Abstract
Background
Stimuli paired with alcohol may evoke conditioned responses that influence consumption and relapse. Understanding extinction of conditioned responses for both alcohol and non-alcoholic reinforcers, and their relation to subsequent consumption, may be useful in identifying methods to maintain abstinence.
Methods
Nine baboons self-administered alcohol (n=4) or a non-alcoholic reinforcer (orange-flavored Tang®, n=5) under a three-component chained schedule of reinforcement (CSR). Each component was associated with distinct stimuli and response requirements, which modeled periods of anticipation (Component 1), seeking (Component 2), and consumption (Component 3). No behavioral contingencies were in effect during Component 1. Responses in Component 2, required to gain access to Component 3, provided indices of seeking behavior. Alcohol or Tang was available only in Component 3. Initial conditions parametrically manipulated the concentration of alcohol (2–6% w/v) or Tang (25–100%) that was available for self-administration. The breaking point (BP) of alcohol- and Tang-seeking responses at each of the concentrations was determined by adding a progressive ratio schedule to Component 2. Extinction of responding under stimulus conditions identical to those during baseline, but with no access to alcohol or Tang, was examined using across- and within-session extinction procedures.
Results
The BP for 2% w/v alcohol was lower than that for 4% and 6%, which were closely similar. For Tang, BPs increased as the concentration increased. When concentrations of alcohol and Tang were adjusted to produce comparable BPs, self-administration of Tang was higher when compared to alcohol; however, alcohol-related cues maintained higher BPs than Tang-related cues when only water was available for self-administration. Alcohol seeking and self-administration responses were more resistant to extinction than those for Tang.
Conclusions
Stimuli paired with alcohol or non-alcoholic reinforcers will gain different motivational properties. Alcohol-related stimuli produced persistent responding that was highly resistant to change, highlighting the role of environmental stimuli in compulsive drinking and relapse.
Keywords: Conditioned Responses, Drug-Seeking, Self-Administration, Alcohol, Extinction, Baboons
INTRODUCTION
Compulsive alcohol drinking involves an extended sequence of behaviors that terminate in alcohol consumption. These behaviors occur in the presence of environmental stimuli that, through classical conditioning, can become associated with the direct effects of alcohol consumption (Childress et al., 1992; See, 2002; Weiss, 2005). Alcohol-associated stimuli can produce physiological (e.g., changes in skin conductance and heart rate) and subjective (e.g., craving and withdrawal) conditioned responses (i.e., “cue reactivity”) that may play a dominant role in the maintenance and perpetuation of compulsive drinking (Drummond et al., 1990; Markou et al., 1993; O’Brien et al., 1998; Rohsenow et al., 1991; Weiss, 2005). As such, understanding the relation between behaviors directed towards obtaining alcohol (seeking or appetitive), the direct consumption of alcohol (self-administration), and the influence of environmental stimuli is an important research focus.
In an effort to model the extended sequence of behaviors involved in human alcohol drinking, our laboratory has developed a nonhuman primate (baboon) procedure that incorporated a chained schedule of reinforcement (CSR; Weerts et al., 2006). The CSR was composed of three sequential components, each of which was associated with distinct stimuli (cues) and behavioral contingencies (schedule requirements). Fulfilling the schedule requirement in each successive component was necessary to progress to the next component, with alcohol available only in the final component. The procedure allowed for alcohol seeking and self-administration to be examined separately. For example, increasing the response requirement for each drink decreased self-administration, but did not alter seeking (Weerts et al., 2006), whereas increasing the response requirement to gain access to alcohol decreased seeking, but did not alter self-administration (Kaminski et al., 2008). However, both seeking and self-administration increased on the first day of drinking following periods of forced abstinence (1 to 14 days) and the magnitude of increase was a direct function of the abstinence duration (Weerts et al., 2006).
Studies that include independent measures of seeking and self-administration can provide important information to increase our understanding of treatment effects on basic behavioral processes thought to be associated with compulsive alcohol drinking. The CSR procedure, in which seeking and consumption can be evaluated in the same session, may be a valid model for the evaluation of novel treatment medications (Duke et al., 2014; Kaminski et al., 2012; Kaminski and Weerts, 2013). In addition, evaluation of the relation between alcohol-maintained behaviors and blood alcohol levels (BAL) in comparison to behaviors maintained by a non alcoholic control allows for the determination of the specificity with which a candidate medication affects alcohol-related behaviors. It is also important to understand how stimuli associated with alcohol versus a non-alcoholic control maintain behavior under different conditions of alcohol availability and abstinence, as alcohol-related cues play a key role in relapse.
Alcohol may be qualitatively different from non-alcoholic reinforcers and, as such, stimuli paired with these reinforcers may gain different incentive–motivational properties (Bindra, 1968; Bindra, 1974; Estes, 1958; Rescorla and Solomon, 1967). For instance, alcohol-dependent subjects report “craving” or “desire to drink” in response to alcohol-related stimuli, while these responses are not elicited by neutral stimuli (Greeley et al., 1993; Kaplan et al., 1985). Thus, the present study sought to extend the investigation of the relation between seeking, self-administration, and environmental stimuli by comparing the self-administration of alcohol to a non-alcoholic control (orange-flavored Tang®) under the CSR. The three-component CSR modeled periods of anticipation (Component 1), seeking (Component 2), and consumption (Component 3). Initial conditions parametrically manipulated the concentration of alcohol or Tang that was available for self-administration. The breaking point (BP) or strength of alcohol- and Tang-related seeking responses at each of the concentrations was determined by adding a progressive ratio (PR) schedule to the seeking component. Next, the persistence of behaviors related to alcohol or Tang seeking and self-administration was examined using across- and within-session extinction procedures. Such extinction of conditioned responses, and their relation to subsequent alcohol consumption, may be particularly useful for maintaining alcohol abstinence and reducing relapse to heavy drinking.
