Abstract
Patch clamp recordings of neurons in the adult rat deep cerebellar nuclei have been limited by the availability of viable brain slices. Using a new slicing technique, this study was designed to explore the maturation of membrane properties of neurons in the deep cerebellar nuclei (DCN), –an area involved in rat eyeblink conditioning. Compared to whole-cell current clamp recordings in DCN in rat pups at postnatal day 16 (P16) to P21, recordings from weanling rats at P22 to P40 revealed a number of significant changes including an increase in the amplitude of the after-hyperpolarization (AHP) – an index of membrane excitability which has been shown to be important for eyeblink conditioning – a prolonged interval between the 1st and 2nd evoked action potential, and an increase in AHP amplitude for hyperpolarization-induced rebound spikes. This is the first report of developmental changes in membrane properties of DCN which may be a major contributor to the ontogeny of eyeblink conditioning in the rat.
Keywords: Cerebellar Nuclear Neurons, Membrane Property, Developmental changes, Maturation, After-hyperpolarization
Introduction
Classical conditioning of the eyeblink response has been widely regarded as one of the best models to study the neural mechanisms underlying learning and memory because of a well-understood neural circuitry and the well-defined parameters influencing the rate and strength of eyeblink conditioning (Freeman, Jr. and Stanton, 1991; Gormezano et al., 1962; Schreurs et al., 1991; Schreurs et al., 1998; Schreurs et al., 2013; Stanton et al., 1992). Deep cerebellar nuclei (DCN), the sole non-vestibular output from the cerebellum and an essential component of this neural circuitry, are critical to the ontogeny of eyeblink conditioned responses and have also been a target area for exploring learning-related synaptic plasticity of both excitatory (Aizenman and Linden, 2000; Pugh and Raman, 2006; Pugh and Raman, 2008) and inhibitory inputs (Bengtsson et al., 2011; Pugh and Raman, 2005; Pugh and Raman, 2008; Witter et al., 2013). Studies in developing rats have shown significant anatomical and functional changes in DCN during the first few postnatal weeks (Freeman, Jr. and Nicholson, 2000; Heinsen, 1977; Nicholson and Freeman, Jr., 2004), and thus, anatomical and functional maturation of DCN may influence the process of associative learning and memory by regulating the induction and maintenance of learning-specific changes within DCN (Freeman, Jr. et al., 1995; Freeman, Jr. and Nicholson, 2004).
Due to the difficulties in obtaining viable DCN slices from adult animals and technical problems obtaining patch clamp recordings from adult DCN neurons caused by an extensive peri-neuronal net (Huang and Uusisaari, 2013; Morales et al., 2004), uncovering the neural mechanisms of learning within DCN has been limited to lesion, inactivation, histological examination, and in vivo extracellular recordings. Characterizing membrane properties and delineating the different forms of synaptic plasticity in the DCN has been limited to patch clamp recordings from early postnatal animals. Therefore, it has been difficult to interpret the discrepancies in spike output between in vitro patch clamp recordings from young animals and in vivo recordings from adult animals and determining which one represents physiologically relevant output. To date, there are no reports showing developmental changes in in-vitro membrane properties of the rat DCN. The current experiments address this question by using a new slicing technique (Huang and Uusisaari, 2013) to obtain healthy acute DCN slices from rats before and after weaning and investigate whether there are developmental changes in membrane properties of DCN during rat maturation.
Materials and Methods
Subjects
Long-Evans rat pups were supplied by Harlan (Indianapolis, IN), housed in a cage with their littermates and mother, given free access to food and water, and maintained on a 12 h light/dark cycle. Rat pups were maintained in accordance with guidelines issued by the National Institutes of Health and the research was approved by the West Virginia University Animal Care and Use Committee.
Slice preparation and patch-clamp recordings
Coronal cerebellar slices were prepared from 25 rats of either sex from 4 litters, aging from postnatal day 16 (P16) to postnatal day 40 (P40). Rats were weaned at P21. Rats were anesthetized with carbon dioxide and then decapitated. Brain slices were prepared by slightly modifying the methods of Huang and Uusisaari (Huang and Uusisaari, 2013) with sucrose modified artificial cerebrospinal fluid (S-ACSF) as the cutting solution which can help improve cell survival during slicing (Person and Raman, 2010; Zheng and Raman, 2011). Briefly, after the brain was removed, slices from the cerebellum were cut at 34 °C on a vibrating slicer (LEICA VT1200S) with S-ACSF containing (in mM) Sucrose 200, KCl 2.5, MgCl2 1.2, CaCl2 0.5, NaH2PO4 1.25, NaHCO3 26 and Dextrose 20, incubated for 1 hour at 34 °C in 95% O2- and 5% CO2-saturated artificial cerebrospinal fluid (ACSF) containing (in mM) NaCl 125, KCl 3.0, MgSO4 1.2, CaCl2 2.0, NaH2PO4 1.2, NaHCO3 26 and Dextrose 10, and then maintained at room temperature until electrophysiological recording. Vertical vibration of the blade was manually adjusted with a Vibrocheck device (Leica) before slice preparation and set to 0 μM.
