy with paclitaxel, 13-cis retinoic acid, and interferon alfa-2b is feasible and safe in treating patients with advanced and recurrent cervical cancer.
Keywords: cervical cancer, paclitaxel, 13-cis retinoic acid, interferon alfa-2b
INTRODUCTION
Cervical cancer is a major worldwide public health problem. Despite improvement in screening utilization, cervical cancer is the fourth most frequent cancer in women worldwide, with an annual incidence of 528,000 and mortality of 266,000 [1,2]. In the United States, cervical cancer is the thirteenth most common malignancy diagnosed in women, with an estimated annual incidence of 12,340 and mortality of 4,030 for 2013 [3].
For patients with distant metastases and recurrent disease that are not amenable to surgery, systemic chemotherapy remains the standard treatment. Different chemotherapy regimens that have demonstrated complete and partial responses include single agent therapy, such as cisplatin (23-30 %), paclitaxel (18%), gemcitabine (5-8%), and topotecan (12.5%) [4-7]. However, chemotherapy results in only temporary control of disease. Most patients who develop metastatic disease have received cisplatin with concurrent radiation and may no longer be sensitive to single-agent therapy. Therefore, cisplatin-based combination chemotherapy regimens have been studied extensively. In 2009, Gynecologic Oncology Group (GOG) 204 compared four cisplatin-based combinations. Although not statistically significant, the combination of cisplatin and paclitaxel regimen had an improved response rate (29.1%), median progression free survival (5.8%) and median overall survival (12.9 months) compared to the other arms [8]. Most recently, Tewari et al. published their GOG 240 results showing that the addition of bevacizumab to a regimen of cisplatin and paclitaxel resulted in an increased overall survival (17.0 months vs 13.3 months) [9]. Although these results are encouraging, it is clear that the eventual development of drug resistance is still a problem in the development of new therapies. Avoiding drug resistance would be pivotal in improving treatments that are active but for a short duration.
Two mechanisms of drug resistance in cervical cancer include human papillomavirus (HPV) inactivation of p53 and over expression of B-cell lymphoma 2 (bcl-2) [10-12]. Agents that downregulate bcl-2 or phosphorylate Raf-1 kinase may decrease the tumor cell threshold for apoptosis and therefore, sensitize tumor cells to chemotherapy [13]. The retinoids and interferons are two groups of biologic agents with promise to alter chemoresistance. For example, retinoids reduce the expression of bcl-2 and induce apoptosis in tumor cells that overexpress bcl-2. Dahiya et al. demonstrated the inhibition of cell growth in cell culture and nude mice with 13-cis retinoic acid (CRA), using LNCaP cell lines [14]. Argawal et al. demonstrated suppression of differentiation on HPV immortalized cervical lines by CRA. They postulated that this inhibition may reduce the extent of viral oncogene transcription and thus be useful in slowing the neoplastic process [15]. Interferon (IFN) may add to the effect of CRA, by enhancing the tumor effects and growth inhibitory effects of retinoids. Goldstein et al. found that interferon inhibited growth of anchorage dependent semiconfluent monolayers and anchorage-dependent colony formation in some cell lines [16].
There are encouraging preclinical and clinical data with combined CRA and IFN in squamous carcinoma of the skin, locally advanced untreated cervical cancer, and renal cell carcinoma [17-20]. In our institution, we studied the combination of CRA, IFN and paclitaxel for patients with prostate cancer and other advanced malignancies in a phase I study and showed the clinical safety of the combination [21]. This work revealed that cancer cells treated invitro with the combination of IFN and CRA decreased bcl-2 expression, with the maximal effect occurring at 72-96 hours. Moreover, the expression of bcl-2 was also decreased in the peripheral blood cells of patients after treatment, with maximal effect occurring on day four. Given prior studies showing effectiveness of this combination regimen, we initiated a phase II trial to evaluate the activity and toxicity of the combination of these three drugs for patients with advanced or recurrent cervical cancer, administering paclitaxel on day four in order to have the maximum bcl-2 inhibition from the other two agents.