MATERIALS AND METHODS
Subjects
Nine adult male baboons (Papio anubis; Southwest Foundation for Biomedical Research, San Antonio, TX), were housed singly in cages that also served as the experimental chambers. All baboons had histories of self-administration under the CSR, but the reinforcer delivered was 2–6% w/v alcohol (N=4) or 25–100% orange-flavored Tang® (N=5). The baboons assigned to the alcohol group only received alcohol and the baboons assigned to the Tang group only received Tang throughout the entire study. Baboons were not food deprived and received primate chow (50–73 kcals/kg), fresh fruit or vegetables, and a children’s chewable multivitamin daily. Water was available ad libitum, except during experimental sessions. The facilities were maintained in accordance with USDA and AAALAC standards. The protocol was approved by the JHU Animal Care and Use Committee and followed the Guide for the Care and Use of Laboratory Animals (2011).
Apparatus
Sessions were conducted in modified cages as described previously (Weerts et al., 2006). Each cage contained a panel with two vertically operated levers, two different colored jewel lights mounted above each lever, and a drinkometer (connected to a calibrated 1,000-ml bottle) with two white and two green lights that surrounded a protruding drink spout. Contact with the spout operated a solenoid valve that delivered fluid for five seconds (1 drink; approximately 25–35 ml). A separate panel contained three colored cue lights. A speaker was mounted above the cages for presentation of auditory stimuli. Experimental events were controlled remotely using Med Associates (East Fairfield, VT) software and hardware interfaced with a computer.
Drugs
All solutions were mixed using reverse osmosis purified drinking water. Ethyl alcohol (190 Proof; Pharmco-AAPER, Brookville, CT) was diluted with reverse osmosis water to concentrations of 2–6% w/v alcohol. Tang powder (Kraft Foods®) was dissolved in reverse osmosis water following package instructions and then diluted from full strength (100%) to concentrations of 25% and 50%.
Chained Schedule of Reinforcement (CSR) Procedure
During all sessions, fluids were only available via the drinkometer. Daily sessions began at the same time (8:30 AM) and were signaled by a 3-s tone. During Component 1, all instrumental responses were recorded but had no programmed consequence. The red cue light was illuminated and a fixed-time (FT) 20-min schedule was in effect. After 20 min elapsed, the red cue light was turned off, and the yellow cue light was illuminated, signaling Component 2.
Component 2 consisted of two links. During the first link, the jewel light over the left lever was illuminated, and an alternate fixed-interval (FI) 10-min, FT 20-min schedule was in effect on the left lever. The first link ended either a) with the first response on the left lever after 10 min elapsed or b) automatically after 20 min, whichever occurred first. During the second link, the jewel light over the left lever flashed and a fixed-ratio (FR) 10 schedule was in effect on the left lever. Completion of the FR 10 ended Component 2; the yellow cue light and the jewel light were turned off and Component 3 was initiated. Failure to complete the FR 10 in Component 2 within 90 min terminated the session (i.e., no access to alcohol or Tang).
During Component 3, the blue cue light and the jewel light over the right lever were illuminated, and the opportunity to orally self-administer alcohol or Tang was available according to an FR 10 schedule on the right lever. Completion of each FR turned the jewel light off and turned on the white lights on the drinkometer faceplate, indicating drink availability. Contact with the drinkometer spout turned off the white lights, turned on the green lights on the drinkometer faceplate, and initiated the delivery of fluid for the duration of spout contact or for a programmed duration (5 s), whichever came first. Following each drink, all drinkometer lights were turned off and the jewel light over the right lever was again illuminated. Component 3 ended after 120 min and all programmed stimuli were turned off.
Before beginning the current study manipulations, the baboons had been exposed to the CSR cues without any access to alcohol or Tang (neutral cues). The alcohol data were published previously (Weerts et al., 2006).
Progressive Ratio (PR) Procedure
The strength of alcohol and Tang seeking was evaluated using an across-sessions PR procedure. The FR 10 requirement in the second link of Component 2 was progressively increased each day by a factor of 2 (i.e., 10, 20, 40, 80, etc.) until the baboon failed to complete the response requirement within 90 min. Completion of the response requirement initiated Component 3 and alcohol or Tang was available as described above. If the response requirement was not completed within 90 min, the session terminated after Component 2 (i.e., no access to alcohol or Tang). The “breaking point” (BP) was defined as the highest response requirement completed in Component 2 that resulted in access to alcohol or Tang. Following each BP determination, the baseline schedule was reestablished (FR 10). After self-administration was stable for three consecutive days, another BP determination began. At least three BPs were determined for each baboon for each concentration of alcohol (2, 4, and 6% w/v) or Tang (25, 50, and 100%) until there were no increasing or decreasing trends in BPs.
