Halothane-induced hepatitis has served as a model for environmentally induced immunopathology for more than three decades and illustrates the paradigm that genetic susceptibility predisposes to a unique immune response and subsequent liver injury (1). Halothane was once considered an ideal anesthetic agent in that it was volatile and non-inflammable, had a high therapeutic index, and no initial manifestation of injury to vital organs such as liver. Not surprisingly, halothane undergoes both oxidative and reductive metabolism by hepatic cytochrome P450 (CYP) with human CYPs 2E1 and 2A6 involved in oxidation and CYPs 2A6 and 3A4 involved in reduction. Hepatotoxicity can result from these CYP-catalyzed transformations (2). Halothane oxidation, the major metabolic pathway, leads to the production of the reactive electrophile trifluoroacetyl chloride and, by subsequent acylation of liver proteins, results in the formation of trifluoroacetylated protein neoantigens. In predisposed individuals, these neoantigens elicit antibody formation and, upon subsequent exposure to halothane, a secondary immune response elicits hepatic necrosis (3, 4).
The reaction of trifluoroacetyl chloride with water produces trifluoroacetic acid (TFA), a clinical marker for halothane oxidation. Metabolic halothane reduction leads to the formation of the 2-chloro-1,1,1-trifluoroethyl radical by hemolysis of the C—Br bond. From this reactive intermediate, the stable metabolites chlorotrifluoroethane (CTE) and chlorodifluoroethene (CDE), formed by additional reduction along with inorganic fluoride are produced; these volatile metabolites, CDE and CTE, are readily detected in exhaled breath of humans exposed to halothane (5). Metabolic reduction to halothane-derived radicals also leads to lipid peroxidation as has been demonstrated in human liver microsomes in vitro (6). Halothane administration reduces hepatic blood flow and this creates the hypoxic conditions required for the reductive metabolism of halothane; consequently CDE and CTE metabolites decline rapidly after halothane exposure is terminated (7).
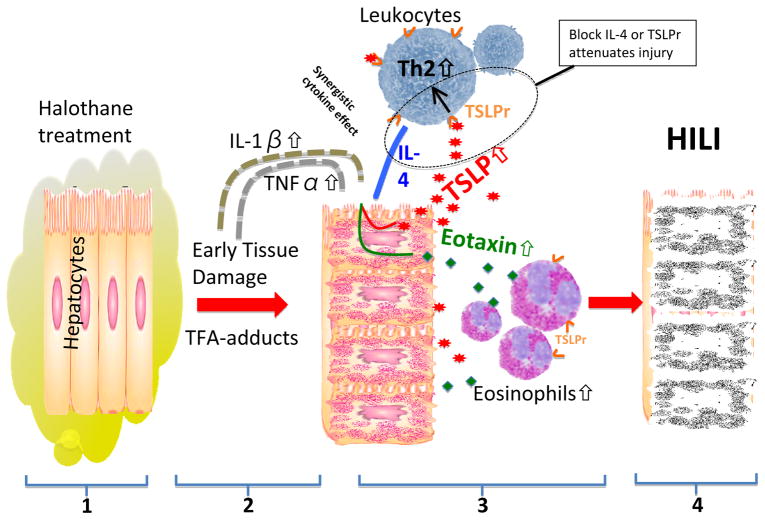
Lance Pohl and his colleagues have a long and distinguished track record of studying the mechanisms involved in HILI, but the molecular basis of immunopathology and the earliest events have remained enigmatic. Interestingly, previous data emphasized the presence of eosinophils at the initiation of liver injury (8). In fact, the presence of eosinophils in these early phases of injury, particularly since they were found exclusively surrounding necrotic areas, with a frequency proportional to the magnitude of injury, suggested that the signaling events that result in eosinophil infiltration will be critical to developing new therapeutic venues. With these comments in mind, the current study (9) has addressed the events that lead to such eosinophilic infiltration and, in particular, provide data that strongly suggests that thymic stromal lymphopoietin (TSLP), derived from hepatic epithelial cells, is the lynch pin along with the concurrent production of IL-4 and other type II cytokines in the events that lead to eosinophil-induced injury (Figure 1).