A slice was placed into a modified recording chamber containing the bath solution (ACSF). Neurons from the cerebellar deep nuclei were identified morphologically through a 40X water immersion objective using DIC-IR optics (Olympus BX50WI, Dulles, VA). Whole-cell patch-clamp recordings were performed using an Axon MultiClamp 700B on cells with diameters of 15-20 μM in the interpositus and the medial portion of the lateral nucleus. These neurons are regarded as large glutamatergic projection neurons (Aizenman et al., 2003; Huang and Uusisaari, 2013). Patch pipettes made from borosilicate glass (catalog #: BF150-86-10; 1.5 mm OD, 0.86 mm ID; Sutter Instrument Company, Novato, CA) were pulled with a P97 Brown-Flaming micropipette puller (Sutter Instrument Company, Novato, CA). The final resistances of pipettes filled with the internal solution [containing (in mM) potassium gluconate (C6H11O7K) 140, MgCl2·6H2O 4.6, HEPES 10, EGTA 10, Na2ATP 4.0, pH 7.3 (KOH)] were between 5 and 8 MΩ. Data were low-pass filtered at 2 kHz and acquired at 20 kHz. Membrane properties were measured when the neuron had stabilized for 5 min after the whole-cell configuration was achieved. Quantitative analysis included resting membrane potential measured directly upon breakthrough in whole-cell configuration, input resistance based on membrane potential changes to depolarizing current injections immediately after whole cell configuration, action potential (AP) threshold, current required for eliciting the first AP, half-width of elicited AP including rising and falling phases, amplitude of elicited AP, the number of elicited APs, latency to the first AP elicited by a 250 ms duration depolarizing current injection, and peak amplitude of the after-hyperpolarization (AHP), first and second interval of evoked action potentials (S1S2 interval), current required for hyperpolarization induced rebound spikes, and the properties of rebound spikes. Recordings were only accepted if the resistance of initial seal formations were greater than 1 GΩ and rejected if their output was unstable or series resistance changed more than 20%. To obtain an accurate measurement of neuronal excitability independent of membrane potential changes, continuous direct current was applied through the recording electrode to hold the cell at a −70 mV baseline.
All recordings were made at room temperature.
All electrophysiological data were recorded online using the Clampex 10.0 software (Axon Instruments). Standard off-line analyses were conducted using Clampfit 10.0.
Data are represented as means ± SEM. One-way ANOVA was used with p < 0.05 as a criterion for significance.
Results
Membrane properties
In order to investigate the development of membrane properties in DCN neurons, we modified a warm slicing technique introduced by Huang and Uusisaari (2013) with S-ACSF as the cutting solution so we could obtain healthy DCN slices from rats both before and after weaning and into early adulthood. Neurons in the DCN were spontaneously active at resting membrane potential and showed action potential firing in response to depolarizing current injections when held at -70mV.
Table 1 summarizes the characteristics of membrane properties from the DCN neurons. As can be seen in the table, DCN neurons from rats pre- and post-weaning exhibited similar resting membrane potentials, input resistance, threshold, current required for an evoked AP, and AP amplitude. However, as shown in Figures 1-2 and confirmed by analysis of variance (ANOVA), DCN neurons from rats pre- and post-weaning had significant differences in APD [F(1,83)=6.381, p<0.05], AP rising phase [F(1,83)=4.13, p<0.05], AP falling phase [F(1,83)=7.425, p<0.01], AHP amplitude[F(1,83)=21.012, p<0.001], and S1S2 interval [F(1,78)=5.318, p<0.05] evoked 200 ms depolarizing current pulse. Measurements of spontaneous APs revealed similar significant differences in APD [F(1,83)=6.365, p<0.05], APD rising phase [F(1,83)=4.498, p<0.05], APD falling phase [F(1,83)=7.477, p<0.01], and AHP amplitude[F(1,83)=12.939, p<0.01] between pre- and post-weaning rats. This indicates there were developmental changes in membrane properties of DCN neurons from pre- to post-weaning, suggesting maturity of membrane properties may be involved in the induction of neural plasticity in the cerebellum, which is thought to be responsible for the increased acquisition of conditioned eye-blink responses at this specific age.