MATERIALS AND METHODS
Eligibility
This protocol was approved by the institutional review boards of each participating institution. Written informed consent was obtained from all patients prior to enrollment. Five institutions participated in the study as part of the Cancer Institute of New Jersey Oncology Group (CINJOG).
Eligible patients had histologically or cytologically proven metastatic, recurrent or persistent cervical cancer. They were not surgical candidates because either they had distant metastases or the pelvic recurrence was not central. All patients had measurable disease by either physical exam or radiographic studies prior to treatment. Patients in whom chemoradiation was given as initial therapy needed to demonstrate progression of disease and be at least four weeks beyond the discontinuation of therapy. Patients with newly diagnosed metastatic or persistent disease who had undergone standard chemoradiation were at least three months beyond discontinuation of treatment. Other inclusion criteria included age greater than 18, and estimated life expectancy of at least six months, an Eastern Cooperative Oncology Group (ECOG) performance status of 0-2, and adequate hematologic (WBC ≥ 3500/μl, platelets ≥100,000/ μl), renal (serum creatinine ≤ 1.5 mg/ dL or creatinine clearance ≥ 50 ml/min) and hepatic function (total bilirubin ≤ 1.5mg/dl and AST, ALT ≤ 2.5x upper limit of the normal range).
Patients were excluded from study participation if they had active infections or known infection with HIV, pregnant, discontinuation of chemoradiation within four weeks, prior use of retinoids, paclitaxel or interferon, or baseline triglyceride levels > Grade I (retinoic acid may increase triglyceride levels).
Treatment Protocol
Treatment consisted of 13-cis retinoic acid 1mg/kg/day PO on days 1-4 of each cycle, interferon alfa-2b SQ 6 mU/m2 on days 1-4 of each cycle and paclitaxel 175 mg/m2 on day 4. Doses were calculated on day 1 of each cycle using the patient’s actual weight in the determination of body surface area. A variance of 5% of the calculated total doses was allowed. Cycles were repeated every 21 days. The dose was kept constant unless there was a 10% change in the patient’s weight, in which case the dose was modified accordingly. Acetaminophen 650mg orally was given prior to each interferon injection. Ketorolac tromethamine 15 mg IV was given to some patients prior to interferon as per physician’s discretion. Prior to paclitaxel administration, patients received premedication with diphenhydramine 50mg IV and ranitidine 50mg IV. Dexamethasone 20mg orally was administered 12 and 6 hours before paclitaxel and 20mg IV 30 minutes prior to paclitaxel. Treatment was discontinued in cases of progressive disease, unacceptable toxicity, or patient’s refusal or non-adherence with treatment plan.
Dose Modification
All patients were required to have an absolute neutrophil count (ANC) of more than 1500/μl on day 1 of treatment. Treatment was held up to 2 weeks if the ANC was still < 1500/μl for 2 weeks, and the patients were removed from the study. Patients with interferon-related grade 3 toxicity had a dose reduction by 50% at the discretion of the principal investigator. Patients who had a fever and ANC < 1000 had a 25% decrease in paclitaxel dose. Patients on retinoic acid who had elevated triglycerides over 2.5 x ULN were treated with gemfibrozil 600mg PO BID.
Response and Toxicity Evaluation
For data analysis, patients were evaluated for response of measurable disease after three courses of protocol therapy according to Response Evaluation Criteria in Solid Tumors (RECIST) version 1.1 criteria [22]. Complete response (CR) was defined as the complete disappearance of all measurable disease maintained for a minimum of one month. Partial response (PR) was defined as a reduction of at least 30% in the sum of the longest diameter of all target lesions maintained for a minimum of 1 month, taking as reference sum at baseline. Progressive disease (PD) was defined as an increase greater than 20% in the sum of the longest diameter of all target lesions or the appearance of one or more new lesions. Stable disease (SD) was defined as neither sufficient shrinkage to qualify for PR nor sufficient increase to qualify for PD. The overall survival (OS) was defined as the date of registration until death (censored by the date of last contact). The progression-free survival (PFS) was defined as the date of registration until disease progression or death, whichever came first (censored by the date of last contact).