Extinction Procedures
Across-session extinction
Prior to extinction procedures, all alcohol and Tang BP determinations were completed and self-administration under the CSR was maintained for two weeks. Water was then substituted for alcohol or Tang as the fluid available for self-administration in Component 3. All stimuli were presented and all behavioral contingencies of the CSR were in effect, but only water was available for self-administration. When the volume of water consumed decreased below the range for alcohol or Tang and intake levels were stable (i.e., + 20% intake volume) for five consecutive days, BPs for water were determined using the PR procedure described above.
Within-session extinction
The resistance of seeking was examined within a single daily session. The stimuli and behavioral contingencies in effect were the same as the baseline CSR sessions except, during the second link of Component 2, the FR 10 schedule was not in effect and responses did not initiate Component 3; responses were recorded until no left lever responses occurred for 30 consecutive minutes (criterion for extinction), and then the session terminated (i.e., no access to alcohol or Tang).
Determination of Blood Alcohol Level
Blood alcohol levels were determined in the alcohol group following self-administration of 2, 4, and 6% w/v alcohol. Immediately following alcohol self-administration sessions, baboons were anesthetized with ketamine and 5-ml blood samples were collected from a saphenous vein. Samples were immediately centrifuged at 3200 rpm for 8–12 min and then the plasma was drawn off, transferred to two separate airtight polypropylene tubes, and frozen until analysis. Double determinations of BAL were completed using a rapid high performance plasma alcohol analysis using alcohol oxidase with AMI analyzer (Analox Instruments USA, Lenenberg, MA) with Analox Kit GMRD-113 as described previously (Kaminski et al., 2008).
Data Analysis
For the CSR, variables of interest were left and right lever presses, drinkometer contacts, the latency to complete the FI requirement in Component 2 (link 1), and the total volume (mL) of fluid (water, alcohol, or Tang) consumed during Component 3. Total g/kg of alcohol was calculated based on individual body weights (kg) and total volume (ml) of alcohol consumed.
As the BPs were not normally distributed, BP values were transformed to number of steps completed (i.e., a BP of 20 = 1 step, a BP of 40 = 2 steps, a BP of 80 = 3 steps, etc.; Kaminski et al., 2008) and the different concentrations were compared using repeated measures analysis of variance (ANOVA) for each group (alcohol or Tang) with concentration as a repeated measure. Bonferroni post hoc tests were used for pairwise comparisons. Tang concentrations (25–50%) were titrated for each baboon, so that the grand mean BP for Tang matched the grand mean BP for 4% w/v alcohol. Breaking points for Tang (25–50%) or alcohol (4% w/v), and water were then compared between and within groups using unpaired and paired t-tests, respectively.
For the across-session extinction procedure, extinction was defined as the session during which the volume of water consumed reached 20% of baseline or below. Days to reach the extinction criterion were then compared between groups using unpaired t-tests. Data for the entire extinction procedure (about 30 days) were summarized as the grand mean of the last 5 days that preceded the extinction condition, the grand means of the first 5 days, days 20–25, and days 30–35 of extinction. Seeking and self-administration behaviors were compared using an ANOVA for each group. Bonferroni post hoc tests were used to compare each 5-day set to alcohol or Tang. For the within-session extinction procedure, total extinction responses and time to extinction were compared between groups using unpaired t-tests. For all statistical analyses, a p-value of .05 or less was considered significant.
RESULTS
Blood Alcohol Levels
Analysis of blood samples verified that the volume of 4% and 6% w/v alcohol consumed under the CSR produced BALs that exceeded 80 mg/dl, whereas the 2% w/v alcohol did not (Figure 1).
Figure 1.
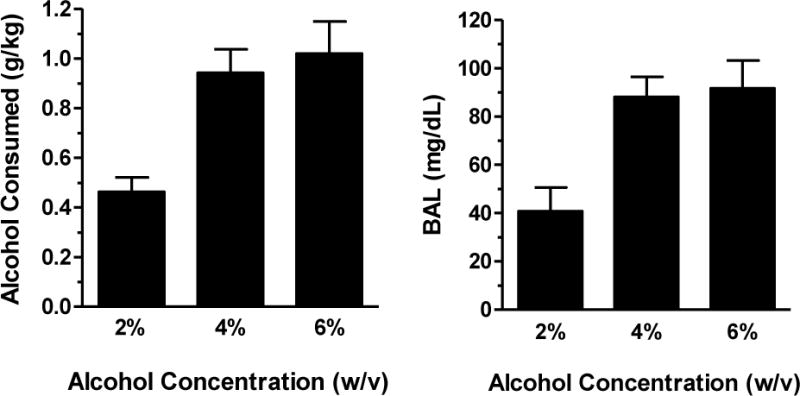
The g/kg alcohol consumed (left panel) and blood alcohol levels (BAL; right panel) from the chained schedule of reinforcement under conditions of alcohol (2, 4, and 6% w/v) availability in Component 3. Total g/kg of alcohol was calculated based on individual body weights (kg) and total volume (ml) of alcohol consumed. Bars show group means (+ SEM).
Progressive Ratio Procedure
The BP for 2% w/v alcohol was lower than that for 4% and 6%, which were closely similar (Figure 2). The BPs for Tang were concentration dependent, with the lowest BP observed for 25% Tang, an intermediate BP for 50%, and the highest BP for 100%. The BPs for 50% and 100% Tang were significantly higher than that for 25% Tang (p < .05; p < .01; respectively).
Figure 2.