Table 1. Characteristics of membrane properties of rat cerebellar DCN neurons aged between p16 and p40.
| Groups | N | Resting Vm (mV) |
Spontaneou sly firing frequency (Hz) |
Input resistance (MΩ) |
Threshold (mV) |
Current required for elicited AP |
Latency for 1st elicited AP (ms) |
AP amplitude (mV) |
APD (ms) | APD rising phase(ms) |
APD falling phase (ms) |
AHP (mV) | S1S2(ms) | S2S3 (ms) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Pre-weaning (p16-21) | 25 | -48.40±0.72 | 14.33±1.77 | 112.32 ±8.35 | -46.29±0.79 | 0.04±0.01 | 20.05±2.26 | 61.74±1.86 | 1.09±0.10 | 0.42±0.03 | 0.68±0.07 | -7.26±1.04 | 11.45±1.46 | 16.66±2.46 |
| Post-weaning (p22-40) | 59 | -48.93±0.46 | 20.04±2.19 | 108.11 ±5.20 | -45.38±0.58 | 0.04±0.01 | 24.27±3.29 | 61.12±1.32 | 0.87±0.04* | 0.36±0.01* | 0.51±0.02** | -12.00±0.51*** | 19.39±2.23* | 22.64±2.27 |
Vm: membrane potential; AP: Action potential; APD: action potential duration; AHP: afterhyperpolarization.
P<0.05 between Pre-weaning and Post-weaning group,
P<0.01,
P<0.001
Fig. 1. Typical recordings of evoked APs by depolarizing current injection in DCN neurons.
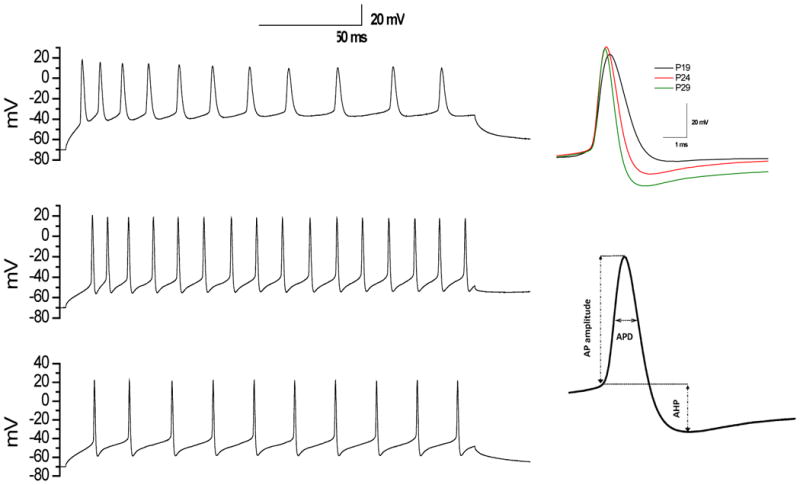
Left panel (Top, middle, and bottom) represents typical whole-cell current clamp recordings with depolarizing current injection of 0.1nA in DCN neurons from P19, P24, and P29 rats, respectively. Right top panel represents the corresponding APs. Right bottom panel represents AP measures taken.
Fig. 2. Developmental changes in APD, AP rising phase, AP falling phase, AHP amplitude, and S1S2 interval.
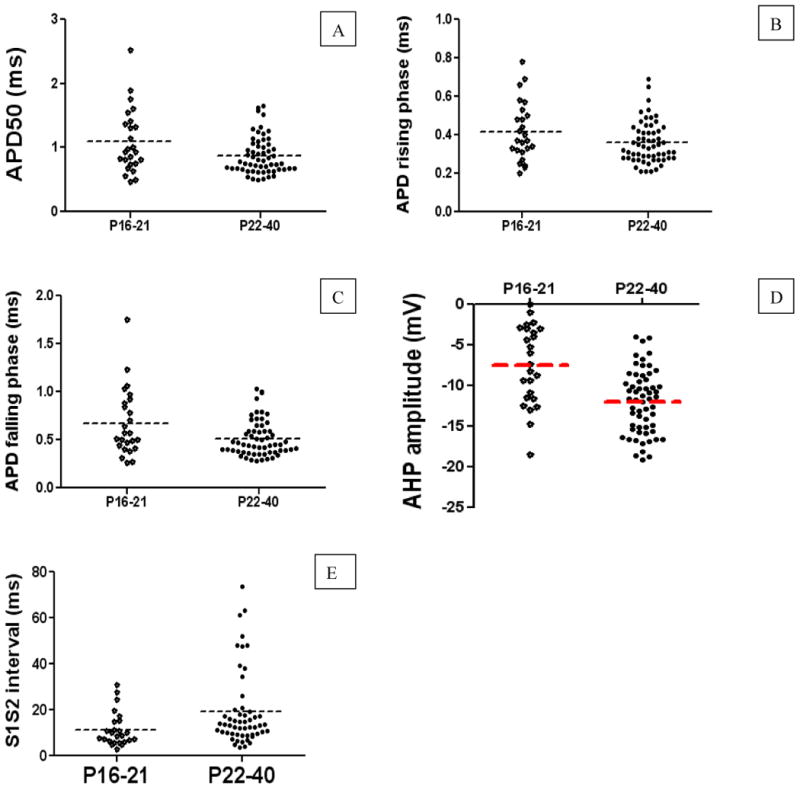
A-E represent the measurements taken for APD, AP rising phase, AP falling phase, AHP amplitude, and S1S2 interval, respectively. Note that each point in the graphs represents the result from a single neuron, and dash line illustrates the average at a given age. A shortening of APD, AP rising phase, and AP falling phases but increased AHP amplitude, prolonged S1S2 interval were observed in post-weaning group.
