Abstract
Background
Nicotine and alcohol co-abuse is highly prevalent. Recently, we have shown that nicotine infusion in the basal forebrain (BF) increases alcohol consumption. Since nucleus accumbens (NAc) is the terminal brain region associated with drug addiction, we hypothesize that nicotine infusion in the BF may enhance alcohol induced activation of NAc.
Methods
Adult male Sprague-Dawley rats were surgically implanted with bilateral guide cannulas in the BF. Following post-operative recovery, rats were divided into four groups: 1) ACSF+W group received artificial cerebrospinal fluid (ACSF; 500 nl/side) in the BF and systemic water [intragastric (ig); 10 ml/kg; N=5], 2) EtOH group received ACSF in the BF (500 nL/side) and systemic alcohol (ig; 3 g/Kg; N=5), 3) NiC group received nicotine in the BF (75 pmole/500nl/side) and systemic water (ig; 10 mL/Kg; N=5) and 4) NiC+EtOH group received nicotine in the BF (75 pmole/500nl/side) and systemic alcohol (ig; 3 g/Kg; N=5). Rats were euthanized two hours after treatment to examine c-Fos expression in the NAc by immunohistochemistry.
Results
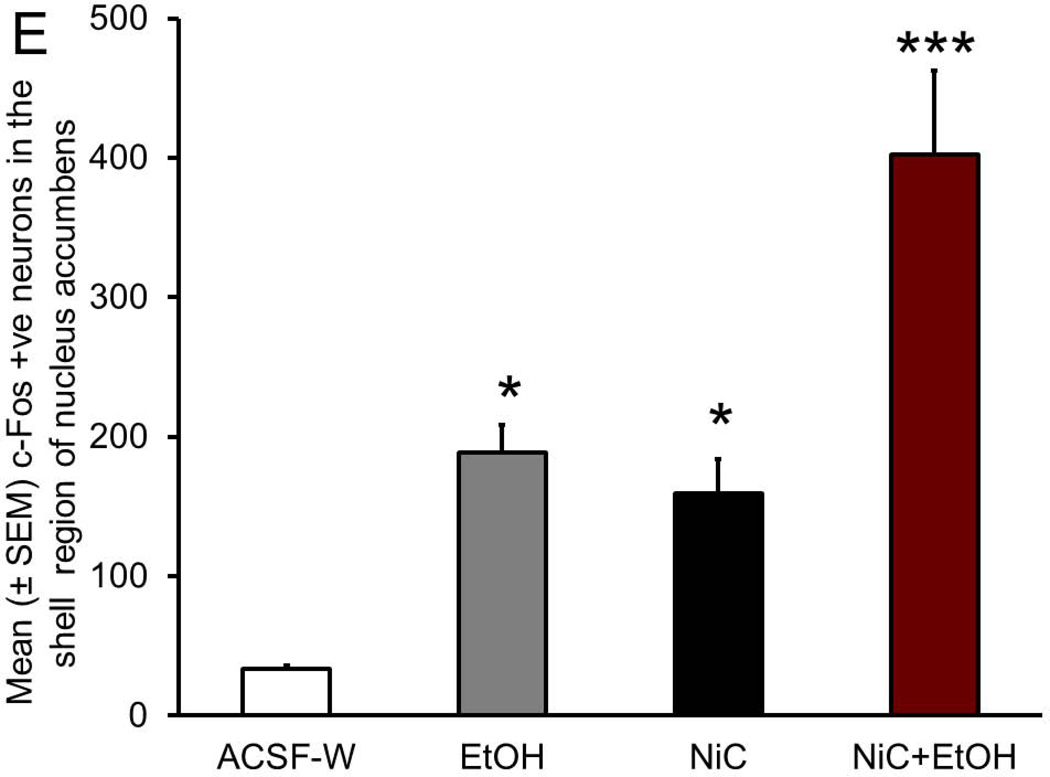
All injections sites were localized in the BF. Two way ANOVA (ig vs infusion) revealed significant main effects of both treatments (ig and infusion, p < 0.001) on c-Fos expression in the NAc shell but not in the core. Subsequent post-hoc test (Bonferroni’s) revealed that as compared to ACSF+W group, c-Fos expression was significantly increased in the shell of NAc of rats in all three (EtOH, NiC and NiC+EtOH) groups with maximal increase observed in NiC+EtOH group.
Conclusions
The results suggests: 1) BF nicotine infusion induced c-Fos in both core and the shell region of NAc at levels comparable to those observed after systemic alcohol administration; 2) BF nicotine infusion with systemic alcohol induced a significant additive increase in c-Fos expression only in the NAc shell region. These findings implicate the BF in alcohol and nicotine co-use.
Keywords: Nicotine, alcohol, basal forebrain, Nucleus accumbens, c-Fos
INTRODUCTION
The use and abuse of alcohol and nicotine, an addictive substance in tobacco, is a serious worldwide health problem. According to the World Health Organization, more than 7 million deaths every year are attributed to alcohol and nicotine use (WHO, 2013; WHO, 2008). Epidemiological studies suggest a shared vulnerability to nicotine and alcohol abuse whereby one substance influences the use of the other (Doyon et al., 2013). Approximately 90% of alcoholics smoke and approximately 60% of smokers consume substantial amount of alcohol (Batel et al., 1995; Dawson, 2000). In addition, alcohol consumption potently increases ad-lib smoking, enhances craving to smoke and enhances positive feelings associated with smoking (Harrison et al., 2009). While the amount of alcohol consumed and the degree of alcohol dependency is positively correlated with the amount of nicotine (tobacco) consumed, drugs used for smoking cessation therapy reduce alcohol consumption (Mitchell et al., 2012; Fucito et al., 2011).
Studies conducted in rodents also demonstrate an intimate relationship between alcohol and nicotine use. For example, rodents exposed to nicotine increase alcohol self-administration (Le et al., 2003; Lopez-Moreno et al., 2004), whereas systemic or central administration of nicotine receptor partial agonist or antagonist reduces alcohol consumption and/or preference (Bito-Onon et al., 2011; Steensland et al., 2007; Blomqvist et al., 1996). Although nicotine is believed to be one of the greatest risk factor for the development of alcoholism, little is known about underlying neurobiological mechanisms responsible for nicotine and alcohol co-abuse (Davis and de Fiebre, 2006).
Compelling clinical and preclinical evidence suggests that nicotine and alcohol may act via a common neuronal substrate: nicotinic acetylcholine receptor (nAChR), especially the α4β2 and α7 nAChR subtypes. Indeed, while alcohol enhances the function of heteromeric α4β2 subtype, it inhibits α7 nAChR subtype (Cardoso et al., 1999; Taslim et al., 2011; Taslim and Saeed, 2010; de Fiebre and de Fiebre, 2005; Narahashi et al., 2001; Blomqvist et al., 1992; Butt et al., 2003). Furthermore, varenicline, a partial agonist of α4β2 containing nAChRs reduces alcohol seeking and consumption in rodents (Steensland et al., 2007; Chatterjee and Bartlett, 2010).
