Abstract
Background
The GABAergic neuroactive steroid (3α,5α)-3-hydroxy-pregnan-20-one (3α,5α-THP, allopregnanolone) has been studied during withdrawal from ethanol in humans, rats and mice. Serum 3α,5α-THP levels decreased and brain levels were not altered following acute ethanol administration (2 g/kg) in male C57BL/6J mice, however the effects of chronic intermittent ethanol (CIE) exposure on 3α,5α-THP levels have not been examined. Given that CIE exposure changes subsequent voluntary ethanol drinking in a time-dependent fashion following repeated cycles of ethanol exposure, we conducted a time-course analysis of CIE effects on 3α,5α-THP levels in specific brain regions known to influence drinking behavior.
Methods
Adult male C57BL/6J mice were exposed to four cycles of CIE to induce ethanol dependence. All mice were sacrificed and perfused at one of two time points, 8 hr or 72 hr following the final exposure cycle. Free floating brain sections (40 μm; 3-5 sections/region/animal) were immunostained and analyzed to determine relative levels of cellular 3α,5α-THP.
Results
Withdrawal from CIE exposure produced time-dependent and region-specific effects on immunohistochemical detection of 3α,5α-THP levels across cortical and limbic brain regions. A transient reduction in 3α,5α-THP immunoreactivity was observed in the central nucleus of the amygdala 8 hr after withdrawal from CIE (-31.4 ± 9.3). Decreases in 3α,5α-THP immunoreactivity were observed 72 hr following withdrawal in the medial prefrontal cortex (−25.0 ± 9.3%), nucleus accumbens core (−29.9 ± 6.6%), and dorsolateral striatum (−18.5 ± 6.0%), while an increase was observed in the CA3 pyramidal cell layer of the hippocampus (+42.8 ± 19.5%). Sustained reductions in 3α,5α-THP immunoreactivity were observed at both time points in the lateral amygdala (8 hr −28.3 ± 12.8%; 72 hr −27.5 ± 12.4%) and in the ventral tegmental area (8 hr −26.5 ± 9.9%; 72 hr −31.6 ± 13.8%).
Conclusions
These data suggest that specific neuroadaptations in 3α,5α-THP levels may be present in regions of brain that mediate anxiety, stress and reinforcement relevant to ethanol dependence. The changes that occur at different time points likely modulate neurocircuitry involved in ethanol withdrawal as well as the elevated drinking observed after CIE exposure.
Keywords: Alcohol, 3α, 5α-THP, allopregnanolone, neuroactive steroid, withdrawal
Introduction
Chronic intermittent ethanol (CIE) exposure and withdrawal produces ethanol dependence and increases subsequent voluntary ethanol consumption in C57BL/6J mice (Becker and Lopez, 2004; Griffin et al., 2009; Lopez et al., 2012). Repeated exposure to ethanol vapor decreases the aversive properties associated with ethanol (Lopez et al., 2012) and induces a persistent blunting of the hypothalamic-pituitary-adrenal (HPA) axis (Becker, 2012). Changes in gene expression are observed in prefrontal cortex, hippocampus and the nucleus accumbens (NAcc) following CIE exposure (Melendez et al., 2012). However, the precise mechanisms mediating the changes in behavior, gene expression and HPA axis functionality are not well understood. Chronic ethanol exposure and withdrawal induces a switch in excitatory and inhibitory tone, with increased excitation and decreased inhibition (Kumar et al., 2009; Olsen and Spigelman, 2012), manifested as a loss of GABAergic tone.