Breaking points completed under a progressive ratio response schedule in Component 2 to initiate Component 3 and gain access to alcohol (2, 4, and 6% w/v; left panel) or Tang (25, 50, and 100%; right panel) for self-administration. The mean of the last three progressive ratio evaluations conducted for each condition in each baboon were determined and results shown are the grand mean (+ SEM). For the statistical analyses, BP values were transformed to number of steps completed (i.e., a BP of 20 = 1 step, a BP of 40 = 2 steps, a BP of 80 = 3 steps, etc.). *Indicates a significant (p < .05) difference from 25% Tang.
A concentration of 4% w/v alcohol was selected for all subsequent conditions for the alcohol group because it maintains higher rates of operant self-administration than high concentrations (e.g., 8–16% w/v), and as demonstrated in our prior studies, is preferred over water by baboons with alcohol drinking experience when both are available concurrently (Ator & Griffiths, 1992), and it produced relevant BALs in the present study. After completion of BP determinations, Tang concentrations were selected for each baboon to match BPs for the alcohol group (4% w/v). A concentration of 25% Tang was selected for two baboons, and a concentration of 50% Tang was selected for three baboons. These concentrations were then maintained for all subsequent conditions. Using these concentrations the grand mean BP for the Tang group matched the grand mean BP for the alcohol group (i.e., Tang and alcohol were functionally equivalent reinforcers).
When water was substituted for alcohol or Tang, alcohol-related cues maintained higher BPs than Tang-related cues (M =128.3, SEM = 39.0; M = 20.0, SEM = 2.1, respectively; p < .01). The mean BP in the extinction condition following alcohol access was significantly lower than the mean BP in the alcohol condition (M =128.3, SEM = 39.0; M =820.0, SEM = 209.2, respectively; p < .05) and the mean BP in the extinction condition following Tang access was significantly lower than the mean BP in the Tang condition (M = 20.0, SEM = 2.1; M = 746.7, SEM = 158.2, respectively; p < .001).
Across-Session Extinction Procedure
When alcohol or Tang was available for self-administration in Component 3, the number of left lever responses and the latency to complete the FI 10-min schedule during Component 2 (i.e., the response latency) were similar for alcohol and Tang (Figure 3). The first link in Component 2 always ended as a result of a response (i.e., the FI contingency) and not the passage of time (i.e., the FT contingency). During the first five days of extinction (when water was substituted for alcohol and Tang), alcohol- and Tang-related cues continued to maintain left lever responses and short response latencies. For alcohol, the number of left lever responses decreased by days 15–20, but was not significantly lower until days 30–35 (p < .05). For Tang, the number of left lever responses was significantly decreased by days 15–20 (p < .01) and further decreased by days 30–35 (p < .001). The response latency increased for both alcohol and Tang as a function of the number of days of extinction; the latency was significantly longer during days 30–35 for both alcohol (p < .05) and Tang (p < .001). The number of sessions during which the first link in Component 2 ended as a result of the passage of time also increased.
Figure 3.
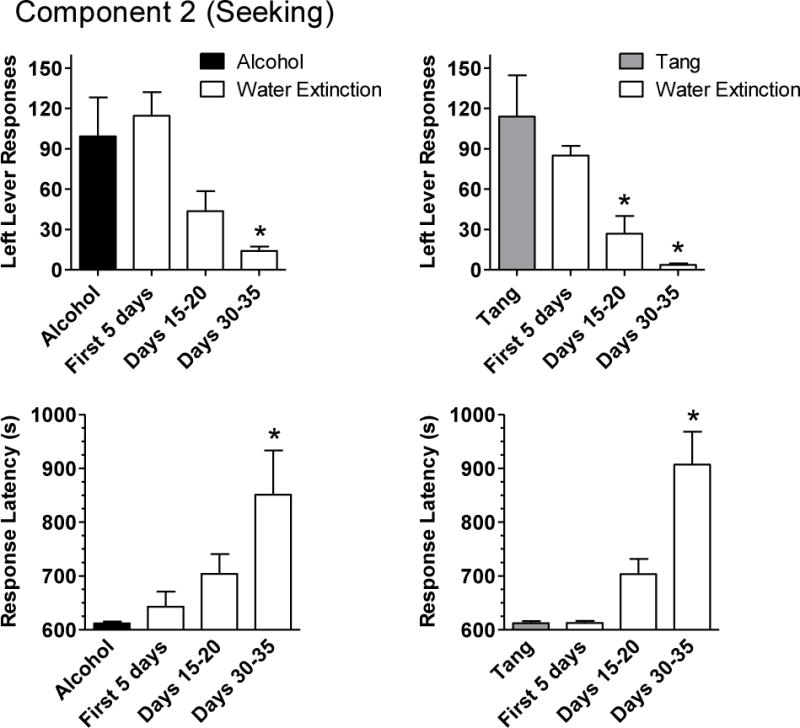
Effects of substituting water for alcohol (4% w/v; left panel) or Tang (25–50%; right panel) on behaviors associated with seeking. Seeking-related behaviors shown are the number of left lever responses (top panel) and the latency to complete the FI 10-min schedule (bottom panel) on the left lever during the fixed-interval (10-min), fixed-time (20-min) schedule in Component 2. The minimum latency to complete the FI schedule was 600 s (i.e., 10 min). Results are the group means (+ SEM) for the last five days of alcohol or Tang availability prior to extinction, the first five days, days 15–20, and days 30–35 of extinction. *Indicates a significant (p < .05) difference from the alcohol or Tang condition.