Figure 1.
(1) Mice treated with halothane produce disruptive trifluoroacetylated (TFA) protein adducts in hepatocytes. (2) This early damage causes an increase in IL-1β and TNFα levels. (3) Along with IL-4, these cytokines synergistically cause an upregulation of thymic stromal lymphopoietin (TSLP) and eotaxin in hepatocytes. Secreted TSLP binds to its receptor (TSLPr) on lymphocytes causing a Th2 response that among other things increases IL-4 secretion. Hepatocyte eotaxin levels are also increased by the elevated trio of cytokines which in turn participates in the recruitment of tissue damaging eosinophils that produce (4) the halothane induced liver injury (HILI). Interfering with IL-4 or TSLPr attenuates the injury in this model.
TSLP is a component of the IL-2 cytokine family and a paralog of IL-7 (10). It is relatively promiscuous in that it has the ability not only to stimulate thymocytes, but it also is integral to B cell differentiation. Importantly, TSLP is produced by a variety of epithelial cells, including not only thymic stroma, but also hepatic epithelial cells, keratinocytes, dendritic cells and mast cells (10–12). In the current study, mice genetically deleted of the TSLP receptor or wild-type controls were exposed to halothane. As expected, in wild-type mice there was a time-dependent appearance of hepatic necrosis demonstrated not only by liver function tests, but also by histochemistry. In contrast, however, in mice deleted of the TSLP receptor, there was a significant reduction in ALT and hepatic necrosis. Interestingly, liver-derived TSLP mRNA was dramatically increased in mice exposed to halothane. IL-4 was also noted to be increased but its production appeared to occur from multiple sources. The reduction in hepatic necrosis in mice deleted of the TSLP receptor was not due to altered metabolism of halothane, i.e. there were no differences in TFA-protein adducts. The decrease in liver pathology was secondary to dramatic reductions in eosinophils concurrent with reductions in IL-4 and the eotaxins, CCL11 and CCL24. Using a variety of cytokine replacements, the authors further demonstrated that TSLP is the initiator of the events that lead to halothane-induced immunopathology. Finally, to expand the data and the potential conclusions, the authors demonstrated a similar role of TSLP in concanavalin-A induced hepatitis.
Although TFA-adduct or halothane-modified macromolecules appear to be an initiating event in HILI, there are other factors required for exacerbation and perpetuation of the injury. Firstly, a specific role for cytokines and/or lymphoid subpopulations continues to remain controversial, but it has been suggested that recruitment of neutrophils or NK cells and changes of the expression levels of IL-17 or IL-10 are essential to the natural history of HILI pathogenesis (13–16). In addition, suppressed miR-106b expression, as well as the subsequent up-regulation of STAT3, may also be critical for the pathogenesis of HILI (17). However, there are no data, including in the current study, that reflect an exclusive factor that provides a mechanism for the exacerbation of HILI. Clearly, there is a multiplicity of TSLP function in the promotion of Th2 responses including the blockade of Th1/Th17 responses (10, 18), the activation of dendritic cells to differentiate Th17-induced inflammation (19), and the requirement of DCs to drive Th17 differentiation via hepatocyte-derived TSLP (20). We would expect therefore that dissection of the TSLP receptor signal cascade would be critical to dissect the natural history of HILI. Since the expression of the TSLP receptor is restricted to mast cells, eosinophils, and myeloid dendritic cells, one would expect that the TSLP receptor signal cascade would be an upstream regulator following the immune response to the xenobiotic. Finally, whether the mechanisms that lead to HILI and/or concanavalin-A induced hepatitis are a paradigm or paradox for xenobiotic-induced immune injury must be further studied.