In order to explore the time window for this developmental change, the data were further analyzed across the following age ranges: P16-21 (Pre-weaning), P22-25 (Weaning), and P26-40 (Post-weaning). As shown in Figure 3, and confirmed by ANOVA, the developmental changes occurred around P22-P25, specifically, there were significant differences in APD [F(2,83)=3.498, P<0.05], AP falling [F(2,83)=4.031, P<0.05], AHP amplitude [F(2,83)=12.484, P<0.001], and S1S2 interval [F(2,78)=3.412, P<0.05] across the three ages. This suggests maturation of membrane properties of DCN neurons happened during a narrow developmental period.
Fig. 3. Developmental changes in membrane properties happened at P22-25.
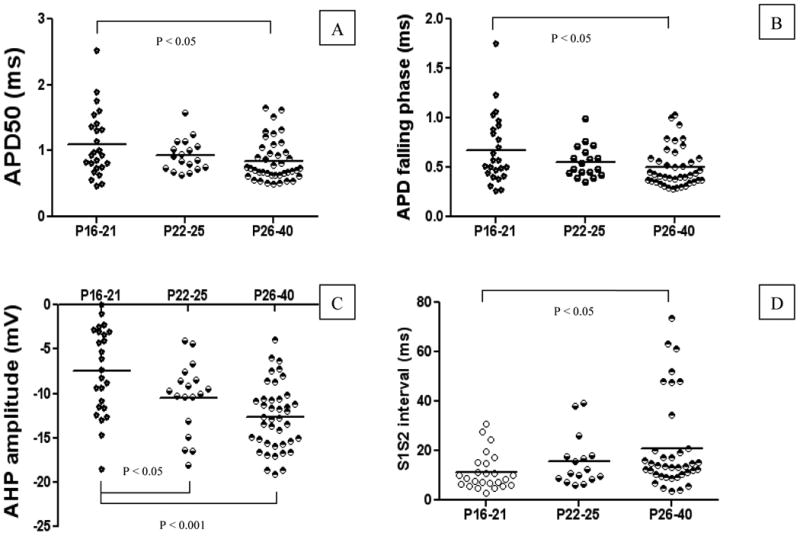
A-D represent the measurements taken for APD, AP falling phase, AHP amplitude, and S1S2 interval, respectively. Note that each point in the graphs represents the result from a single neuron, and dash line illustrates the average at a given age. The developmental changes happened on P22-25 (weaning age).
Rebound spikes
Table 2 summarizes the electrophysiological properties of rebound spikes from DCN neurons. As can be seen in Table 2, DCN neurons from pre- and post-weaning rats exhibited similar threshold, current required for eliciting rebound spikes, and amplitude and duration for rebound spikes. However, Figure 4 shows and ANOVA confirmed significant differences in AHP amplitude [F(1,75)=14.59, p<0.001] of rebound spikes elicited by a 200 ms hyperpolarizing current pulse. This developmental change in the AHP amplitude of rebound spikes that occurred in DCN neurons in the post-weaning group indicates the maturation of synaptic input especially inhibitory inputs from cerebellar Purkinje cells, which may contribute to the synaptic plasticity and enhanced learning seen on the rats at this specific age. Further data analysis for P16-21 (Pre-weaning), P22-25 (Weaning), and P26-40 (Post-weaning) revealed significant group differences in AHP amplitude of rebound spikes [F (2,75)=12.030, P<0.001], and this change occurred at P22-25 and peaked at post-weaning P26-40 [P<0.0001 and P<0.001 if compared to P16-21, P22-25, respectively]. This indicates the maturation of synaptic input may increase with aging and may be involved in the process of learning and memory mediated by synaptic plasticity.