Tang maintained significantly more right lever responses (p <.01) and drinkometer contacts (p < .01) than alcohol, resulting in different intake volumes for alcohol and Tang (p < .0001; Figure 4). When water was substituted for alcohol, the number of right lever responses was significantly decreased by the first 5 days of extinction (p < .01) and then remained at a slightly lower level during days 15–20 (p < .001) and 30–35 (p < .001). Drinkometer contacts were significantly decreased by days 15–20 (p < .001) and remained at a similar level during days 30–35 (p < .001). The volume of water consumed was not significantly decreased until days 30–35 (p < .05). For Tang, right lever responses, drinkometer contacts, and volume of water consumed were significantly decreased by the first 5 days of extinction and continued to decrease to low levels across the remaining days (for all pairwise comparisons, p < .001). A greater number of days were required to reach the extinction criterion for the alcohol group than the Tang group (p < .0001; Figure 5). Alcohol-related responding required about 17 more days to reach the extinction criterion than Tang-related responding.
Figure 4.

Effects of substituting water for alcohol (4% w/v; left panel) or Tang (25–50%; right panel) on behaviors associated with consumption. Consumption-related behaviors shown are the number of right lever responses completed to activate the drinkometer (top panel), the number of drinkometer contacts (middle panel), and the volume consumed (bottom panel) in Component 3. Results are the group means (+ SEM) for the last five days of alcohol or Tang availability prior to extinction, the first five days, days 15–20, and days 30–35 of extinction. *Indicates a significant (p < .05) difference from the alcohol or Tang condition.
Figure 5.

The left panel shows the number of days to reach the extinction criterion (i.e., the volume of water consumed reached 20% of baseline or below) during the across-session extinction tests in which water was substituted for alcohol (4% w/v) or Tang (25–50%). The middle and right panels show the total number of extinction responses (i.e., the sum of the left lever responses, right lever responses, and drinkometer contacts for each baboon) and the time to meet the extinction criteria during the within-session extinction tests in Component 2, Link 2 for alcohol (4% w/v) or Tang (25–50%). Results are the group means (+ SEM) for alcohol or Tang. *Indicates a significant (p < .05) difference between the alcohol and Tang conditions.
Within-Session Extinction Procedure
Alcohol-related cues produced significantly more responses during the within-session extinction test than Tang-related cues (p < .01; Figure 5). Time to reach the extinction criterion was longer for the alcohol group than the Tang group; however, the groups did not differ significantly.
DISCUSSION
The present study examined the relation between seeking, self-administration, and environmental cues by comparing the self-administration of alcohol to a preferred non-alcoholic reinforcer under the CSR. In our previous research (Weerts et al., 2006), we have shown that exposure to the CSR cues alone (i.e., neutral cues) did not maintain behavior. When the same cues were then paired with alcohol, the CSR procedure was effective in establishing and maintaining alcohol seeking and self-administration behaviors in baboons at pharmacologically relevant BALs (>80 mg/dl) (Kaminski et al., 2008; Weerts et al., 2006). The present study expanded upon this previous research by extending the procedure to a non-alcoholic control. Because the present study was concerned with comparisons of conditioned behavior for alcohol versus a non-alcoholic reinforcer, we elected to use a group design so that subjects in each group were only ever exposed to either alcohol or Tang. Examination of the strength of alcohol and Tang seeking showed that alcohol-related cues maintained higher BPs than Tang-related cues when only water was available for self-administration. During the across- and within-session extinction procedures, alcohol seeking and self-administration were both more resistant to extinction than those for Tang. Overall, these findings suggest that stimuli paired with alcohol and non-alcoholic reinforcers will gain different motivational properties. This is consistent with research showing that drug-associated stimuli produce a greater tendency to “grab attention” as they engender attentional and approach biases (Field et al., 2004; Field and Cox, 2008; Ostafin et al., 2003; Palfai and Ostafin, 2003) and produce higher levels of “craving” or “desire” in human subjects (Greeley et al., 1993; Kaplan et al., 1985).
The persistence of seeking behaviors during extinction provides a measure of the extent to which stimuli previously associated with a reinforcer maintain responding in its absence. During the across- and within-session extinction tests, seeking responses persisted for both groups, albeit at a reduced rate. These findings indicate that reinforcer-associated stimuli can maintain seeking for both alcohol and non-alcoholic reinforcers; however, the strength of these behaviors was greater for alcohol. After over 30 days of only water being available, and throughout the subsequent BP determinations, alcohol-related cues engendered BPs as high as 320 to gain access to water, whereas a BP of 40 was the highest engendered by the Tang-related cues. During the within-session extinction tests, alcohol-related cues produced approximately five times as many seeking responses as the Tang-related cues. These results are consistent with theoretical and empirical work suggesting that compulsive drug seeking may be a conditioned habit in which seeking is increasingly controlled by drug-associated stimuli (e.g., Everitt and Robbins, 2005). According to this view, the transition to compulsive drug seeking at the neural level is associated with a shift in control processes from the prefrontal cortex to the striatum, and within the striatum from ventral to dorsal domains (Cardinal and Everitt, 2004; Everitt et al., 2008; Vollstädt Klein et al., 2010). Given the strength of the seeking response in the presence of alcohol-related cues, treatments for alcohol dependence must address alcohol seeking as a means to maintain alcohol abstinence and reduce relapse to heavy drinking. The recent addition of cravings, or a strong desire to drink, as one of the DSM-5 criteria for alcohol use disorder (American Psychiatric Association, 2013) highlights the importance of these conditioned states.