Recently, we have observed that bilateral nicotine infusion in the wake-promoting cholinergic basal forebrain (BF), which exhibits high expression of α4β2 and α7 nAChR, increases binge alcohol consumption in C57BL/6J mice (Sharma et al., 2014a). Since rewarding or reinforcing properties of alcohol and/or nicotine are associated with the activation of nucleus accumbens (NAc) (Koob and Volkow, 2010; Pontieri et al., 1996; Pontieri et al., 1998), we hypothesized that bilateral nicotine infusion in the BF will enhance alcohol induced activation of NAc.
MATERIALS AND METHODS
Experimental Design
Neuronal activation is indicated by induction of c-Fos (Thakkar et al., 2010; Shiromani et al., 1992; Morgan and Curran, 1991; McKenna et al., 2009). Thus, activation of NAc was examined by monitoring c-Fos expression with immunohistochemistry (IH).
Both the BF and NAc are implicated in sleep regulation (Sharma et al., 2010; Thakkar et al., 2003; Qiu et al., 2012). Therefore, to prevent any confounds, all experiments were performed during the dark period when rats are maximally active.
Animals
Adult male Sprague–Dawley rats (350 – 450 g) were obtained from Charles River Laboratories (Willmington, MA) and housed in the Harry S. Truman Memorial Veterans Hospital vivarium, under constant room temperature, and 12:12 hour light-dark cycle with food and water ad libitum. All animals were allowed to recover from transportation stress and habituated to the light:dark cycle for at least 7 days and before any experimentation was begun.
All animals were treated in accordance with the American Association for Accreditation of Laboratory Animal Care’s policy on care and use of laboratory animals. All experiments were conducted in accordance with the Guide for the Care and Use of Laboratory Animals and all experimental protocols were approved by the Committee for Animal Studies of the Harry S. Truman Memorial Veterans Hospital
Chemicals
Alcohol (35% v/v) was prepared by mixing ethanol (200 proof, Fisher Scientific, Pittsburgh, PA) in sterile water. 1 mM stock solution of (-)-Nicotine hydrogen tartrate (or Nicotine; Sigma-Aldrich Co. LLC, St Louis, MO) was prepared with artificial cerebrospinal fluid (ACSF = 147 mM NaCl, 3 mM, KCl, 1.2 mM CaCl2 and 1.0 mM MgCl2; pH 7.0), aliquoted and stored at −20°C. The working solution was prepared fresh on the day of the experiment.
Surgery
Stereotaxic surgery was performed to implant chronic bilateral guide cannulas above the BF region. Under isoflurane anesthesia, rats were mounted onto the stereotaxic apparatus (Model 900, David Kopf Instruments, Tujunga CA) and 23 gauge stainless steel guide cannulas were bilaterally implanted at 90 degree angle 2 mm above the substantia innominata/horizontal limb of diagonal band (SI/HDB) region of the BF [co-ordinates: AP = + 0.4, ML= 2.0, DV= 8.5: relative to bregma; see (Paxinos and Watson, 2007)] along with two anchor screws. Stylets were inserted in guide cannulas to maintain patency (Thakkar et al., 2001). The entire assembly was secured to the skull with dental cement (Thakkar et al., 2008b).
Post- surgical recovery and habituation
To prevent any damage to the head-post and/or guide cannulas, immediately after surgery, each rat was housed individually in an experimental cage [similar to home cage except taller with open top and a grommeted hole on one (shorter) side of the cage for the dispensing water] and allowed to undergo post-operative recovery for three days followed by three days of habituation with the insertion of the gavage needle (for systemic administration of alcohol or water) and sham microinjections (Thakkar et al., 2008a).
Experimental Protocol
After completion of the habituation, the experiment was begun at dark onset. All treatments were performed in wake animals. Rats were divided in four groups: ACSF+W, EtOH, NiC and NiC+EtOH. ACSF (500 nl/side) was bilaterally microinjected into the BF of ACSF+W and EtOH group, whereas nicotine [75 pmol (equivalent to 11 ng nicotine free base)/500 nl/side] was bilaterally microinjected into the BF of the NiC and NiC+EtOH groups as described previously (Thakkar et al., 2008a, 2010). In brief, the rat was gently swaddled in a towel. The stylus was removed. Custom fabricated stainless steel, internal cannula [27 G: length = 20 mm; extends 2 mm out of the guide] connected to a 0.5 µl Hamilton syringe (Hamilton, Reno, NV) via FEP connector [Eicom, San Diego, CA] was gently inserted. Once the injector was in place, and after waiting for approximately 1 min, nicotine (or ACSF) was slowly (manually) injected. On completion, the internal cannula was kept in place for 1 min before retracting. The same protocol was repeated on the contralateral side.
Immediately after completion of microinjections, alcohol (ig; 3 g/Kg) was administered to EtOH and NiC+EtOH groups. Rats in NiC and ACSF+W group were administered water (ig; 10 ml/kg). Thus, while rats in the ACSF+W group received ACSF in the BF and systemic water, rats in the EtOH group received ACSF in the BF and systemic alcohol. Similarly, while NiC group rats received nicotine in the BF and systemic water, NiC+EtOH group rats received nicotine in the BF followed by systemic alcohol. Subsequently, rats were left undisturbed for two hours and then euthanized under deep phenobarbital anesthesia and perfused transcardially with 200 mL of 0.9% saline followed by perfusion of 200 mL 10% formalin (Sigma Chemicals, St Louis, MO). Brains were removed and post-fixed in the same fixative overnight and equilibrated in 20% sucrose at 4°C. Once equilibrated, brains were blocked and sectioned into 40 µm coronal sections using a freezing microtome (Reichert Scientific Instruments, Buffalo, NY).
Localization of BF injection sites
Nissl (cresyl violet) staining was performed to localize microinjection sites in the BF as described previously (Thakkar et al., 2002). In brief: A series of one-in four sections containing the SI/HDB region was mounted on subbed slides and dried overnight. On the following day, sections were rehydrated by immersing slides in each of the following solutions (5 min/solution): Histoclear, 100%, 95%, 70%, 50%, and 30% alcohol followed by distilled water and subsequent staining in 0.5 % cresyl violet (Sigma) in acetate buffer (pH 3.9) for 20 minutes. Next, the sections were differentiated in distilled water for 5 minutes and then dehydrated through 70, 95 and 100 % alcohol followed by Histoclear. On completion, slides were cover-slipped using Permount mounting medium (Fisher Scientific).
c-Fos Expression in the NAc
A series of one-in four sections containing the NAc region was selected for c-Fos IH. All washings were performed thrice (10 min each time) in 100 mM phosphate buffered saline (PBS; pH 7.4). The entire procedure was performed at room temperature. Day 1: Sections were washed and incubated in PBS containing 0.3% hydrogen peroxide and 20% methanol for 20 min followed by 2 hours incubation in blocking solution [3% normal donkey serum (Jackson ImmunoResearch, West Grove PA) and 0.3% Triton X-100 (Sigma Chemicals, St. Louis MO)]. Next, sections were incubated overnight in a blocking solution containing primary, rabbit anti-c-Fos, antibody (1: 5000; Calbiochem, Rockland MA). Day 2: Sections were washed and incubated in biotinylated donkey anti-rabbit secondary antibody (1:400; Jackson ImmunoResearch) for 2 hours followed by washing and incubation in Avidin Biotin Complex (ABC; Vector, Burlingame CA) for 1 hour. Next, sections were washed and incubated in 0.05% 3, 3'-diaminobenzidine solution containing 0.05% nickel chloride and 0.015% hydrogen peroxide until desired black color intensity was confirmed under a microscope. Next, sections were mounted on subbed slides, dried, processed through alcohols, and cover-slipped as described above. Control IH was performed by eliminating primary antibodies.