All patients who received one dose of protocol therapy were evaluated for toxicity. Adverse events were assessed prior to each cycle according to the National Cancer Institute’s Common Terminology Criteria for Adverse Events version 2.0 [23]. The toxicities were graded based on the greatest severity.
Statistical analysis
The primary objective of this study was to determine the overall response (CR+PR) of the protocol regimen in women with Stage IVB or recurrent cervical cancer, using measurable disease. Secondary endpoints included adverse effects, progression free survival and overall survival. Since this was a combination therapy of paclitaxel, CRA, and IFN, the response rate was expected to be greater than that of paclitaxel alone in the referenced historical studies of 18%. The combination therapy was considered clinically interesting if the response rate was greater than 30%. A two-stage study design was applied. The two-stage design required 27 patients for the first stage and a cumulative accrual of 66 patients to the second stage. The trial needed 5/27 responses to proceed to the second stage and more than 15/66 patients for the combination therapy to be accepted. Even though the first part of the accrual was completed, patient accrual was closed prematurely prior to completion of the second phase because of slow patient registration. Given that 33 patients participated in the trial, we report the findings.
RESULTS
Patient Characteristics
Thirty-three patients were eligible for this study from five participating institutions between March 2001 and June 2009. Two patients were not evaluable for response, as one patient had insufficient assessment of tumor response, and the other patient died after having one day of treatment from a non-cancer related cause.
The baseline characteristics are presented in Table 1. The majority of patients were white, had an ECOG performance status of 0-1 and in the age range of 30-69 years old. Twenty patients (61%) had squamous histology, and twelve (36%) had either adenocarcinoma or adenosquamous carcinoma. Twenty-seven patients (82%) received prior concurrent chemoradiation or radiotherapy alone before study enrollment. Twenty-four patients (73%) had at least one extra-pelvis target lesion, including liver, lymph nodes, psoas muscle, or lung metastases.
Table 1.
Patient Characteristics
| Characteristics | No. of patients | % of patients |
|---|---|---|
| Age, years | ||
| 30-49 | 12 | 36 |
| 50-69 | 16 | 48 |
| 70-79 | 5 | 15 |
| Race | ||
| Asian | 1 | 3 |
| Black or African American | 7 | 21 |
| White | 25 | 76 |
| ECOG performance status | ||
| 0 | 19 | 58 |
| 1 | 10 | 30 |
| 2 | 4 | 12 |
| Histology | ||
| SCC | 20 | 61 |
| ASCC | 1 | 3 |
| ACC | 11 | 33 |
| Unknown | 1 | 3 |
| Prior Radiation | ||
| Yes | 27 | 82 |
| No | 6 | 18 |
| Sites of disease | ||
| Intra-pelvis alone | 9 | 27 |
| Extra-pelvis | 24 | 73 |
Abbreviations: ECOG, Eastern Cooperative Oncology Group;
SCC, squamous cell carcinoma;
ASCC, adenosquamous cell carcinoma;
ACC, adenocarcinoma cell.
A total of 184 treatment cycles were administered. A median of three cycles was administered, with a range of 1 to 22 cycles. Twenty nine patients did not require dose reductions. One patient had 25% dose reduction of paclitaxel due to prolonged neutropenia for two weeks. Three patients had dose reduction for Grade 2 peripheral neuropathy. One patient had two delays in treatment for social reasons, and one patient had one postponement in treatment because of a delay in obtaining the prescription medication. Total follow-up time is 583 person-months. The most common reason for discontinuing treatment was disease progression in 16/33 patients (48%) and complete response in 6 patients (18%).