There are also a number of additional questions that remain unanswered. Firstly, it is not clear that these data can be extrapolated from mice to humans. For example, it is still unknown why some patients are susceptible to halothane-induced injury and others are not. Second, the dichotomy of Th1/Th2/Th17 cytokines in humans is not as clear as it is in inbred mice. Third, although eosinophils appear to be the major component of immunopathology, it is equally clear that there may well be other innate mechanisms that contribute, particularly in those individuals who have genetic variation and/or polymorphisms in the TSLP receptor gene. Recently, at least two independent eosinophil functions have been identified, including firstly as an effector cell and secondly as an antigen presenting cell; these functions may well be the basis for inter-individual differences (21). These comments notwithstanding, this current paper is an important contribution to understanding the cellular events and signaling pathways that drive the earliest stages of HILI. Future studies need to focus on the genetics and, in particular, to define whether HILI is an isolated example or can be generically applied to other models of drug induced liver disease.
Acknowledgments
Funding support provided by a grant from the National Institutes of Health, DK067003
Abbreviations
- CDE
chlorodifluorethene
- CTE
chlorotrifluorethane
- HILI
halothane-induced liver injury
- TSLP
thymic stromal lymphopoietin
References
- 1.Bogdanos DP, Smyk DS, Rigopoulou EI, Mytilinaiou MG, Heneghan MA, Selmi C, Gershwin ME. Twin studies in autoimmune disease: genetics, gender and environment. J Autoimmun. 2012;38:J156–169. doi: 10.1016/j.jaut.2011.11.003. [DOI] [PubMed] [Google Scholar]
- 2.Ray DC, Drummond GB. Halothane hepatitis. Br J Anaesth. 1991;67:84–99. doi: 10.1093/bja/67.1.84. [DOI] [PubMed] [Google Scholar]
- 3.Bourdi M, Chen W, Peter RM, Martin JL, Buters JT, Nelson SD, Pohl LR. Human cytochrome P450 2E1 is a major autoantigen associated with halothane hepatitis. Chem Res Toxicol. 1996;9:1159–1166. doi: 10.1021/tx960083q. [DOI] [PubMed] [Google Scholar]
- 4.Furst SM, Gandolfi AJ. Interaction of lymphocytes with Kupffer cells from halothane-exposed guinea pigs. Int Arch Allergy Immunol. 1997;114:46–53. doi: 10.1159/000237642. [DOI] [PubMed] [Google Scholar]
- 5.Jenner MA, Plummer JL, Cousins MJ. Halothane reductive metabolism in an adult surgical population. Anaesth Intensive Care. 1990;18:395–399. doi: 10.1177/0310057X9001800318. [DOI] [PubMed] [Google Scholar]
- 6.Minoda Y, Kharasch ED. Halothane-dependent lipid peroxidation in human liver microsomes is catalyzed by cytochrome P4502A6 (CYP2A6) Anesthesiology. 2001;95:509–514. doi: 10.1097/00000542-200108000-00037. [DOI] [PubMed] [Google Scholar]
- 7.Kharasch ED, Hankins DC, Fenstamaker K, Cox K. Human halothane metabolism, lipid peroxidation, and cytochromes P(450)2A6 and P(450)3A4. Eur J Clin Pharmacol. 2000;55:853–859. doi: 10.1007/s002280050707. [DOI] [PubMed] [Google Scholar]