Quantification of c-Fos Expression in NAc
A single person blind to the treatment conditions performed counting of immunoreactive neurons in NAc. In each animal, three representative sections [between AP = +1.8 and +0.9 mm from bregma; see (Paxinos and Watson, 2007)] were selected and used for the quantification of c-Fos expression. Under low magnification (5× objective, Leica microscope, DM 4000B), one photomicrograph of the entire NAc (both shell and core) was taken with Spot camera (version 4.0.9; Diagnostic Instruments, Inc., Sterling Heights, MI, USA) and all c-Fos +ve neurons (brown/black nucleus) were manually and separately counted for NAc shell and core regions with the Adobe Photoshop CS3 software counting tool, summed and tabulated for further analysis.
Statistical analysis
Two-way ANOVA followed by Bonferroni’s post hoc test (GraphPad Prism; Graphpad Software, San Diego, CA) was used to determine the effects of BF nicotine and systemic alcohol on the c-Fos expression in the NAc with α = 0.05.
Blood alcohol concentration (BAC)
BAC was examined in EtOH and NiC+EtOH group to verify that activation of NAc was not associated with BAC. The protocol for BAC measurement was as follows: Immediately before the animal was euthanized, the blood (approximately 400 µl) was removed from the tail vein. The plasma was separated and BAC was analyzed using an Ethanol Assay Kit (Biomedical Research Service, Buffalo, NY) as per manufacturer’s instructions (Sharma et al., 2014b).
RESULTS
Statistical power analysis and sample size
Pilot studies conducted to calculate the sample size suggested an N = 5/group was sufficient to provide statistical power > 0.8 at α = 0.05 with two way ANOVA.
Localization of the injection sites in the BF
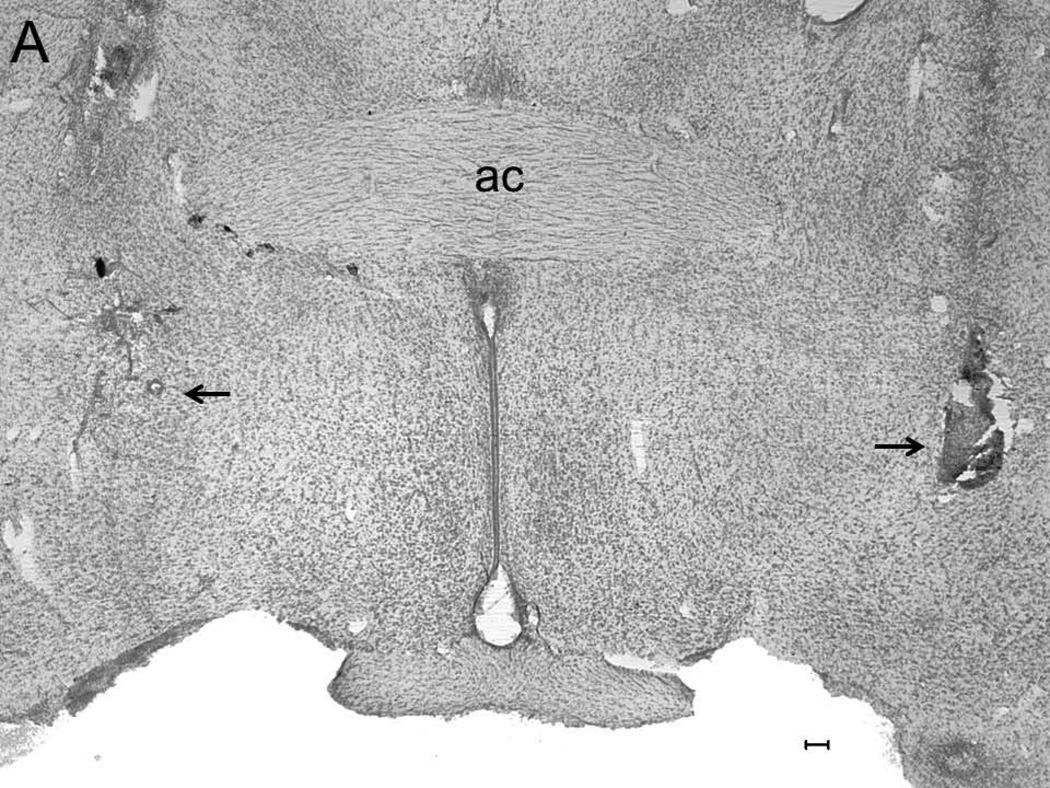
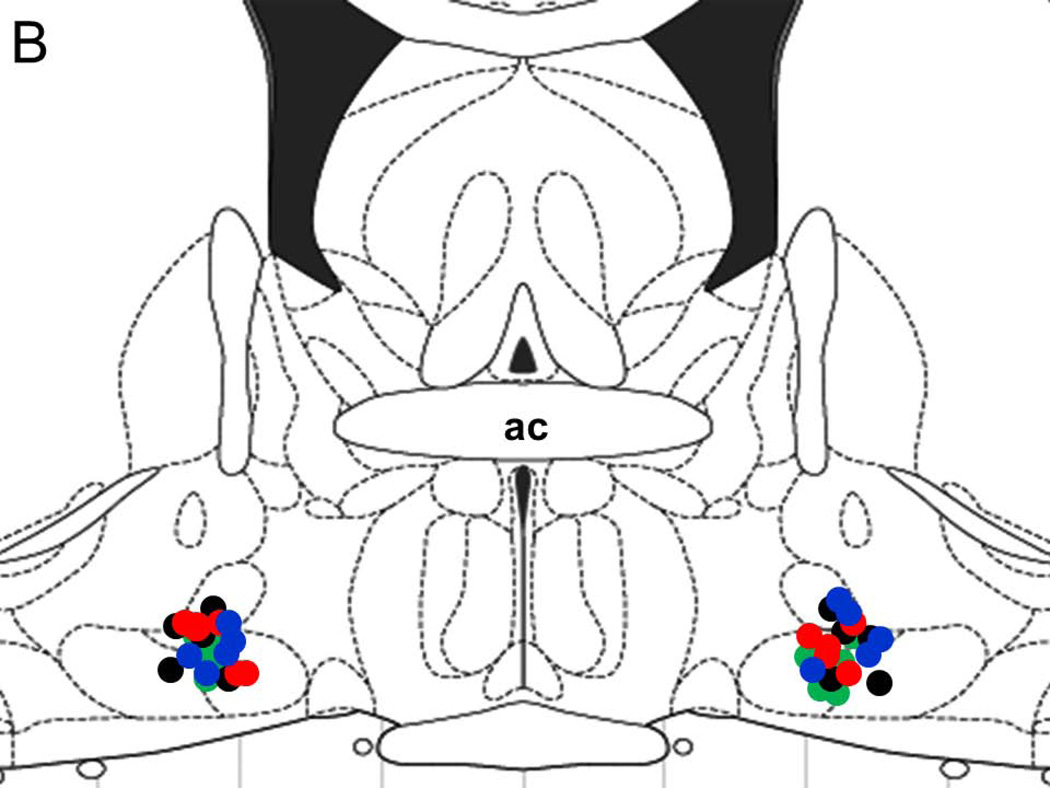
A representative photomicrograph describing lesions caused by placement of microinjectors for injections into the BF is described in Figure 1A. All injection sites (N=20; 5 rats/group) were localized in the SI/HDB region of the BF and are mapped onto one coronal schematic [AP = −0.34; adapted from (Paxinos and Watson, 2007)] in Figure 1B.