Efficacy
The response evaluation is presented in Table 2. The protocol specified a two-stage design as stated in the statistical methods. During the first stage of the protocol, 5/27 patients were needed to have a CR or PR in order to move to the second stage. Because 9/27 patients responded, the protocol then moved to second stage. However, because of slow accrual, the protocol was closed after only 6 additional patients were added. 1/6 patients on the second stage had a partial response. The overall response rate (CR + PR) was 10/33 (30%); six patients (18%) had complete response, and 4 (12%) had partial response. Because this trial used biologic response drugs, we also report on the stable disease found in seven patients (27%), ranging from two to six months until disease progression. Therapeutic benefit (CR + PR + SD) was noted in 17 patients (51%). 36% (4/11) of patients with adenocarcinoma had either complete (3/11, 27%) or partial (1/11, 9%) response, while 30% (6/20) of patients with squamous cell carcinoma had either complete (3/20, 15%) or partial (3/20, 15%) response. 30% of patients who had either complete or partial response (3/10) had target lesions inside of the prior irradiated field. The median PFS was 3.4 months (95% C.I., 2.0-7.4 months; Fig. 1). The OS was 11.2 months (95% C.I., 7.5-26.2 months; Fig.2).
Table 2.
Response (n=33)
| No. of patients | % of patients | |
|---|---|---|
| Complete Response | 6 | 18 |
| Partial Response | 4 | 12 |
| Stable Disease | 7 | 21 |
| Progressive Disease | 14 | 42 |
| Not Evaluable | 2 | 6 |
Figure 1.
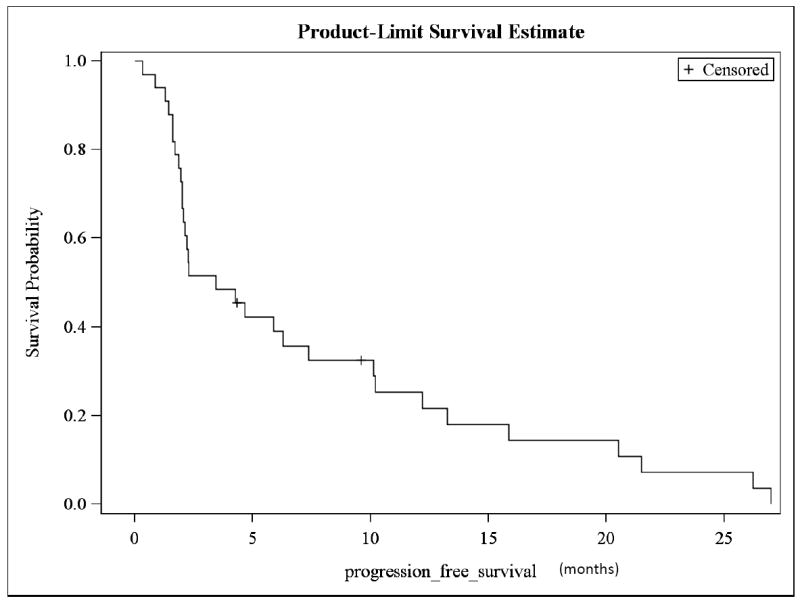
Kaplan-Meier survival curve of progression-free survival (PFS)
Figure 2.