Neuroactive steroids are endogenous modulators of neuronal activity that act as modulators of ion channels, including GABAA receptors. The 5α-reduced pregnane steroids (3α,5α)-3-hydroxy-pregnan-20-one (3α,5α-THP) and (3α,5α)-3,21-dihydroxypregnan-20-one (3α,5α-THDOC), are two of the most potent positive allosteric modulators of GABAA receptors, which can alter GABAergic activity at nanomolar concentrations (Morrow et al., 1987). GABAergic neuroactive steroids, including 3α,5α-THP, (3α,5β)-3-hydroxy-pregnan-20-one (3α,5β-THP) and 3α,5α-THDOC act at known potentiating sites within α subunits of GABAA receptors (Hosie et al., 2006) to enhance GABAergic activity, producing similar pharmacological effects as ethanol.
Effects of acute ethanol exposure on neuroactive steroids differ in rat and mouse models. Sprague Dawley rats have higher basal serum pregnenolone (a precursor to 3α,5α-THP) and lower basal serum 3α,5α-THP levels compared to C57BL/6J mice (Porcu et al., 2010). Acute ethanol administration increases serum, plasma, cortical, and hippocampal 3α,5α-THP levels in Sprague Dawley rats (Morrow et al., 1999; Porcu et al., 2010; Serra et al., 2003; VanDoren et al., 2000). Using immunohistochemistry (IHC), we recently showed that acute ethanol increases 3α,5α-THP levels in medial prefrontal cortex (mPFC), CA1 pyramidal cell layer of the hippocampus, polymorph cell layer of the dentate gyrus, paraventricular hypothalamic nucleus (PVN), bed nucleus of the stria terminals (BNST), and decreases 3α,5α-THP in the NAcc shore and central nucleus of the amygdala (CeA) in Wistar rats (Cook et al., 2014). In the Sprague Dawley rat hippocampal slice, acute ethanol increased neurosteroidogenesis in CA1 pyramidal cells in the hippocampus, while functionally decreasing long-term potentiation (LTP) (Follesa et al., 2006; Sanna et al., 2004; Tokuda et al., 2011). In contrast, acute ethanol administration decreases 3α,5α-THP levels in serum and causes no change in whole brain, cortical or hippocampal 3α,5α-THP levels in C57BL/6J mice (Finn et al., 2004; Porcu et al., 2014; Porcu et al., 2010). However, no studies of ethanol effects on changes in 3α,5α-THP levels in discrete mesolimbic, amygdalar and hippocampal brain regions in C57BL/6J mice have been conducted to date.
Neuroactive steroid administration has biphasic effects on voluntary ethanol self-administration (Ford et al., 2005) and consumption in male mice (Sinnott et al., 2002) and rats (Janak et al., 1998; Morrow et al., 2001b). Pregnenolone administration decreased ethanol self-administration and increased cerebral 3α,5α-THP levels in alcohol-preferring (P) rats (Besheer et al., 2010). In contrast, intracerebroventricular administration of 3α,5α-THP increased ethanol drinking in C57BL/6J mice (Ford et al., 2007). Together, these data indicate that 3α,5α-THP levels can influence drinking behavior in rodent models, but different effects are observed across strains of rodents and dose of neurosteroid.
Chronic ethanol exposure and withdrawal decreases hippocampal and cortical 3α,5α-THP levels in male rats (Cagetti et al., 2004; Janis et al., 1998) and in human alcoholic patient serum (Romeo et al., 1996). Our laboratory found that cerebral cortical 3α,5α-THP levels were elevated 72 hours following CIE exposure, but were not different following 8 hr withdrawal in C57BL/6J mice (Morrow et al., 2009). However, little is known about brain region-specific changes in endogenous neuroactive steroid levels following CIE exposure using this mouse model. Many brain regions likely contribute to elevated ethanol consumption following CIE exposure in ethanol dependent mice (Griffin, 2014). Many of these regions were chosen to examine the effects of CIE on neuronal 3α,5α-THP immunoreactivity, including mPFC, NAcc, dorsal striatum, hippocampus, BNST, amygdala and ventral tegmental area (VTA). Therefore, the present set of experiments were designed to assess brain-region and time-dependent specific changes in 3α,5α-THP immunoreactivity following 8-hr or 72-hr withdrawal from CIE exposure in male C57BL/6J mice.