Tang maintained more self-administration responses and higher intake volumes than alcohol during baseline. Because of this, one might suggest that responding for Tang extinguished more quickly than that for alcohol solely because of the different rates of self-administration during baseline (akin to a rate-dependency effect, but cf. Branch, 1984; Pitts, 2014 for a discussion of the limitations of the rate-dependency concept). However, research has questioned the generality of the idea that high rate behaviors extinguish more quickly than lower rate behaviors (see Nevin, 1979). A large body of research examining resistance to extinction has found that behavior occurring in an environment that provides a larger magnitude of reinforcement is more resistant to extinction, regardless of response rates, than behavior occurring in an environment with a smaller magnitude of reinforcement (Nevin, 1974; Nevin et al., 1990; Nevin, 1992). Resistance to extinction depends on the Pavlovian relation between the stimulus context in which the behavior occurs and the magnitude of reinforcement delivered within that context (e.g., stimulus–reinforcer relation). In the present study, alcohol self-administration was more resistant to extinction. Specifically, during the across-session extinction procedure for alcohol, right lever responses and drinkometer contacts decreased by only 30% and 16%, respectively, during the first five days of extinction, and both by only 55% during days 30–35. In comparison, for Tang, right lever responses and drinkometer contacts decreased by 80% and 81%, respectively, during the first five days, and both by 98% during days 30–35. Furthermore, alcohol-related responding required a greater number of days to reach the extinction criterion. The fact that alcohol self-administration was more resistant to extinction than Tang self-administration further supports the notion that stimuli paired with alcohol or Tang gained different incentive–motivational properties (Bindra, 1968; Bindra, 1974; Estes, 1958; Rescorla and Solomon, 1967) and may be associated with different behavioral control processes at the neural level (Everitt et al., 2008; Vollstädt Klein et al., 2010).
The concentration of Tang was directly related to its BP, as the concentration of Tang was increased, BPs increased as well. In comparison, this direct relation was not observed for the concentration of alcohol, but was related to BALs in excess of 80 mg/dl. The BP for 4% and 6% w/v alcohol were closely similar, as were BALs, for these concentrations. In contrast, the BP for 2% w/v alcohol was lower than the BP for either 4% or 6%. These findings are consistent with a previous study that employed the rodent appetitive model of alcohol drinking (Czachowski et al., 2003), in which rats responded on an FR schedule to gain access to a 20-min period in which alcohol or sucrose solutions were freely available for consumption. When BPs to gain access to 2–20% alcohol or 1–10% sucrose were determined, BPs increased in concert with the concentration of sucrose, whereas BPs for 2% alcohol were lower than BPs for any of the other alcohol concentration, which were closely similar. While BALs were not determined in that study, it seems likely that there is a decreased tendency to seek alcohol when the concentration is so low as to make it difficult to achieve intoxication.
The present results provide information that may be useful in identifying methods to maintain abstinence in alcohol-dependent individuals. Given that alcohol-related cues produced responding that was highly resistant to extinction and given that cue-induced cravings can promote relapse, the present results suggest that interventions aimed at reducing the frequency and intensity of cue-induced cravings may need to employ methods beyond drug cue exposure extinction. For example, interventions may benefit from the incorporation of aversive consequences for drug seeking, such as counter-conditioning and contingency management (for a review, see Peck and Ranaldi, 2014). Nevertheless, cue exposure therapies may benefit from newer applications that incorporate computer-based virtual environments (e.g., Culbertson et al., 2010) as exposure therapy can be conducted in an environment that resembles the natural setting in which an individual may use drugs.
Animal models, such as the CSR, are useful tools to further evaluate the interactions between environmental cues and behaviors associated with seeking and consumption. A strength of the non-human primate CSR model is that it permits concurrent evaluations of changes in cue reactivity, alcohol seeking, reinforcement and consumption during long-term exposure (years) to alcohol-associated cues in the drinking environment that are not possible with rodent models. The non-human primate CSR models several key features of alcohol use disorder. First, baboons are large non-human primates that metabolize alcohol in a manner similar to humans (Weerts et al., 2007). Second, baboons spend a great deal of time in activities directed towards obtaining and drinking alcohol. Third, the CSR model captures the too much, too fast, too often drinking pattern associated with “at risk” problem drinking as defined by NIAAA (2007). Binge drinking (drinking too much, too fast) is defined as alcohol consumption sufficient to achieve a blood alcohol level (BAL) of 80 mg/dL (0.08%) or more within a 2–3 hour drinking period. This corresponds to consumption of 0.8–1 g/kg or about 5 drinks for men and 4 drinks for women. This level of drinking is also defined by NIAA as a “heavy drinking day.” “At risk” drinking also includes drinking an average of more than 14 drinks per week for men and more than 7 drinks per week for women (drinking too much, drinking too often). The CSR models the too much, too fast, too often drinking pattern by achieving BALs exceeding 0.08% in a 2-hr period and alcohol intake ~1 g/kg per day, 7 days per week. In contrast, rats and mice metabolize alcohol at substantially faster rates and intake levels using bodyweights (g/kg) in rodents do not parallel BAL in a manner comparable to humans. Thus, the CSR procedure produces regular heavy drinking that also meets binge criteria. Since non-human primates are closer in phylogenetic origin, and thus more genetically, physiologically, and behaviorally similar to humans, they can help bridge the gap between rodent and human data. The use of animal models to increase our understanding of basic behavioral processes involved in long-term exposure to stimuli associated with heavy alcohol drinking and to model the extended sequence of behaviors involved in human alcohol drinking will lead to better treatments for alcohol-dependent individuals.