Table 2. Electrophysiological properties of rebound spikes of rat cerebellar DCN neurons aged between P16 and P40.
| Groups | N | Threshold (mV) | Current required for elicited rebound spikes (nA) | Latency for 1st elicited RD (ms) | RD amplitude (mV) | RD duration (ms) | RD rising phase (ms) | RD falling phase (ms) | AHP (mV) |
|---|---|---|---|---|---|---|---|---|---|
| Pre-weaning (p16-21) | 22 | -52.76±1.09 | -0.46±0.07 | 114.34±16.42 | 62.83±2.03 | 1.09±0.10 | 0.43±0.03 | 0.67±0.07 | -7.23±1.30 |
| Post-weaning(p22-40) | 54 | -50.93±0.66 | -0.43±0.05 | 121.93±9.88 | 60.69±1.48 | 0.91±0.05 | 0.37±0.02 | 0.54±0.03 | -12.25±0.65** |
RD: rebound spike; AHP: afterhyperpolarization.
P<0.001 between Pre-weaning and Post-weaning group
Fig. 4. Developmental changes in AHP amplitude of DCN rebound spikes elicited by hyperpolarization pulses.
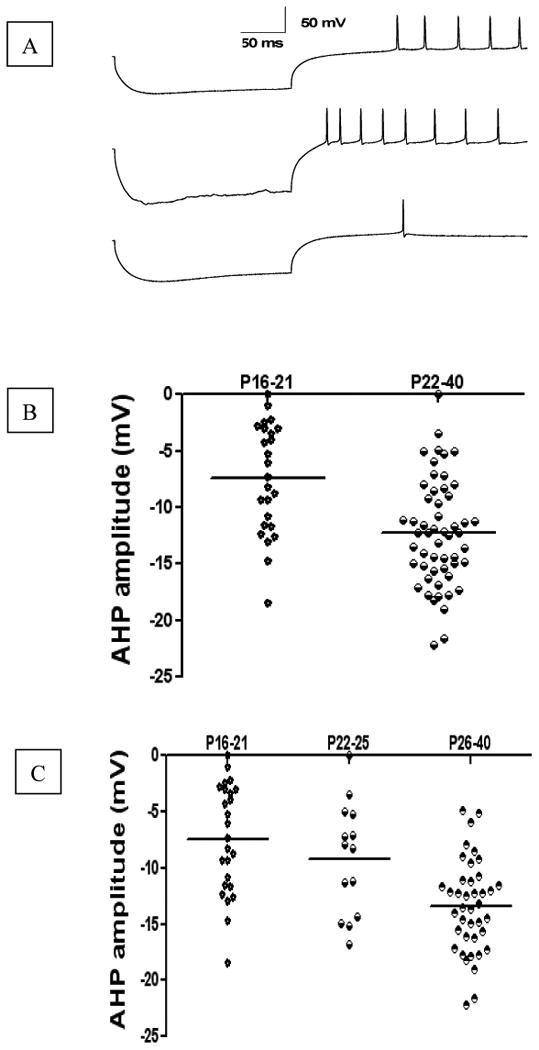
A represents typical rebound spikes elicited by hyperpolarization current injection of -0.5nA in DCN neurons from P19, P24, and P29 rats, respectively. B represents AHP amplitude of rebound spikes from P16-21 group, and P22-40 group, respectively. C represents AHP amplitude of rebound spikes from P16-21 group, P22-25 group, and P26-40 group, respectively. Note that each point in the graphs represents the result from a single neuron, and dash line illustrates the average at a given age. The developmental changes happened on P22-25 (weaning age).
Discussion
In the present study we found developmental changes in the membrane properties of DCN for rats after weaning and these occurred during a narrow developmental window starting at P22-25. The changes were characterized by a shortening of the APD, and the AP rising and falling phase as a function of maturation. In addition, there was an increase in AHP amplitude – an index of membrane excitability shown to be important for classical conditioning (Coulter et al., 1989; Schreurs et al., 1998; Wang and Schreurs, 2010) which was accompanied by a prolonged S1S2 interval. Interestingly, an increased AHP amplitude was also observed for the hyperpolarization-elicited rebound spikes, which are modulated by synaptic inputs on DCN especially inputs from Purkinje cells and climbing fibers (Aizenman and Linden, 1999; Bengtsson et al., 2011; Person and Raman, 2012; Zheng and Raman, 2010) and define the accuracy and timing of cerebellar motor performance (Witter et al., 2013). Taken together, these developmental changes represent the maturation of membrane properties of rat DCN neurons and may help explain the enhanced acquisition of conditioned eyeblink responses at this specific period (Freeman, Jr. et al., 1995; Freeman, Jr. and Nicholson, 2004).