Materials and Methods
Subjects
Adult male C57BL/6J mice were obtained from Jackson Laboratories (Bar Harbor, ME) at 7-8 weeks of age. Animals were between 9-11 weeks of age at the beginning of the experiment. All mice were allowed to acclimate to the colony before initial exposure to vapor inhalation chambers. Animals were group-housed 4-5/cage with free access to food and water for the duration of the experiment. Body weight was recorded daily before introduction to the vapor inhalation chamber. All mice were maintained in a temperature (23 ± 0.06 °C) and humidity (40 ± 10%) controlled room with lights on from 0700-1900 hr. Animal care followed National Institutes of Health Guidelines under University of North Carolina at Chapel Hill Institutional Animal Care and Use Committee approved protocols.
Chronic Intermittent Vapor Inhalation Chamber Exposure
Mice were exposed to repeated intermittent ethanol or air vapor for four cycles over four weeks as described below. Mice were transported from the colony to the procedure room at approximately 1630 hr. Animals were weighed and administered an ip. injection (0.02 ml/g) of the alcohol dehydrogenase inhibitor pyrazole (1 mmol/kg) combined with saline for control animals or combined with 1.6 g/kg ethanol (8% w/v) and immediately placed in inhalation chambers (La Jolla Alcohol Research Inc., San Diego, CA). Mice remained in the vapor inhalation chambers for 16 hr overnight with room air (control group) or volatized ethanol (ethanol group) delivered to the chambers at a rate of 10 liters/min. These procedures were designed to maintain blood ethanol concentrations (BECs) at 150-250 mg/dl throughout the exposure period. Ethanol (95%) was volatilized by passing air through an air stone (gas diffuser) submerged in ethanol. The following morning, at 0900 hr, mice were removed from the vapor inhalation chamber and returned to the home cage for 8 hr. One cycle consisted of four 16-hr exposures. Following each cycle, mice were returned to the home cage and colony and remained undisturbed for three days. On the morning following initial exposure to the vapor inhalation chamber and following the third exposure of each cycle, mice were removed from the chamber and 50 μL of blood was collected from the submandibular space for BEC assessment (Table 1). BECs were analyzed by gas chromatography with 10 μL of blood diluted in 375 μL of ddH2O and 500 mg of NaCl in a sealed 10×75 test tube. The sample was volatized in a water bath set at 55°C and 1.5 mL of volatized sample was removed from the tube and injected.
Table 1.
Blood ethanol concentrations (mg/dl) across CIE cycles
| Cycle | 8 hr Withdrawal | 72 hr Withdrawal |
|---|---|---|
| 1 (Day 1) | 232.1 ± 16.3 | 219.4 ± 10.1 |
| 1 (Day 3) | 176.9 ± 17.6 | 139.7 ± 11.3 |
| 2 (Day 3) | 155.0 ± 18.9 | 164.3 ± 16.8 |
| 3 (Day 3) | 154.2 ± 17.3 | 188.7 ± 18.6 |
| 4 (Day 3) | 132.3 ± 10.7** | 213.2 ± 12.4 |
Data are presented as mean blood ethanol concentration (mg/dl) ± SEM across CIE exposure cycles for each experiment.
p<0.01 indicates 72 hr withdrawal significantly greater than 8 hr withdrawal.
Tissue Processing and Immunohistochemistry
All mice were euthanized with an overdose of pentobarbital sodium (150 mg/kg) and transcardially perfused 8 hr or 72 hr after the last ethanol vapor or air exposure cycle. Mice were perfused at a flow rate of 3 ml/min with a total volume of 6 ml of 0.1M phosphate-buffered saline (PBS) followed by 12 ml of 4% paraformaldehyde (PFA). Following perfusion, brains were extracted and post-fixed in 4% PFA for 24 hr at 4° C and then stored in PBS until they were sectioned coronally at 40 μm on a vibrating microtome and stored at −30° C until IHC analysis.