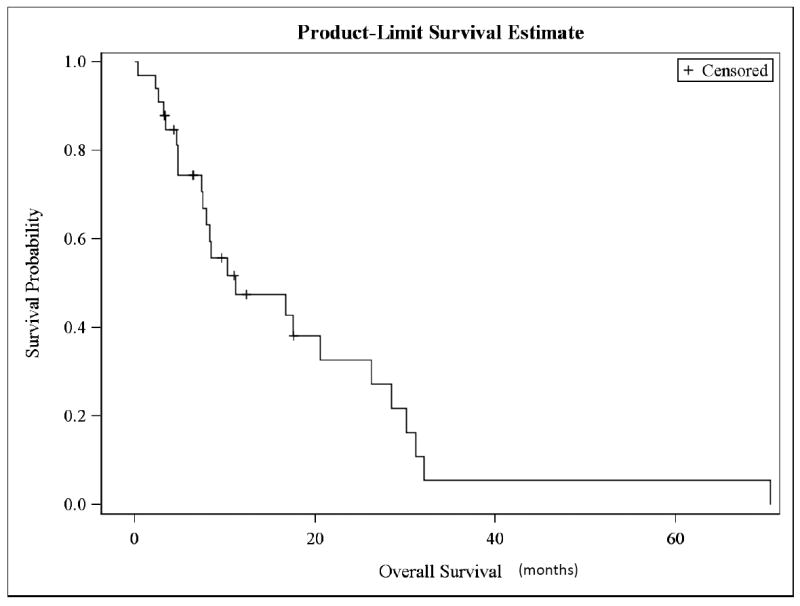
Kaplan- Meier survival curve for overall survival (OS)
Safety
A summary of major treatment related toxicities is presented in Table 3. Major adverse toxicities were hematologic, with 16 patients (48%) having grade 3 and 4 neutropenia, one patient having grade 4 febrile neutropenia, and another patient having a grade 3 anemia who required blood transfusion. Any grade non-hematologic toxicities included nausea (94%), fatigue (85%), and neuropathy (52%). Major non-hematologic toxicities included grade 3 fever in two patients and grade 3 and 4 infection in three patients. Two patients with grade 4 infection were removed from study. There were six hospital admissions while on treatment. Two patients were admitted for dehydration, two patients for infection, one patient for neck fracture after a fall, and one patient for bleeding into a mass in the psoas muscle. There were no treatment-related deaths. However, there were two deaths that were not treatment related; one was an accidental death secondary to a neck fracture and another due to cardiac disease.
Table 3.
Adverse Events
| Maximum grade (no. of patients) | ||||
|---|---|---|---|---|
|
| ||||
| Toxicity | Grade 1 | Grade 2 | Grade 3 | Grade 4 |
| Hematologic | ||||
| Neutropenia | 1 | 16 | 14 | 2 |
| Febrile Neutropenia | 1 | 1 | 0 | 1 |
| Anemia | 0 | 13 | 1 | 0 |
| Thrombocytopenia | 0 | 0 | 0 | 0 |
| Non-hematologic | ||||
| Allergy | 1 | 0 | 0 | 0 |
| Fatigue | 19 | 9 | 0 | 0 |
| Fever | 8 | 4 | 2 | 0 |
| Rigors, chills | 8 | 3 | 0 | 0 |
| Alopecia | 9 | 13 | 0 | 0 |
| Constipation | 9 | 0 | 0 | 0 |
| Diarrhea | 12 | 0 | 0 | 0 |
| Nausea | 28 | 3 | 0 | 0 |
| Vomiting | 7 | 3 | 1 | 0 |
| Sensory Neuropathy | 11 | 6 | 0 | 0 |
| Myalgia / arthralgia | 6 | 8 | 0 | 0 |
| Infection | 0 | 0 | 1 | 2 |
DISCUSSION
The study’s combination regimen has an extensive safety profile and is well tolerated by patients. The toxicities of the individual agents are not worsened by concurrent administration. The predominant toxicities are mostly grade 1 and 2 and related to interferon with flu-like syndrome with fever, myalgia, and fatigue. Only 48% of patients had neutropenia as the most common adverse event. There were no treatment-related deaths, and patients experiencing grade 3 or 4 non-hematologic toxicities were rare.
In this multi-institutional trial, the combination of paclitaxel, 13-cis retinoic acid, and interferon alfa-2b had a response rate (RR) of 30%, with an OS of 11.2 months. Remarkably, there are twelve patients (36%) who had an overall survival of 18 months or more with this combination regimen, of which three patients lived five years or more. However, because our study did not meet its planned enrollment of 66 patients, we can only assess feasibility of the regimen and cannot make definitive conclusions.