Neural function is dependent on the basal membrane properties and its responsive properties to synaptic inputs. DCN neurons, unlike hippocampal CA1 cells, which are silent at resting membrane potential and actively driven by synaptic pulses, fire action potentials spontaneously at rest though there are extensive basal and driven inhibitions from Purkinje cells. This unique basal state and its response to synaptic inputs may represent a functional convergence of both intrinsic responses and synaptic integration in DCN, and thus encode the signal information for cerebellar motor learning and memory. The AHP increases seen in rats post-weaning and the accompanying adaptation of spike frequency provide evidence that functional immaturity in membrane properties may limit the induction of neural plasticity in the cerebellum and thereby limit the development of conditioned eye-blink responses. A smaller AHP amplitude observed in pre-weanling rats indicates the DCN neurons are more excitable, suggesting they may be capable of establishing learning related plasticity before weaning and cerebellar learning can happen in rats as early as P12 using pontine stimulation as the conditioned stimulus (Campolattaro and Freeman, 2008). However, the expression of robust long-term potentiation in DCN, unlike in the hippocampus that can be easily evoked by high frequency stimulation (Teyler et al., 1989), requires high frequency stimulation applied with hyperpolarization (Person and Raman, 2010; Pugh and Raman, 2008). Therefore, maturation of the membrane properties of DCN could help elicit a steady and strong synaptic plasticity (Pugh and Raman, 2008) that may underlie the enhanced learning acquisition and memory recall during this narrow development period (Freeman, Jr. and Nicholson, 1999; Freeman, Jr. and Nicholson, 2000). Rebound spiking, another intrinsic property of DCN neurons, is regarded as encoding the amplitude of inhibitory synapses in cerebellar circuit and serving to transfer inhibitory signals (Pedroarena, 2010). Interestingly, AHP increases were also seen in rebound spikes from post-weaning rats, which suggests maturation of synaptic inputs especially the inhibitory inputs (Waters et al., 2006) may be required for associative cerebellar learning (Bengtsson et al., 2011; Person and Raman, 2012; Witter et al., 2013).
In addition to the AHP increases noted above, we also found a shortening of APD, AP rising phase, and AP falling phase among post weanling rats. These maturational changes may be encoded by several different kinds of ion channels including voltage-gated sodium channels (Raman et al., 2000), and channels responsible for spike repolarization such as Kv3.1 potassium channels (Pedroarena, 2011), Kv3.3 potassium channels (Waters et al., 2006) , sodium activated potassium channels (Bhattacharjee et al., 2002), BK channels (Alvina and Khodakhah, 2008; Pedroarena, 2011), and N-type voltage-gated calcium channels (Alvina and Khodakhah, 2008).
Finally, it is worthwhile noting that the changes in membrane properties of adult rat DCN neurons may be attributable to maturation (Wilber et al., 2007) rather than that triggered by the cessation of maternal care brought about by separation from the dam at weaning (Gudsnuk and Champagne, 2011). This maturation may underlie the enhanced acquisition of eye-blink conditioning seen most clearly at or just after weaning (Wilber et al., 2007) by maintaining the neurons in a relatively stable state for better signal integration and regulating the maturation process of synaptic formation as reported in cerebellar granular cells (Okazawa et al., 2009).
In summary, this is the first demonstration that developmental changes in membrane properties of rat DCN neurons occur after weaning, specifically starting at P22-25. Maturation of membrane properties of DCN neurons can explain the facilitated conditioned eye-blink response observed at this specific age in behavioral studies.
Acknowledgments
This project was supported by NIH Grant NS061103 and BRNI funds to Bernard G. Schreurs. The contents of this manuscript are solely the responsibility of the authors and do not necessarily represent the official views of the NINDS.
We are grateful to Carrie L. Smith-Bell for animal care and Wen Zheng for reading the manuscript.