Acknowledgments
Supported by NIH/NIAAA R01 AA015971.
References
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. Fifth. Washington, DC: American Psychiatric Association; 2013. [Google Scholar]
- Ator NA, Griffiths RR. Oral self-administration of triazolam, diazepam and ethanol in the baboon: Drug reinforcement and benzodiazepine physical dependence. Psychopharm. 1992;108(3):301–312. doi: 10.1007/BF02245116. [DOI] [PubMed] [Google Scholar]
- Bindra D. Neuropsychological interpretation of the effects of drive and incentive-motivation on general activity and instrumental behavior. Psychol Rev. 1968;75(1):1–22. [Google Scholar]
- Bindra D. A motivational view of learning, performance, and behavior modification. Psychol Rev. 1974;81(3):199–213. doi: 10.1037/h0036330. [DOI] [PubMed] [Google Scholar]
- Branch MN. Rate dependency, behavioral mechanisms, and behavioral pharmacology. J Exp Anal Behav. 1984;42:511–522. doi: 10.1901/jeab.1984.42-511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cardinal RN, Everitt BJ. Neural and psychological mechanisms underlying appetitive learning: links to drug addiction. Curr Opin Neurobiol. 2004;14(2):156–162. doi: 10.1016/j.conb.2004.03.004. [DOI] [PubMed] [Google Scholar]
- Childress AR, Ehrman R, Rohsenow DJ, Robbins SJ, O’Brien CP. Classically conditioned factors in drug dependence. In: Lowenstein JH, Ruiz P, Millman RB, editors. Substance Abuse: A Comprehensive Textbook. 2. Williams and Wilkins; Baltimore, MD, USA: 1992. pp. 56–69. [Google Scholar]
- Culbertson C, Nicolas S, Zaharovits I, London ED, De La Garza R, 2nd, Brody AL, Newton TF. Methamphetamine craving induced in an online virtual reality environment. Pharmacol Biochem Behav. 2010;96:454–460. doi: 10.1016/j.pbb.2010.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Czachowski CL, Legg BH, Samson HH. Assessment of sucrose and ethanol reinforcement: The across-session breakpoint procedure. Physiol Behav. 2003;78(1):51–59. doi: 10.1016/s0031-9384(02)00963-0. [DOI] [PubMed] [Google Scholar]
- Drummond DC, Cooper T, Glautier SP. Conditioned learning in alcohol dependence: Implications for cue exposure treatment. Brit J Addict. 1990;85(6):725–743. doi: 10.1111/j.1360-0443.1990.tb01685.x. [DOI] [PubMed] [Google Scholar]
- Duke AN, Kaminski BJ, Weerts EM. Baclofen effects on alcohol seeking, self-administration and extinction of seeking responses in a within-session design in baboons. Addict Biol. 2014;19(1):16–26. doi: 10.1111/j.1369-1600.2012.00448.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Estes W. Stimulus-response theory of drive. University of Nebraska Press; Lincoln: 1958. [Google Scholar]
- Everitt BJ, Robbins TW. Neural systems of reinforcement for drug addiction: from actions to habits to compulsion. Nat Neurosci. 2005;8(11):1481–1489. doi: 10.1038/nn1579. [DOI] [PubMed] [Google Scholar]
- Everitt BJ, Belin D, Economidou D, Pelloux Y, Dalley JW, Robbins TW. Neural mechanisms underlying the vulnerability to develop compulsive drug-seeking habits and addiction. Phil Trans R Soc B. 2008;363(1507):3125–3135. doi: 10.1098/rstb.2008.0089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Field M, Cox WM. Attentional bias in addictive behaviors: A review of its development, causes, and consequences. Drug Alcohol Depend. 2008;97(1):1–20. doi: 10.1016/j.drugalcdep.2008.03.030. [DOI] [PubMed] [Google Scholar]
- Field M, Mogg K, Zetteler J, Bradley BP. Attentional biases for alcohol cues in heavy and light social drinkers: The roles of initial orienting and maintained attention. Psychopharm. 2004;176(1):88–93. doi: 10.1007/s00213-004-1855-1. [DOI] [PubMed] [Google Scholar]
- Greeley JD, Swift W, Prescott J, Heather N. Reactivity to alcohol-related cues in heavy and light drinkers. J Stud Alcohol Drugs. 1993;54(3):359–368. doi: 10.15288/jsa.1993.54.359. [DOI] [PubMed] [Google Scholar]
- Kaminski BJ, Duke AN, Weerts EM. Effects of naltrexone on alcohol drinking patterns and extinction of alcohol seeking in baboons. Psychopharm. 2012;223(1):55–66. doi: 10.1007/s00213-012-2688-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaminski BJ, Goodwin AK, Wand G, Weerts EM. Dissociation of Alcohol-Seeking and consumption under a chained schedule of oral alcohol reinforcement in baboons. Alcohol Clin Exp Res. 2008;32(6):1014–1022. doi: 10.1111/j.1530-0277.2008.00662.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaminski BJ, Weerts EM. The effects of varenicline on alcohol seeking and Self-Administration in baboons. Alcohol Clin Exp Res. 2013;38(2):376–383. doi: 10.1111/acer.12233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaplan RF, Cooney NL, Baker LH, Gillespie RA, Meyer RE, Pomerleau OF. Reactivity to alcohol-related cues: Physiological and subjective responses in alcoholics and nonproblem drinkers. J Stud Alcohol Drugs. 1985;46(4):267–272. doi: 10.15288/jsa.1985.46.267. [DOI] [PubMed] [Google Scholar]