Monk et al. were the first ones to investigate prospectively the prognostic impact of the location of measurable disease [8]. They found that lesions in the irradiated field have higher risk of death. However, in the current trial, 30% of patients who had either complete or partial response had biopsy proven target lesions inside of the prior irradiated field. One hypothesis for the improved efficacy may be that although patients had prior concomitant chemo-radiotherapy with platinum administration, the intra-pelvic target lesions are naïve to paclitaxel and are not intrinsically resistant to the new combination regimen.
In this study, patients with adenocarcinoma had a 36% response rate (27% complete and 9% partial). Multiple studies have shown that patients with adenocarcinoma have poorer response rate and survival rate in both early and advanced-stage carcinoma when compared to patients with squamous cell carcinoma [24-25]. The improved response rate in adenocarcinoma in the present study could indicate that a taxane-containing regimen might be more effective for non-squamous cell histology. Curtin et al. reported a 31% response rate in patients with persistent or recurrent adenocarcinoma after administering paclitaxel [26]. Kastritis et al. showed that patients with non-squamous cell tumors who were treated with paclitaxel had a higher median survival compared to those without paclitaxel (20.3 vs. 11.7 months) [27]. Given the small sample size due to the rarity of non-squamous carcinomas, further studies, including meta-analyses, evaluating response to chemotherapy of these tumors need to be considered.
Our study tests the addition of chemotherapy to immunotherapy to enhance efficacy. In a Southwest Oncology Group Phase II trial that studied CRA plus IFN in recurrent cervical cancer, all confirmed responses were partial, and there was a response rate of only 8% [28]. Although this was not a randomized phase II trial, the addition of paclitaxel to the same immunotherapy in our study makes the utilization of biochemotherapy in advanced or recurrent cervical cancer an intriguing potential treatment modality.
This trial demonstrates that the combination therapy with paclitaxel, 13-cis retinoic acid, and interferon alfa-2b is safe in treating patients with advanced and recurrent cervical cancer. In the era of precision medicine where targeting tumor vulnerabilities is the key, our study combines drugs that target the anti-apoptotic phenotype of bcl-2 overexpression in cervical cancer and combines agents that promote tumor death through apoptosis. Given our encouraging results, newer anti-apoptotic drugs and immune modulators should be explored in this setting.
Acknowledgments
This trial was supported by National Cancer Institute grants CA 66077 and the following shared resources: Laboratory Support Services and Biometrics; Biospecimen Repository Service; and the Office of Human Research Services.
The following Cancer Institute of New Jersey Oncology Group (CINJOG) member institutions participated in this study: Rutgers Cancer Institute of New Jersey; University Hospital, Newark; Somerset Medical Center; Robert Wood Johnson University Hospital Hamilton; and the St. Peter’s University Hospital.
Footnotes
Conflict of interest statement
The authors report that there are no conflicts of interest to declare.