- 8.Proctor WR, Chakraborty M, Chea LS, Morrison JC, Berkson JD, Semple K, Bourdi M, et al. Eosinophils mediate the pathogenesis of halothane-induced liver injury in mice. Hepatology. 2013;57:2026–2036. doi: 10.1002/hep.26196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Proctor WR, Chakraborty M, Fullerton AM, Korrapati MC, Ryan PM, Semple K, Morrison JC, et al. Thymic Stromal Lymphopoietin and Interleukin-4 Mediate the Pathogenesis of Halothane-Induced Liver Injury in Mice. Hepatology. 2014 doi: 10.1002/hep.27169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ziegler SF, Roan F, Bell BD, Stoklasek TA, Kitajima M, Han H. The biology of thymic stromal lymphopoietin (TSLP) Adv Pharmacol. 2013;66:129–155. doi: 10.1016/B978-0-12-404717-4.00004-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kashyap M, Rochman Y, Spolski R, Samsel L, Leonard WJ. Thymic stromal lymphopoietin is produced by dendritic cells. J Immunol. 2011;187:1207–1211. doi: 10.4049/jimmunol.1100355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Moon PD, Choi IH, Kim HM. Naringenin suppresses the production of thymic stromal lymphopoietin through the blockade of RIP2 and caspase-1 signal cascade in mast cells. Eur J Pharmacol. 2011;671:128–132. doi: 10.1016/j.ejphar.2011.09.163. [DOI] [PubMed] [Google Scholar]
- 13.Cheng L, You Q, Yin H, Holt MP, Ju C. Involvement of natural killer T cells in halothane-induced liver injury in mice. Biochem Pharmacol. 2010;80:255–261. doi: 10.1016/j.bcp.2010.03.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Feng D, Wang Y, Xu Y, Luo Q, Lan B, Xu L. Interleukin 10 deficiency exacerbates halothane induced liver injury by increasing interleukin 8 expression and neutrophil infiltration. Biochem Pharmacol. 2009;77:277–284. doi: 10.1016/j.bcp.2008.09.028. [DOI] [PubMed] [Google Scholar]
- 15.Kobayashi E, Kobayashi M, Tsuneyama K, Fukami T, Nakajima M, Yokoi T. Halothane-induced liver injury is mediated by interleukin-17 in mice. Toxicol Sci. 2009;111:302–310. doi: 10.1093/toxsci/kfp165. [DOI] [PubMed] [Google Scholar]
- 16.You Q, Cheng L, Reilly TP, Wegmann D, Ju C. Role of neutrophils in a mouse model of halothane-induced liver injury. Hepatology. 2006;44:1421–1431. doi: 10.1002/hep.21425. [DOI] [PubMed] [Google Scholar]
- 17.Endo S, Yano A, Fukami T, Nakajima M, Yokoi T. Involvement of miRNAs in the early phase of halothane-induced liver injury. Toxicology. 2014;319:75–84. doi: 10.1016/j.tox.2014.02.011. [DOI] [PubMed] [Google Scholar]
- 18.Bogiatzi SI, Guillot-Delost M, Cappuccio A, Bichet JC, Chouchane-Mlik O, Donnadieu MH, Barillot E, et al. Multiple-checkpoint inhibition of thymic stromal lymphopoietin-induced TH2 response by TH17-related cytokines. J Allergy Clin Immunol. 2012;130:233–240. e235. doi: 10.1016/j.jaci.2012.04.038. [DOI] [PubMed] [Google Scholar]
- 19.Lin J, Chang W, Dong J, Zhang F, Mohabeer N, Kushwaha KK, Wang L, et al. Thymic stromal lymphopoietin over-expressed in human atherosclerosis: potential role in Th17 differentiation. Cell Physiol Biochem. 2013;31:305–318. doi: 10.1159/000343369. [DOI] [PubMed] [Google Scholar]
- 20.Lee HC, Sung SS, Krueger PD, Jo YA, Rosen HR, Ziegler SF, Hahn YS. Hepatitis C virus promotes T-helper (Th)17 responses through thymic stromal lymphopoietin production by infected hepatocytes. Hepatology. 2013;57:1314–1324. doi: 10.1002/hep.26128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Siracusa MC, Kim BS, Spergel JM, Artis D. Basophils and allergic inflammation. J Allergy Clin Immunol. 2013;132:789–801. doi: 10.1016/j.jaci.2013.07.046. quiz 788. [DOI] [PMC free article] [PubMed] [Google Scholar]