References
- Aizenman CD, Huang EJ, Linden DJ. Morphological correlates of intrinsic electrical excitability in neurons of the deep cerebellar nuclei. J Neurophysiol. 2003;89:1738–1747. doi: 10.1152/jn.01043.2002. [DOI] [PubMed] [Google Scholar]
- Aizenman CD, Linden DJ. Regulation of the rebound depolarization and spontaneous firing patterns of deep nuclear neurons in slices of rat cerebellum. J Neurophysiol. 1999;82:1697–1709. doi: 10.1152/jn.1999.82.4.1697. [DOI] [PubMed] [Google Scholar]
- Aizenman CD, Linden DJ. Rapid, synaptically driven increases in the intrinsic excitability of cerebellar deep nuclear neurons. Nat Neurosci. 2000;3:109–111. doi: 10.1038/72049. [DOI] [PubMed] [Google Scholar]
- Alvina K, Khodakhah K. Selective regulation of spontaneous activity of neurons of the deep cerebellar nuclei by N-type calcium channels in juvenile rats. J Physiol. 2008;586:2523–2538. doi: 10.1113/jphysiol.2007.148197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bengtsson F, Ekerot CF, Jorntell H. In vivo analysis of inhibitory synaptic inputs and rebounds in deep cerebellar nuclear neurons. PLoS One. 2011;6:e18822. doi: 10.1371/journal.pone.0018822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhattacharjee A, Gan L, Kaczmarek LK. Localization of the Slack potassium channel in the rat central nervous system. J Comp Neurol. 2002;454:241–254. doi: 10.1002/cne.10439. [DOI] [PubMed] [Google Scholar]
- Campolattaro MM, Freeman JH. Eyeblink conditioning in 12-day-old rats using pontine stimulation as the conditioned stimulus. Proc Natl Acad Sci U S A. 2008;105:8120–8123. doi: 10.1073/pnas.0712006105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coulter DA, Lo Turco JJ, Kubota M, Disterhoft JF, Moore JW, Alkon DL. Classical conditioning reduces amplitude and duration of calcium-dependent afterhyperpolarization in rabbit hippocampal pyramidal cells. J Neurophysiol. 1989;61:971–981. doi: 10.1152/jn.1989.61.5.971. [DOI] [PubMed] [Google Scholar]
- Freeman JH, Jr, Barone S, Jr, Stanton ME. Disruption of cerebellar maturation by an antimitotic agent impairs the ontogeny of eyeblink conditioning in rats. J Neurosci. 1995;15:7301–7314. doi: 10.1523/JNEUROSCI.15-11-07301.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freeman JH, Jr, Nicholson DA. Neuronal activity in the cerebellar interpositus and lateral pontine nuclei during inhibitory classical conditioning of the eyeblink response. Brain Res. 1999;833:225–233. doi: 10.1016/s0006-8993(99)01547-4. [DOI] [PubMed] [Google Scholar]
- Freeman JH, Jr, Nicholson DA. Developmental changes in eye-blink conditioning and neuronal activity in the cerebellar interpositus nucleus. J Neurosci. 2000;20:813–819. doi: 10.1523/JNEUROSCI.20-02-00813.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freeman JH, Jr, Nicholson DA. Developmental changes in the neural mechanisms of eyeblink conditioning. Behav Cogn Neurosci Rev. 2004;3:3–13. doi: 10.1177/1534582304265865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freeman JH, Jr, Stanton ME. Fimbria-fornix transections disrupt the ontogeny of delayed alternation but not position discrimination in the rat. Behav Neurosci. 1991;105:386–395. doi: 10.1037//0735-7044.105.3.386. [DOI] [PubMed] [Google Scholar]
- Gormezano I, Schneiderman N, Deaux E, Fuentes I. Nictitating membrane: classical conditioning and extinction in the albino rabbit. Science. 1962;138:33–34. doi: 10.1126/science.138.3536.33. [DOI] [PubMed] [Google Scholar]
- Gudsnuk KM, Champagne FA. Epigenetic effects of early developmental experiences. Clin Perinatol. 2011;38:703–717. doi: 10.1016/j.clp.2011.08.005. [DOI] [PubMed] [Google Scholar]
- Heinsen H. Quantitative anatomical studies on the postnatal development of the cerebellum of the albino rat. Anat Embryol (Berl) 1977;151:201–218. doi: 10.1007/BF00297481. [DOI] [PubMed] [Google Scholar]
- Huang S, Uusisaari MY. Physiological temperature during brain slicing enhances the quality of acute slice preparations. Front Cell Neurosci. 2013;7:48. doi: 10.3389/fncel.2013.00048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morales E, Fernandez FR, Sinclair S, Molineux ML, Mehaffey WH, Turner RW. Releasing the peri-neuronal net to patch-clamp neurons in adult CNS. Pflugers Arch. 2004;448:248–258. doi: 10.1007/s00424-004-1246-9. [DOI] [PubMed] [Google Scholar]
- Nicholson DA, Freeman JH., Jr Selective developmental increase in the climbing fiber input to the cerebellar interpositus nucleus in rats. Behav Neurosci. 2004;118:1111–1116. doi: 10.1037/0735-7044.118.5.1111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okazawa M, Abe H, Katsukawa M, Iijima K, Kiwada T, Nakanishi S. Role of calcineurin signaling in membrane potential-regulated maturation of cerebellar granule cells. J Neurosci. 2009;29:2938–2947. doi: 10.1523/JNEUROSCI.5932-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pedroarena CM. Mechanisms supporting transfer of inhibitory signals into the spike output of spontaneously firing cerebellar nuclear neurons in vitro. Cerebellum. 2010;9:67–76. doi: 10.1007/s12311-009-0153-1. [DOI] [PubMed] [Google Scholar]