References
- 1.Ferlay J, Soerjomatarm I, Ervik M, et al. Lyon, France: International Agency for Research on Cancer; 2013. [March 15, 2014]. GLOBOCAN 20012 v1.0, Cancer Incidence and Mortality Worldwide: IARC CancerBase No. 11. Available at: http://globocan.iarc.fr. [Google Scholar]
- 2.Arbyn M, Castellsague X, de Sanjose S, et al. Worldwide burden of cervical cancer in 2008. Ann Oncol. 2011;22:2675–86. doi: 10.1093/annonc/mdr015. [DOI] [PubMed] [Google Scholar]
- 3.Siegel R, Naishadham D, Jemal A. Cancer statistics, 2013. CA Cancer J Clin. 2013;63:11–30. doi: 10.3322/caac.21166. [DOI] [PubMed] [Google Scholar]
- 4.Bonomi P, Blessing JA, Stehman FB, et al. Randomized trial of three cisplatin dose schedules in squamous-cell carcinoma of the cervix: a Gynecologic Oncology Group study. J Clin Oncol. 1985;3:1079–85. doi: 10.1200/JCO.1985.3.8.1079. [DOI] [PubMed] [Google Scholar]
- 5.McGuire WP, Blessing JA, Moore D, et al. Paclitaxel has moderate activity in squamous cervix cancer. A Gynecologic Oncology Group study. J Clin Oncol. 1996;14:792–5. doi: 10.1200/JCO.1996.14.3.792. [DOI] [PubMed] [Google Scholar]
- 6.Schilder RJ, Blessing JA, Morgan M, et al. Evaluation of gemcitabine in patients with squamous cell carcinoma of the cervix: a Phase II study of the gynecologic oncology group. Gynecol Oncol. 2000;76:204–7. doi: 10.1006/gyno.1999.5671. [DOI] [PubMed] [Google Scholar]
- 7.Bookman MA, Blessing JA, Hanjani P, et al. Topotecan in squamous cell carcinoma of the cervix: a Phase II study of the Gynecologic Oncology Group. Gynecol Oncol. 2000;77:446–9. doi: 10.1006/gyno.2000.5807. [DOI] [PubMed] [Google Scholar]
- 8.Monk BJ, Sill MW, McMeekin DS, et al. Phase III trial of four cisplatin-containing doublet combinations in Stage IVB, recurrent, or persistent cervical carcinoma: a Gynecologic Oncology Group study. J Clin Oncol. 2009;27:4649–55. doi: 10.1200/JCO.2009.21.8909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tewari KS, Sill MW, Long HJ, 3rd, et al. Improved survival with bevacizumab in advanced cervical cancer. N Engl J Med. 2014;370:734–43. doi: 10.1056/NEJMoa1309748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pillai MR, Halabi S, McKalip A, et al. The presence of human papillomavirus-16/-18 E6, p53, and Bcl-2 protein in cervicovaginal smears from patients with invasive cervical cancer. Cancer Epidemiol Biomarkers Prev. 1996;5:329–35. [PubMed] [Google Scholar]
- 11.Uehara T, Kuwashima Y, Izumo T, et al. Expression of the proto-oncogene bcl-2 in uterine cervical squamous cell carcinoma: its relationship to clinical outcome. Eur J of Gynaecol Oncol. 1995;16:453–60. [PubMed] [Google Scholar]
- 12.Liang XH, Mungal S, Ayscue A, et al. Bcl-2 protooncogene expression in cervical carcinoma cell lines containing inactive p53. J Cell Biochem. 1995;57:509–21. doi: 10.1002/jcb.240570316. [DOI] [PubMed] [Google Scholar]
- 13.Adam L, Crepin M, Israel L. Tumor growth inhibition, apoptosis and Bcl-2 down-regulation of MCF-7ras tumors by sodium phenlyacetate and tamoxifen combination. Cancer Res. 1997;57:1023–9. [PubMed] [Google Scholar]
- 14.Dahiya R, Park HD, Cusick J, et al. Inhibition of tumorigenic potential and prostate-specific antigen expression in LNCap human prostate cancer cell line by 13-cis-retinoic acid. Int J Cancer. 1994;59:126–32. doi: 10.1002/ijc.2910590122. [DOI] [PubMed] [Google Scholar]