Figure 1.
A representative photomicrograph depicting lesions (arrows) caused due to bilateral microinjections in the basal forebrain (BF). Abbreviations: ac, anterior commissure; Calibration bar: 100 µm (Panel A). All injection sites were localized in the BF and a schematic representation of all injection sites (N=20; 5/group) mapped on one coronal schematic (AP = −0.3; adapted from Paxinos and Watson 2007) is described in Panel B. Circles denote injection sites. Blue circle = ACSF infusion in ACSF+W group; Black circle = ACSF infusion in EtOH group; Green circle = nicotine infusion in NiC group and Red circle = nicotine infusion in NiC+EtOH group.
c-Fos expression in the shell region of NAc
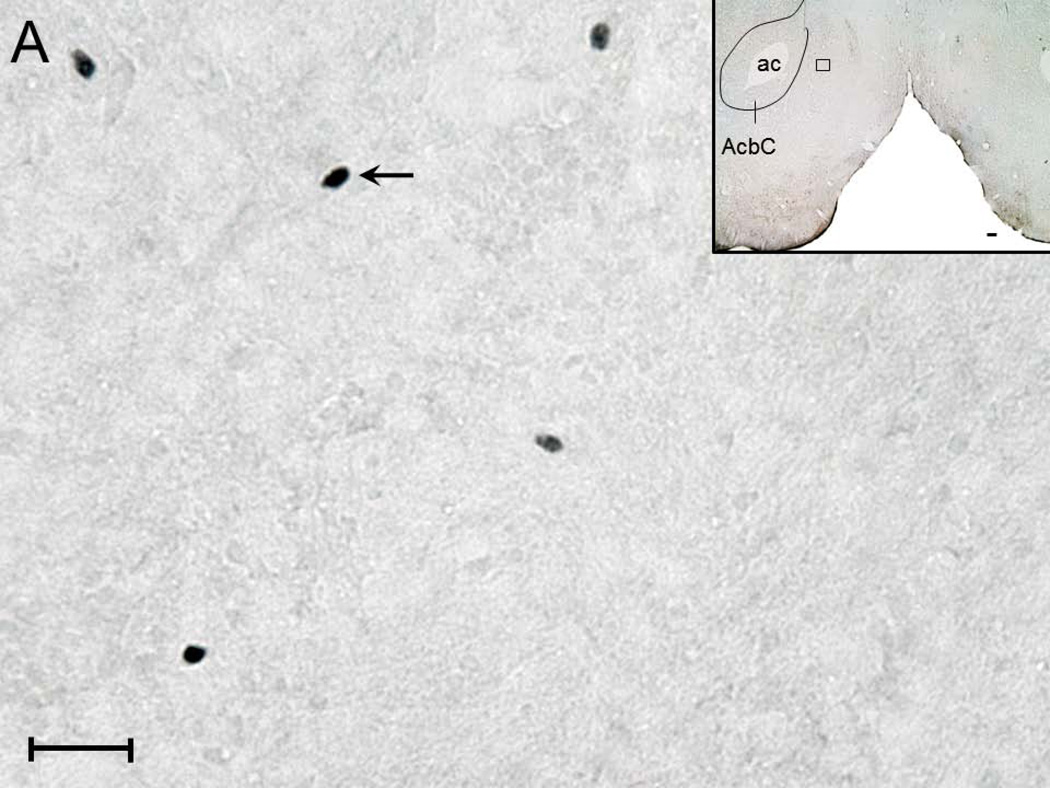
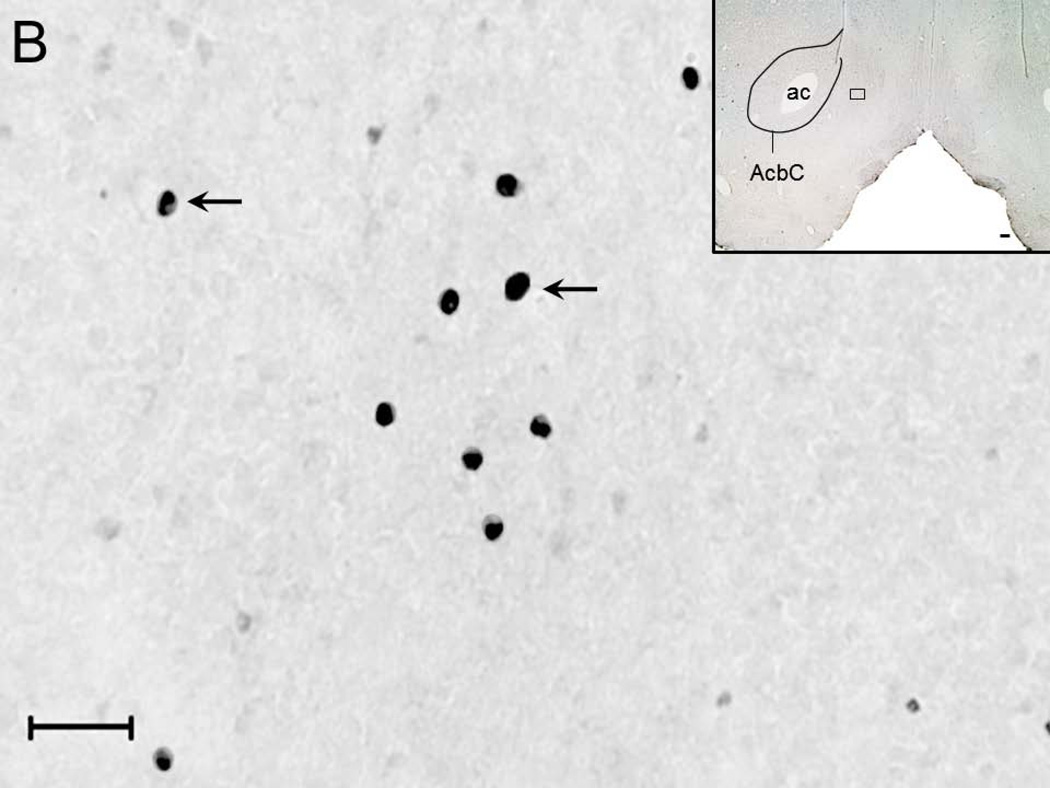
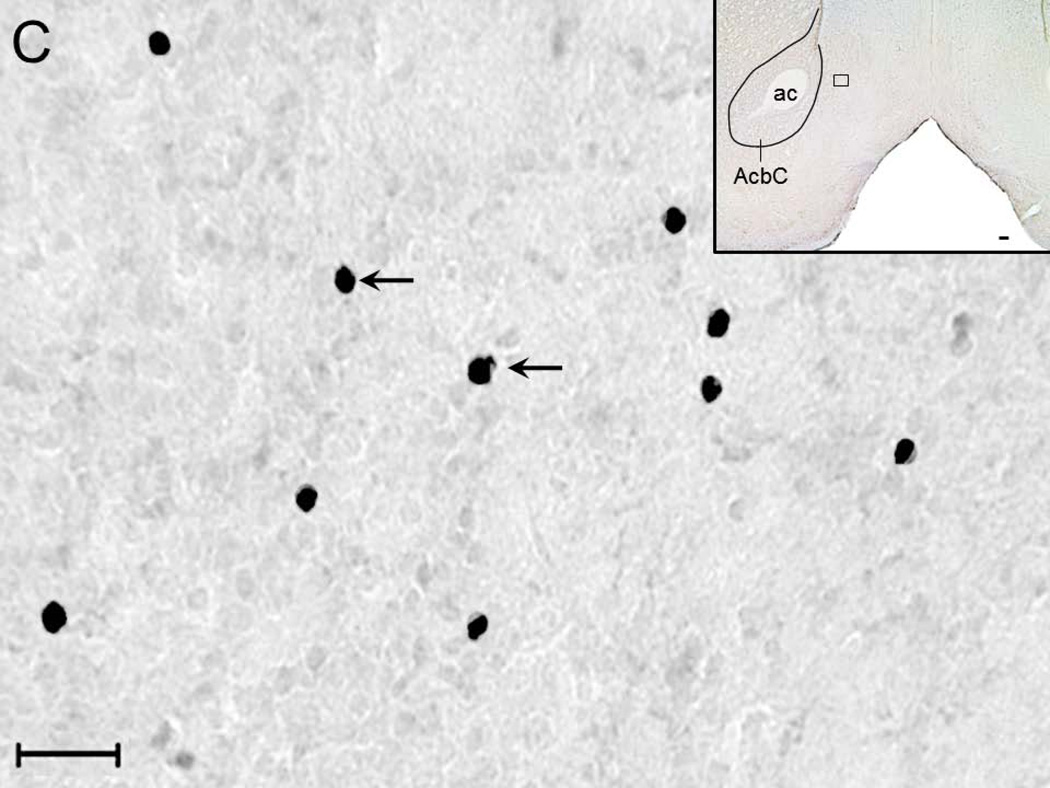
A representative photomicrograph (high magnification; 40x objective) depicting c-Fos expression in the NAc shell region of rats from ACSF+W (Figure 2A), EtOH (Figure 2B), NiC (Figure 2C) and NiC+EtOH (Figure 2D) group is described. Two-way ANOVA (intragastric vs infusion) revealed a significant main effect of both treatments (intragastric, F1,16 = 34.5; p < 0.0001; infusion F1,16 = 25.0; p = 0.0001) on c-Fos expression in the NAc shell region. Subsequent post-hoc (Bonferroni’s) analysis revealed that as compared to ACSF+W group, c-Fos expression was significantly increased in the NAc of rats in EtOH (p < 0.05), NiC (p < 0.05) and NiC+EtOH (p < 0.001) groups. However, c-Fos expression was comparable between EtOH and NiC groups (Figure 2E).
Figure 2.
Representative high magnification photomicrograph depicting c-Fos +ve nuclei (arrows) in the NAc shell region of rats exposed to: systemic water + BF ACSF administration (Panel A); systemic alcohol + BF ACSF administration (Panel B); systemic water + BF nicotine administration (Panel C) and systemic alcohol + BF nicotine administration (Panel D). Calibration bar: 30 µm. The boxes in the insets describe the localization of the photomicrograph in the NAc shell. Calibration bar: 100 µm. Abbreviations: ac, anterior commissure; AcbC, core region of NAc. The graphical representation of the results is described in Panel E.
A total of 168 (Mean ± SEM = 34 ± 1.9; N=5) c-Fos +ve neurons were counted from the NAc shell region of the rats in ASCF+W group; 944 (Mean ± SEM = 189 ± 19.8; N=5) in rats of EtOH group; 796 (Mean ± SEM = 159 ± 24.4; N=5) in rats of NiC group. In contrast, the shell region of the NAc in the rats of NiC+EtOH group showed a total of 2011 (Mean ± SEM = 402 ± 60; N=5) c-Fos +ve neurons. Thus, as compared to single nicotine or alcohol administration combined BF nicotine and systemic alcohol administration more than doubled c-Fos expression in the shell region of NAc.