IHC was performed on free-floating sections (3-5 sections/animal/brain region) using an affinity purified 3α,5α-THP sheep antibody, that we have previously characterized by radioimmunoassay (VanDoren et al., 2000) and IHC (Cook et al., 2014). We have shown low cross-reactivity of this antibody with other 5α-reduced pregnane neurosteroids and their precursors (Cook et al., 2014). No detergents or organic solvents were used to prevent the possibility of neuroactive steroid leeching. Sections were rinsed in PBS, followed by incubation in 1% H202 to block endogenous peroxidase activity. The tissue was blocked in 10% normal rabbit serum (Vector Laboratories, Burlingame, CA) in PBS following by incubation in the sheep affinity purified anti-3α,5α-THP antiserum (purchased from Dr. R.H. Purdy) at a 1:2500 dilution for 48 hr at 4° C. Sections were rinsed in PBS and then incubated in a rabbit-antisheep biotinylated secondary antibody (1:200, Vector Laboratories, preabsorbed in 2% mouse serum; Sigma-Aldrich, St. Louis, MO) for 60 min. The secondary antibody was preabsorbed in 2% mouse serum 60 min before the initial PBS rinse to decrease non-specific binding. Sections were then rinsed in PBS, avidin biotin amplification was performed using a Vectastain Elite ABC kit (Vector Laboratories, Burlingame, CA) and immunoreactivity was visualized with 3,3’-diaminobenzidine (DAB, Sigma-Aldrich, St Louis, MO) using the manufacturers’ recommended protocol.
Immunohistochemical Analyses
Brain region immunoreactivity was visualized with an Olympus CX41 light microscope (Olympus America, Center Valley, PA), images were captured with a digital camera (Regita model, QImaging, Burnaby, BC), and analyzed using Bioquant (Nashville, TN) image analysis to obtain linear integrated optical density for immunoreactivity assessment. The microscope, camera, and software were background corrected to eliminate non-specific labeling and normalized to preset light levels to ensure fidelity of data acquisition. Positive pixel count of immunoreactivity was quantified from a circumscribed field, delineated as a brain region, divided by the area of the region in square millimeters, and expressed as pixels/mm2. Data from 3-5 alternate sequential sections per animal per brain region from both hemispheres were used to average one value per mouse. If tissue damage from IHC processing resulted in fewer than 3 sections/brain region, the mouse was eliminated from analyses. The experimenter was blind to the condition of each animal when analyses were conducted. Brain regions analyzed included mPFC (+1.98 to +1.70 AP), striatum [including dorsomedial striatum (DMS); dorsolateral striatum (DLS) and NAcc shell and core (+1.54 to +0.98 AP)], BNST [dorsal and ventral (+0.38 to +0.02 AP)], PVN (−0.46 to −1.06 AP), amygdala [lateral amygdala (LA), basolateral amygdala (BLA) and CeA (−0.94 to −1.58 AP)], hippocampus [CA1, CA3 and polymorph cell layer of the dentate gyrus (−1.34 to −1.82 AP)] and VTA (−2.80 to −3.28 AP). All coordinates were expressed relative to bregma in the Mouse Brain Atlas in Stereotaxic Coordinates (Franklin and Paxinos, 2007).
Statistical Analysis
Given the two experiments (8-hr withdrawal, 72-hr withdrawal) were conducted independently and tissue samples and image analysis were processed separately, data for each brain region were transformed as a percent of control [(individual mean/control group mean)*100] and analyzed using a two-way between-subjects design ANOVA (Statistica, StatSoft Inc., Tulsa, OK, USA), with CIE Exposure (Air, Ethanol) and Withdrawal Time Point (8 hr, 72 hr withdrawal) as factors. In the presence of significant main effects or interactions, Fisher's planned least significant difference (PLSD) test was conducted to isolate effects. Additionally, within each experiment (8 hr withdrawal, 72 hr withdrawal), raw pixel densities (pixels/mm2) were analyzed using Student's t-test for comparisons between ethanol-exposed and air-exposed mice (Table 2). BECs were analyzed using a two-factor mixed-model design ANOVA with Withdrawal Time Point (8 hr, 72 hr withdrawal) as a between-subjects factor and Cycle as a repeated measure. The level of significance was set at p≤ 0.05.