- 15.Agarwal C, Rorke EA, Irwin JC, et al. Immortalization by human papillomavirus type 16 alters retinoid regulation of human ectocervical epithelial cell differentiation. Cancer Res. 1991;51:3982–9. [PubMed] [Google Scholar]
- 16.Goldstein D, O’Leary M, Mitchen J, et al. Effects of interferon beta ser and transforming growth factor beta on prostatic cell lines. J Urol. 1991;146:1173–7. doi: 10.1016/s0022-5347(17)38034-5. [DOI] [PubMed] [Google Scholar]
- 17.Lippman SM, Parkinson DR, Itri LM, et al. 13-cis-retinoic acid and interferon alpha-2a: effective combination therapy for advanced squamous cell carcinoma of the skin. J Natl Cancer Inst. 1992;84:235–41. doi: 10.1093/jnci/84.4.235. [DOI] [PubMed] [Google Scholar]
- 18.Toma S, Palumbo R, Vincenti M, et al. Efficacy of recombinant interferon alpha and 13-cis-retinoic acid in the treatment of squamous cell carcinoma. Ann Oncol. 1994;5:463–65. doi: 10.1093/oxfordjournals.annonc.a058881. [DOI] [PubMed] [Google Scholar]
- 19.Lippman SM, Kavanagh JJ, Paredez-Espinoza M, et al. 13-cis-retinoic acid plus interferon-alpha 2a in locally advanced squamous cell carcinoma of the cervix. J Natl Cancer Inst. 1993;85:499–500. doi: 10.1093/jnci/85.6.499. [DOI] [PubMed] [Google Scholar]
- 20.Motzer RJ, Schwartz L, Law TM, et al. Interferon alpha-2a and 13-cis-retinoic acid in renal cell carcinoma: antitumor activity in a phase II trial and interactions in vitro. J Clin Oncol. 1995;13:1950–57. doi: 10.1200/JCO.1995.13.8.1950. [DOI] [PubMed] [Google Scholar]
- 21.DiPaola RS, Rafi MM, Vyas V, et al. Phase I clinical and pharmacologic study of 13-cis-retinoic acid, interferon alfa, and paclitaxel in patients with prostate cancer and other advanced malignancies. J Clin Oncol. 1999;17:2213–8. doi: 10.1200/JCO.1999.17.7.2213. [DOI] [PubMed] [Google Scholar]
- 22.Eisenhauer EA, Therasse P, Bogaerts J, et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1) Eur J Cancer. 2009;45:228–47. doi: 10.1016/j.ejca.2008.10.026. [DOI] [PubMed] [Google Scholar]
- 23.National Cancer Institute. Common terminology criteria for adverse events v2.0. doi: 10.1200/JOP.2015.006106. http://ctep.cancer.gov/protocolDevelopment/electronic_applications/docs/ctcv20_4-30-992.pdf. [DOI] [PMC free article] [PubMed]
- 24.Katanyoo K, Sanguanrungsirikul S, Manusirivithaya S. Comparison of treatment outcomes between squamous cell carcinoma and adenocarcinoma in locally advanced cervical cancer. Gynecol Oncol. 2012;125:292–6. doi: 10.1016/j.ygyno.2012.01.034. [DOI] [PubMed] [Google Scholar]
- 25.Galic V, Herzog TJ, Lewin SN, et al. Prognostic significance of adenocarcinoma histology in women with cervical cancer. Gynecol Oncol. 2012;125:287–291. doi: 10.1016/j.ygyno.2012.01.012. [DOI] [PubMed] [Google Scholar]
- 26.Curtin JP, Blessing JA, Webster KD, et al. Paclitaxel, an active agent in nonsquamous carcinoma of the uterine cervix: a Gynecologic Oncology Group study. J Clin Oncol. 2001;19:1275–8. doi: 10.1200/JCO.2001.19.5.1275. [DOI] [PubMed] [Google Scholar]
- 27.Kastritis E, Bamias A, Efstathiou E, et al. The outcome of advanced or recurrent non-squamous carcinoma of the uterine cervix after platinum-based combination chemotherapy. Gynecol Oncol. 2005;99:376–82. doi: 10.1016/j.ygyno.2005.06.024. [DOI] [PubMed] [Google Scholar]
- 28.Weiss GR, Liu PY, Alberts DS, et al. 13-cis-retinoic acid or all-trans-retinoic acid plus interferon-alpha in recurrent cervical cancer: a Southwest Oncology Group phase II randomized trial. Gynecol Oncol. 1998;71:386–90. doi: 10.1006/gyno.1998.5204. [DOI] [PubMed] [Google Scholar]