c-Fos expression in the core region of NAc
In contrast to the NAc shell region, no significant effect of treatments (intragastric F1,16 = 0.4; p = 0.5; infusion F1,16 = 0.39; p = 0.5, Two-way ANOVA; N=5/group) was observed on c-Fos expression in NAc core. A total of 1307 (Mean ± SEM = 261 ± 35; N=5) c-Fos +ve neurons were counted from the core region of rats in the ACSF+W group; 1295 (Mean ± SEM = 259 ± 38; N=5) the core region of rats in the EtOH group; 1289 (Mean ± SEM = 258 ± 66; N=5) from NAc core of rats in NiC groups and 1600 (Mean ± SEM = 320 ± 39; N=5) from NAc core of rats in NiC+EtOH group.
BAC
BAC levels were comparable in EtOH (Mean ± SEM = 140.4 ± 11.4; N=5) and NiC+EtOH (Mean ± SEM = 128.7 ± 6.4; N=5) groups suggesting that the change in the c-Fos expression was not associated with BAC.
DISCUSSION
The major findings of this study are: 1) Nicotine infusion in the BF activated NAc as evident by induction of c-Fos in both core and the shell region of NAc at levels comparable to those observed after systemic alcohol administration but significantly higher than ACSF+W controls in the shell region; 2) local BF nicotine infusion coupled with systemic alcohol administration induced a significant, additive, increase in c-Fos expression only in the shell region of the NAc as compared to single BF nicotine or systemic alcohol. The c-Fos expression in the NAc core region was comparable across all four groups. BAC values were comparable between EtOH and NiC+EtOH group suggesting that increase in c-Fos expression observed in NiC+EtOH was not associated with BAC. Based on these finding, we suggest that the BF may have an important role in alcohol and nicotine co-use.
The strength of our study is simple experimental design to demonstrate the role of BF in alcohol and nicotine co-abuse. We chose to infuse nicotine in the BF, instead of systemic or central administration, for specificity, to examine the role of BF in nicotine and alcohol co-abuse. We chose the 150 pmol/rat dose of nicotine based on our unpublished observations which suggest that infusion of 75 pmol/side dose of nicotine in the BF of freely behaving rats attenuates the aversive, sleep promoting effects of alcohol. Furthermore, in vitro and in vivo studies have used similar doses of nicotine to examine neuronal activation as well as alcohol seeking in rats (Saint-Mleux et al., 2004; Hauser et al., 2014).