- Pedroarena CM. BK and Kv3.1 potassium channels control different aspects of deep cerebellar nuclear neurons action potentials and spiking activity. Cerebellum. 2011;10:647–658. doi: 10.1007/s12311-011-0279-9. [DOI] [PubMed] [Google Scholar]
- Person AL, Raman IM. Deactivation of L-type Ca current by inhibition controls LTP at excitatory synapses in the cerebellar nuclei. Neuron. 2010;66:550–559. doi: 10.1016/j.neuron.2010.04.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Person AL, Raman IM. Purkinje neuron synchrony elicits time-locked spiking in the cerebellar nuclei. Nature. 2012;481:502–505. doi: 10.1038/nature10732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pugh JR, Raman IM. GABAA receptor kinetics in the cerebellar nuclei: evidence for detection of transmitter from distant release sites. Biophys J. 2005;88:1740–1754. doi: 10.1529/biophysj.104.055814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pugh JR, Raman IM. Potentiation of mossy fiber EPSCs in the cerebellar nuclei by NMDA receptor activation followed by postinhibitory rebound current. Neuron. 2006;51:113–123. doi: 10.1016/j.neuron.2006.05.021. [DOI] [PubMed] [Google Scholar]
- Pugh JR, Raman IM. Mechanisms of potentiation of mossy fiber EPSCs in the cerebellar nuclei by coincident synaptic excitation and inhibition. J Neurosci. 2008;28:10549–10560. doi: 10.1523/JNEUROSCI.2061-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raman IM, Gustafson AE, Padgett D. Ionic currents and spontaneous firing in neurons isolated from the cerebellar nuclei. J Neurosci. 2000;20:9004–9016. doi: 10.1523/JNEUROSCI.20-24-09004.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schreurs BG, Burhans LB, Smith-Bell CA, Mrowka SW, Wang D. Ontogeny of trace eyeblink conditioning to shock-shock pairings in the rat pup. Behav Neurosci. 2013;127:114–120. doi: 10.1037/a0031298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schreurs BG, Gusev PA, Tomsic D, Alkon DL, Shi T. Intracellular correlates of acquisition and long-term memory of classical conditioning in Purkinje cell dendrites in slices of rabbit cerebellar lobule HVI. J Neurosci. 1998;18:5498–5507. doi: 10.1523/JNEUROSCI.18-14-05498.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schreurs BG, Sanchez-Andres JV, Alkon DL. Learning-specific differences in Purkinje-cell dendrites of lobule HVI (Lobulus simplex): intracellular recording in a rabbit cerebellar slice. Brain Res. 1991;548:18–22. doi: 10.1016/0006-8993(91)91100-f. [DOI] [PubMed] [Google Scholar]
- Stanton ME, Freeman JH, Jr, Skelton RW. Eyeblink conditioning in the developing rat. Behav Neurosci. 1992;106:657–665. doi: 10.1037//0735-7044.106.4.657. [DOI] [PubMed] [Google Scholar]
- Teyler TJ, Perkins AT, Harris KM. The development of long-term potentiation in hippocampus and neocortex. Neuropsychologia. 1989;27:31–39. doi: 10.1016/0028-3932(89)90088-2. [DOI] [PubMed] [Google Scholar]
- Wang D, Schreurs BG. Dietary cholesterol modulates the excitability of rabbit hippocampal CA1 pyramidal neurons. Neurosci Lett. 2010;479:327–331. doi: 10.1016/j.neulet.2010.05.090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waters MF, Minassian NA, Stevanin G, Figueroa KP, Bannister JP, Nolte D, Mock AF, Evidente VG, Fee DB, Muller U, Durr A, Brice A, Papazian DM, Pulst SM. Mutations in voltage-gated potassium channel KCNC3 cause degenerative and developmental central nervous system phenotypes. Nat Genet. 2006;38:447–451. doi: 10.1038/ng1758. [DOI] [PubMed] [Google Scholar]
- Wilber AA, Southwood CJ, Sokoloff G, Steinmetz JE, Wellman CL. Neonatal maternal separation alters adult eyeblink conditioning and glucocorticoid receptor expression in the interpositus nucleus of the cerebellum. Dev Neurobiol. 2007;67:1751–1764. doi: 10.1002/dneu.20549. [DOI] [PubMed] [Google Scholar]
- Witter L, Canto CB, Hoogland TM, de G, Jr, De Zeeuw CI. Strength and timing of motor responses mediated by rebound firing in the cerebellar nuclei after Purkinje cell activation. Front Neural Circuits. 2013;7:133. doi: 10.3389/fncir.2013.00133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng N, Raman IM. Synaptic inhibition, excitation, and plasticity in neurons of the cerebellar nuclei. Cerebellum. 2010;9:56–66. doi: 10.1007/s12311-009-0140-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng N, Raman IM. Prolonged postinhibitory rebound firing in the cerebellar nuclei mediated by group I metabotropic glutamate receptor potentiation of L-type calcium currents. J Neurosci. 2011;31:10283–10292. doi: 10.1523/JNEUROSCI.1834-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]