- Markou A, Weiss F, Gold LH, Caine SB, Schulteis G, Koob GF. Animal models of drug craving. Psychopharm. 1993;112(2–3):163–182. doi: 10.1007/BF02244907. [DOI] [PubMed] [Google Scholar]
- National Research Council. Guide for the Care and Use of Laboratory Animals. Eighth. Washington, DC: The National Academies Press; 2011. [PubMed] [Google Scholar]
- Nevin JA. Response strength in multiple schedules I. J Exp Anal Behav. 1974;21(3):389–408. doi: 10.1901/jeab.1974.21-389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nevin JA. Reinforcement schedules and response strength. In: Zeiler MD, Harzem P, editors. Advances in analysis of behavior: Vol. I. Reinforcement and the organization of behavior. London: Wiley; 1979. pp. 117–158. [Google Scholar]
- Nevin JA. An integrative model for the study of behavioral momentum. J Exp Anal Behav. 1992;57(3):301–316. doi: 10.1901/jeab.1992.57-301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nevin JA, Tota ME, Torquato RD, Shull RL. Alternative reinforcement increases resistance to change: Pavlovian or operant contingencies? J Exp Anal Behav. 1990;53(3):359–379. doi: 10.1901/jeab.1990.53-359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Brien CP, Childress AR, Ehrman R, Robbins SJ. Conditioning factors in drug abuse: Can they explain compulsion? J Psychopharm. 1998;12(1):15–22. doi: 10.1177/026988119801200103. [DOI] [PubMed] [Google Scholar]
- Ostafin BD, Palfai TP, Wechsler CE. The accessibility of motivational tendencies toward alcohol: Approach, avoidance, and disinhibited drinking. Exp Clin Psychopharm. 2003;11(4):294–301. doi: 10.1037/1064-1297.11.4.294. [DOI] [PubMed] [Google Scholar]
- Palfai TP, Ostafin BD. Alcohol-related motivational tendencies in hazardous drinkers: Assessing implicit response tendencies using the modified-IAT. Behav Res Ther. 2003;41(10):1149–1162. doi: 10.1016/s0005-7967(03)00018-4. [DOI] [PubMed] [Google Scholar]
- Peck JA, Ranaldi R. Drug abstinence: exploring animal models and behavioral treatment strategies. Psychopharmacology. 2014;231(10):2045–2058. doi: 10.1007/s00213-014-3517-2. [DOI] [PubMed] [Google Scholar]
- Pitts RC. Reconsidering the concept of behavioral mechanisms of drug action. J Exp Anal Behav. 2014;101(3):422–441. doi: 10.1002/jeab.80. [DOI] [PubMed] [Google Scholar]
- Rescorla RA, Solomon RL. Two-process learning theory: Relationships between pavlovian conditioning and instrumental learning. Psychol Rev. 1967;74(3):151–182. doi: 10.1037/h0024475. [DOI] [PubMed] [Google Scholar]
- Rohsenow DJ, Childress AR, Monti PM, Niaura RS, Abrams DB. Cue reactivity in addictive behaviors: Theoretical and treatment implications. Subst Use Misuse. 1991;25(S7–S8):957–993. doi: 10.3109/10826089109071030. [DOI] [PubMed] [Google Scholar]
- See RE. Neural substrates of conditioned-cued relapse to drug-seeking behavior. Pharmacol Biochem Behav. 2002;71(3):517–529. doi: 10.1016/s0091-3057(01)00682-7. [DOI] [PubMed] [Google Scholar]
- Vollstädt Klein S, Wichert S, Rabinstein J, Bühler M, Klein O, Ende G, Mann K. Initial, habitual and compulsive alcohol use is characterized by a shift of cue processing from ventral to dorsal striatum. Addiction. 2010;105(10):1741–1749. doi: 10.1111/j.1360-0443.2010.03022.x. [DOI] [PubMed] [Google Scholar]
- Weerts EM, Fantegrossi WE, Goodwin AK. The value of nonhuman primates in drug abuse research. Exp Clin Psychopharmacol. 2007;15(4):309–327. doi: 10.1037/1064-1297.15.4.309. [DOI] [PubMed] [Google Scholar]
- Weerts EM, Goodwin AK, Kaminski BJ, Hienz RD. Environmental cues, alcohol seeking, and consumption in baboons: Effects of response requirement and duration of alcohol abstinence. Alcohol Clin Exp Res. 2006;30(12):2026–2036. doi: 10.1111/j.1530-0277.2006.00249.x. [DOI] [PubMed] [Google Scholar]
- Weiss F. Neurobiology of craving, conditioned reward and relapse. Curr Opin Pharmacol. 2005;5(1):9–19. doi: 10.1016/j.coph.2004.11.001. [DOI] [PubMed] [Google Scholar]