Since lab rats do not readily consume alcohol, we chose the ig route to administer alcohol because this is similar to the method of alcohol consumption in humans and does not involve pain which is inherent in the intraperitoneal method of alcohol administration. We chose the 3 g/Kg dose of alcohol because this dose has been shown to promote sleep and activate NAc (Thakkar et al., 2010; Alaux-Cantin et al., 2013). However, one limitation of this route (ig) of alcohol administration is that rats do not develop conditioned place preference (Fidler et al., 2004).
In this study, c-Fos expression was monitored to examine the activation of NAc. Although the relationship between measures of c-Fos expression and measures of spike activity in single units is unresolved, induction of c-Fos expression is widely used as a tool to delineate neurons activation and neuronal pathways in response to a wide variety of stimuli including alcohol and/or nicotine co-abuse (Vilpoux et al., 2009; Bachtell and Ryabinin, 2001; Pich et al., 1997). We did not determine the phenotype of activated neurons in the NAc and this may be considered as a limitation of this study. However, since majority of neurons (~90%) in the NAc use GABA as the neurotransmitter (Gonzales et al., 2004), it is very likely that the activated neurons are GABAergic.
Strong and converging evidence suggest that the mesocorticolimbic dopaminergic system is the primary neuronal system mediating drug addiction and/or reinforcing properties of drugs of abuse (Koob and Volkow, 2010; Hyman et al., 2006).Within the mesocorticolimbic system, the ventral tegmental area (VTA) contains dopaminergic neurons and is the source of dopamine inputs to the NAc (Koob and Volkow, 2010). All addictive drugs, including alcohol and nicotine, increase dopamine and c-Fos expression preferentially in the shell region of the NAc (Pontieri et al., 1996; Pich et al., 1997; Bachtell and Ryabinin, 2001; Tizabi et al., 2007).
One important reason why people begin to use “recreational drugs” is to enjoy “recreational, euphoric and/or pleasurable sensations” and activation of the NAc is associated with feelings of pleasure and/or euphoria (Chatterjee and Bartlett, 2010; Robinson and Berridge, 2001). Thus, the result of this study may suggest that nicotine via its action on the BF enhances alcohol induced activation of NAc, a critical anatomical substrate in the mesolimbic “reward pathway”. Furthermore, since the BF is also involved in control of sleep-wakefulness, cognition and attention, nicotine via its activation of the BF may mitigate the “aversive effects” of alcohol including sleep promotion, attention deficits and cognitive impairments (Froehlich and Li, 1991; Chatterjee and Bartlett, 2010; Thakkar et al., 2010). Indeed, recently we have observed that nicotine infusion in the BF increases alcohol consumption in mice exposed to binge alcohol drinking under the “drinking in the dark” protocol (Sharma et al., 2014a). Thus, taken together, these results may suggest that nicotine, when consumed with alcohol, may act via the BF to attenuate the aversive sleep promoting effects while enhancing the activation of the NAc, the reward center. While BF nicotine and systemic alcohol increased c-Fos expression in the shell region, no significant change was observed in the core region of the NAc. Although the precise role of core and shell regions of NAc in reward-dependent behaviors is being debated, it has been suggested the NAc shell may mediate the initiation or induction of drug-seeking behavior, where the core region may regulate the conditioning processes associated with drug-seeking behavior (Ito et al., 2004; Gonzales et al., 2004).
Although the mechanism of how nicotine infusion in the BF enhances the activation of NAc is unclear and under investigation, previous studies suggests the BF has strong cholinergic and non-cholinergic projections to the cortex and activation of cortex results in increased glutamate in the NAc. Increase glutamate in NAc has been implicated in both, alcohol and nicotine addiction (Liechti et al., 2007; Griffin Iii et al., 2014; D'Souza and Markou, 2014). Ongoing studies in the lab are examining glutamate release in the NAc to delineate the mechanism of how nicotine in the BF enhances alcohol induced activation of the NAc.
In summary, the results of this study suggest that nicotine infusion in the BF enhances alcohol induced increases in c-Fos expression in the NAc implicating the BF in alcohol and nicotine co-use.
ACKNOWLEDGEMENTS
This work is supported by resources, including the use of facilities, from Research Services Harry S. Truman Memorial Veterans Hospital, and funded by research grants (AA020334 and AA0174720) from National Institute of Alcohol Abuse and Alcoholism. We thank Carrie Harris for animal care and Shafi Lodhi and Tamarea Jones for helping with cell counting.
REFERENCE LIST
- Alaux-Cantin S, Warnault V, Legastelois R, Botia B, Pierrefiche O, Vilpoux C, Naassila M. Alcohol intoxications during adolescence increase motivation for alcohol in adult rats and induce neuroadaptations in the nucleus accumbens. Neuropharmacology. 2013;67:521–531. doi: 10.1016/j.neuropharm.2012.12.007. [DOI] [PubMed] [Google Scholar]
- Bachtell RK, Ryabinin AE. Interactive effects of nicotine and alcohol co-administration on expression of inducible transcription factors in mouse brain. Neuroscience. 2001;103:941–954. doi: 10.1016/s0306-4522(01)00042-2. [DOI] [PubMed] [Google Scholar]
- Batel P, Pessione F, Maitre C, Rueff B. Relationship between alcohol and tobacco dependencies among alcoholics who smoke. Addiction. 1995;90:977–980. doi: 10.1046/j.1360-0443.1995.90797711.x. [DOI] [PubMed] [Google Scholar]
- Bito-Onon JJ, Simms JA, Chatterjee S, Holgate J, Bartlett SE. Varenicline, a partial agonist at neuronal nicotinic acetylcholine receptors, reduces nicotine-induced increases in 20% ethanol operant self-administration in Sprague-Dawley rats. Addict Biol. 2011;16:440–449. doi: 10.1111/j.1369-1600.2010.00309.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blomqvist O, Ericson M, Johnson DH, Engel JA, Soderpalm B. Voluntary ethanol intake in the rat: effects of nicotinic acetylcholine receptor blockade or subchronic nicotine treatment. Eur J Pharmacol. 1996;314:257–267. doi: 10.1016/s0014-2999(96)00583-3. [DOI] [PubMed] [Google Scholar]
- Blomqvist O, Soderpalm B, Engel JA. Ethanol-induced locomotor activity: involvement of central nicotinic acetylcholine receptors? Brain Res Bull. 1992;29:173–178. doi: 10.1016/0361-9230(92)90023-q. [DOI] [PubMed] [Google Scholar]
- Butt CM, Hutton SR, Stitzel JA, Balogh SA, Owens JC, Collins AC. A polymorphism in the alpha4 nicotinic receptor gene (Chrna4) modulates enhancement of nicotinic receptor function by ethanol. Alcohol Clin Exp Res. 2003;27:733–742. doi: 10.1097/01.ALC.0000067973.41153.BC. [DOI] [PubMed] [Google Scholar]
- Cardoso RA, Brozowski SJ, Chavez-Noriega LE, Harpold M, Valenzuela CF, Harris RA. Effects of ethanol on recombinant human neuronal nicotinic acetylcholine receptors expressed in Xenopus oocytes. J Pharmacol Exp Ther. 1999;289:774–780. [PubMed] [Google Scholar]
- Chatterjee S, Bartlett SE. Neuronal nicotinic acetylcholine receptors as pharmacotherapeutic targets for the treatment of alcohol use disorders. CNS Neurol Disord Drug Targets. 2010;9:60–76. doi: 10.2174/187152710790966597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D'Souza MS, Markou A. Differential role of N-methyl-D-aspartate receptor-mediated glutamate transmission in the nucleus accumbens shell and core in nicotine seeking in rats. Eur J Neurosci. 2014 doi: 10.1111/ejn.12491. [DOI] [PubMed] [Google Scholar]
- Davis TJ, de Fiebre CM. Alcohol's actions on neuronal nicotinic acetylcholine receptors. Alcohol Res Health. 2006;29:179–185. [PMC free article] [PubMed] [Google Scholar]
- Dawson DA. Drinking as a risk factor for sustained smoking. Drug and Alcohol Dependence. 2000;59:235–249. doi: 10.1016/s0376-8716(99)00130-1. [DOI] [PubMed] [Google Scholar]
- de Fiebre NC, de Fiebre CM. alpha7 Nicotinic acetylcholine receptor knockout selectively enhances ethanol-, but not beta-amyloid-induced neurotoxicity. Neurosci Lett. 2005;373:42–47. doi: 10.1016/j.neulet.2004.09.054. [DOI] [PubMed] [Google Scholar]
- Doyon WM, Thomas AM, Ostroumov A, Dong Y, Dani JA. Potential substrates for nicotine and alcohol interactions: a focus on the mesocorticolimbic dopamine system. Biochem Pharmacol. 2013;86:1181–1193. doi: 10.1016/j.bcp.2013.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fidler TL, Bakner L, Cunningham CL. Conditioned place aversion induced by intragastric administration of ethanol in rats. Pharmacol Biochem Behav. 2004;77:731–743. doi: 10.1016/j.pbb.2004.01.010. [DOI] [PubMed] [Google Scholar]
- Froehlich JC, Li TK. Animal models for the study of alcoholism: utility of selected lines. J Addict Dis. 1991;10:61–71. doi: 10.1300/J069v10n01_05. [DOI] [PubMed] [Google Scholar]
- Fucito LM, Toll BA, Wu R, Romano DM, Tek E, O'Malley SS. A preliminary investigation of varenicline for heavy drinking smokers. Psychopharmacology (Berl) 2011;215:655–663. doi: 10.1007/s00213-010-2160-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzales RA, Job MO, Doyon WM. The role of mesolimbic dopamine in the development and maintenance of ethanol reinforcement. Pharmacol Ther. 2004;103:121–146. doi: 10.1016/j.pharmthera.2004.06.002. [DOI] [PubMed] [Google Scholar]
- Griffin Iii WC, Haun HL, Hazelbaker CL, Ramachandra VS, Becker HC. Increased extracellular glutamate in the nucleus accumbens promotes excessive ethanol drinking in ethanol dependent mice. Neuropsychopharmacol. 2014;39:707–717. doi: 10.1038/npp.2013.256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrison EL, Hinson RE, McKee SA. Experimenting and daily smokers: episodic patterns of alcohol and cigarette use. Addict Behav. 2009;34:484–486. doi: 10.1016/j.addbeh.2008.12.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hauser SR, Deehan GA, Jr, Toalston JE, Bell RL, McBride WJ, Rodd ZA. Enhanced alcohol-seeking behavior by nicotine in the posterior ventral tegmental area of female alcohol-preferring (P) rats: modulation by serotonin-3 and nicotinic cholinergic receptors. Psychopharmacology (Berl) 2014 Mar 6; doi: 10.1007/s00213-014-3508-3. [Epub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hyman SE, Malenka RC, Nestler EJ. Neural mechanisms of addiction: the role of reward-related learning and memory. Annu Rev Neurosci. 2006;29:565–598. doi: 10.1146/annurev.neuro.29.051605.113009. [DOI] [PubMed] [Google Scholar]
- Ito R, Robbins TW, Everitt BJ. Differential control over cocaine-seeking behavior by nucleus accumbens core and shell. Nat Neurosci. 2004;7:389–397. doi: 10.1038/nn1217. [DOI] [PubMed] [Google Scholar]
- Koob GF, Volkow ND. Neurocircuitry of addiction. Neuropsychopharmacol. 2010;35:217–238. doi: 10.1038/npp.2009.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le AD, Wang A, Harding S, Juzytsch W, Shaham Y. Nicotine increases alcohol self-administration and reinstates alcohol seeking in rats. Psychopharmacology (Berl) 2003;168:216–221. doi: 10.1007/s00213-002-1330-9. [DOI] [PubMed] [Google Scholar]
- Liechti ME, Lhuillier L, Kaupmann K, Markou A. Metabotropic glutamate 2/3 receptors in the ventral tegmental area and the nucleus accumbens shell are involved in behaviors relating to nicotine dependence. J Neurosci. 2007;27:9077–9085. doi: 10.1523/JNEUROSCI.1766-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopez-Moreno JA, Trigo-Diaz JM, Rodriguez de FF, Gonzalez CG, Gomez de HR, Crespo GI, Navarro M. Nicotine in alcohol deprivation increases alcohol operant self-administration during reinstatement. Neuropharmacology. 2004;47:1036–1044. doi: 10.1016/j.neuropharm.2004.08.002. [DOI] [PubMed] [Google Scholar]
- McKenna JT, Cordeira JW, Jeffrey BA, Ward CP, Winston S, McCarley RW, Strecker RE. c-Fos protein expression is increased in cholinergic neurons of the rodent basal forebrain during spontaneous and induced wakefulness. Brain Res Bull. 2009;80:382–388. doi: 10.1016/j.brainresbull.2009.08.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell JM, Teague CH, Kayser AS, Bartlett SE, Fields HL. Varenicline decreases alcohol consumption in heavy-drinking smokers. Psychopharmacology (Berl) 2012;223:299–306. doi: 10.1007/s00213-012-2717-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgan JI, Curran T. Stimulus-transcription coupling in the nervous system: involvement of the inducible proto-oncogenes fos and jun. Annu Rev Neurosci. 1991;14:421–451. doi: 10.1146/annurev.ne.14.030191.002225. [DOI] [PubMed] [Google Scholar]
- Narahashi T, Soderpalm B, Ericson M, Olausson P, Engel JA, Zhang X, Nordberg A, Marszalec W, Aistrup GL, Schmidt LG, Kalouti U, Smolka AM, Hedlund L. Mechanisms of alcohol-nicotine interactions: alcoholics versus smokers. Alcohol Clin Exp Res. 2001;25:152S–156S. doi: 10.1097/00000374-200105051-00026. [DOI] [PubMed] [Google Scholar]
- Paxinos G, Watson C. The rat brain in stereotaxic coordinates. Boston: Academic Press; 2007. [DOI] [PubMed] [Google Scholar]
- Pich EM, Pagliusi SR, Tessari M, Talabot-Ayer D, Hooft van HR, Chiamulera C. Common neural substrates for the addictive properties of nicotine and cocaine. Science. 1997;275:83–86. doi: 10.1126/science.275.5296.83. [DOI] [PubMed] [Google Scholar]
- Pontieri FE, Passarelli F, Calo L, Caronti B. Functional correlates of nicotine administration: similarity with drugs of abuse. J Mol Med (Berl) 1998;76:193–201. doi: 10.1007/s001090050208. [DOI] [PubMed] [Google Scholar]
- Pontieri FE, Tanda G, Orzi F, Di CG. Effects of nicotine on the nucleus accumbens and similarity to those of addictive drugs. Nature. 1996;382:255–257. doi: 10.1038/382255a0. [DOI] [PubMed] [Google Scholar]
- Qiu MH, Liu W, Qu WM, Urade Y, Lu J, Huang ZL. The role of nucleus accumbens core/shell in sleep-wake regulation and their involvement in modafinil-induced arousal. PLoS ONE. 2012;7:e45471. doi: 10.1371/journal.pone.0045471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson TE, Berridge KC. Incentive-sensitization and addiction. Addiction. 2001;96:103–114. doi: 10.1046/j.1360-0443.2001.9611038.x. [DOI] [PubMed] [Google Scholar]
- Saint-Mleux B, Eggermann E, Bisetti A, Bayer L, Machard D, Jones BE, Muhlethaler M, Serafin M. Nicotinic enhancement of the noradrenergic inhibition of sleep-promoting neurons in the ventrolateral preoptic area. J Neurosci. 2004;24:63–67. doi: 10.1523/JNEUROSCI.0232-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharma R, Engemann S, Sahota P, Thakkar MM. Role of adenosine and wake-promoting basal forebrain in insomnia and associated sleep disruptions caused by ethanol dependence. J Neurochem. 2010;115:782–794. doi: 10.1111/j.1471-4159.2010.06980.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharma R, Sahota P, Thakkar MM. Nicotine administration in the cholinergic basal forebrain increases alcohol consumption in C57BL/6J mice. Alcohol Clin Exp Res. 2014a;38:1315–1320. doi: 10.1111/acer.12353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharma R, Sahota P, Thakkar MM. Role of adenosine and the orexinergic perifornical hypothalamus in sleep-promoting effects of ethanol. Sleep. 2014b;37:525–533. doi: 10.5665/sleep.3490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shiromani PJ, Kilduff TS, Bloom FE, McCarley RW. Cholinergically induced REM sleep triggers Fos-like immunoreactivity in dorsolateral pontine regions associated with REM sleep. Brain Res. 1992;580:351–357. doi: 10.1016/0006-8993(92)90968-f. [DOI] [PubMed] [Google Scholar]
- Steensland P, Simms JA, Holgate J, Richards JK, Bartlett SE. Varenicline, an alpha4beta2 nicotinic acetylcholine receptor partial agonist, selectively decreases ethanol consumption and seeking. Proc Natl Acad Sci U S A. 2007;104:12518–12523. doi: 10.1073/pnas.0705368104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taslim N, Saeed DM. The Role of Nicotinic Acetylcholine Receptor (nAChR) alpha(7) Subtype in the Functional Interaction Between Nicotine and Ethanol in Mouse Cerebellum. Alcohol Clin Exp Res. 2010;35:540–549. doi: 10.1111/j.1530-0277.2010.01371.x. [DOI] [PubMed] [Google Scholar]
- Taslim N, Soderstrom K, Dar MS. Role of mouse cerebellar nicotinic acetylcholine receptor (nAChR) alpha(4)beta(2)- and alpha(7) subtypes in the behavioral cross-tolerance between nicotine and ethanol-induced ataxia. Behav Brain Res. 2011;217:282–292. doi: 10.1016/j.bbr.2010.10.026. [DOI] [PubMed] [Google Scholar]
- Thakkar MM, Delgiacco RA, Strecker RE, McCarley RW. Adenosinergic inhibition of basal forebrain wakefulness - active neurons: A simultaneous unit recording and microdialysis study in freely behaving cats. Neuroscience. 2003;22:1107–1113. doi: 10.1016/j.neuroscience.2003.08.006. [DOI] [PubMed] [Google Scholar]
- Thakkar MM, Engemann SC, Sharma R, Sahota P. Role of wake-promoting basal forebrain and adenosinergic mechanisms in sleep-promoting effects of ethanol. Alcohol Clin Exp Res. 2010;34:997–1005. doi: 10.1111/j.1530-0277.2010.01174.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thakkar MM, Engemann SC, Walsh KM, Sahota PK. Adenosine and the homeostatic control of sleep: Effects of A1 receptor blockade in the perifornical lateral hypothalamus on sleep-wakefulness. Neuroscience. 2008a;153:875–880. doi: 10.1016/j.neuroscience.2008.01.017. [DOI] [PubMed] [Google Scholar]
- Thakkar MM, Ramesh V, Strecker RE, McCarley RW. Microdialysis perfusion of orexin-A in the basal forebrain increases wakefulness in freely behaving rats. Arch Ital Biol. 2001;139:313–328. [PubMed] [Google Scholar]
- Thakkar MM, Strecker RE, McCarley RW. Phasic but not tonic REM-selective discharge of periaqueductal gray neurons in freely behaving animals: relevance to postulates of GABAergic inhibition of monoaminergic neurons. Brain Res. 2002;945:276–280. doi: 10.1016/s0006-8993(02)02914-1. [DOI] [PubMed] [Google Scholar]
- Thakkar MM, Winston S, McCarley RW. Effect of microdialysis perfusion of 4,5,6,7-tetrahydroisoxazolo-[5,4-c]pyridine-3-ol in the perifornical hypothalamus on sleep-wakefulness: role of delta-subunit containing extra synaptic GABAA receptors. Neuroscience. 2008b;153:551–555. doi: 10.1016/j.neuroscience.2008.02.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tizabi Y, Bai L, Copeland RL, Jr, Taylor RE. Combined effects of systemic alcohol and nicotine on dopamine release in the nucleus accumbens shell. Alcohol Alcohol. 2007;42:413–416. doi: 10.1093/alcalc/agm057. [DOI] [PubMed] [Google Scholar]
- Vilpoux C, Warnault V, Pierrefiche O, Daoust M, Naassila M. Ethanol-sensitive brain regions in rat and mouse: a cartographic review, using immediate early gene expression. Alcohol Clin Exp Res. 2009;33:945–969. doi: 10.1111/j.1530-0277.2009.00916.x. [DOI] [PubMed] [Google Scholar]
- WHO. WHO report on the global tobacco epidemic. World Health Organization. 2008:1–342.
- WHO. Global status report on alcohol and health. World Health Organization. 2013:1–286.