Table 2.
CIE alters neuronal 3α,5α-THP levels across various brain structures following 8 hr or 72 hr withdrawal.
| 8 hr Withdrawal | 72 hr Withdrawal | |||
|---|---|---|---|---|
| Air | Ethanol | Air | Ethanol | |
| mPFC | 54,711 ± 4990 | 45,671 ± 2486 | 53,897 ± 3448 | 40,415 ± 3622* |
| CA1 | 58,952 ± 7703 | 58,544 ± 10774 | 142,514 ± 15,040 | 113,555 ± 11,952 |
| CA3 | 82,170 ± 9349 | 78,888 ± 11321 | 70,094 ± 4820 | 110,420 ± 14,116* |
| Polymorph Cell Layer of Dentate Gyrus | 25,781 ± 1997 | 23,289 ± 2356 | 21,324 ± 2350 | 21,378 ± 2424 |
| VTA | 14,941 ± 1081 | 10,980 ± 1421* | 25,032 ± 3143 | 17,134 ± 1582* |
| NAcc core | 72,394 ± 2121 | 70,288 ± 4898 | 48,175 ± 2465 | 33,718 ± 2230# |
| NAcc shell | 70,666 ± 2213 | 66,353 ± 4138 | 42,134 ± 2917 | 43,094 ± 2017 |
| DLS | 43,683 ± 1195 | 41,419 ± 3764 | 37,084 ± 1504 | 30,209 ± 1614** |
| DMS | 52,310 ± 1539 | 46,504 ± 3130 | 45,132 ± 1504 | 44,681 ± 1614 |
| LA | 24,606 ± 1949 | 17,653 ± 2440* | 22,301 ± 2372 | 16,214 ± 1569* |
| CeA | 23,474 ± 2291 | 16,582 ± 2015* | 12,936 ± 884 | 13,017 ± 1491 |
| BLA | 28,390 ± 2305 | 24,932 ± 3711 | 19,016 ± 1687 | 18,108 ± 1663 |
| PVN | 33,149 ± 2800 | 28,693 ± 2507 | 23,576 ± 2134 | 21,038 ±1991 |
| Dorsal BNST | 13,824 ± 1802 | 12,326 ± 1287 | 11,928 ± 2500 | 10,540 ± 1464 |
| Ventral BNST | 13,474 ± 1573 | 11,367 ± 1049 | 19,520 ± 2635 | 15,723 ± 1818 |
Data are presented as mean positive pixels/mm2 ± SEM of air-exposed or ethanol exposed mice following 8 hr or 72 hr withdrawal (air-exposed n= 10-12; ethanol-exposed n=10-13)
p< 0.05
p<0.01
p<0.0005 compared to respective air-exposed control.
Results
Blood ethanol concentrations across CIE cycles
In general, mice were exposed to ethanol concentrations (>130 mg/dl) across the four CIE cycles [Table 1, Cycle by Withdrawal Time Point interaction [F(4, 92) = 6.18, p< 0.0005] and a significant main effect of Cycle [F(4, 92) = 9.22, p< 0.0001]]. Comparable BECs were observed across CIE cycles 1 through 3 (Table 1) between the mice in the 8 hr and 72 hr withdrawal experiments. During the 4th CIE cycle, mice in the 8 hr withdrawal experiment fell to 132 mg/dl, compared to 213 mg/dl in the 72 hour withdrawal experiment (Table 1, p< 0.01).
CIE exposure alters 3α,5α-THP levels in mPFC and hippocampus 72-hr after withdrawal, but not 8-hr after withdrawal
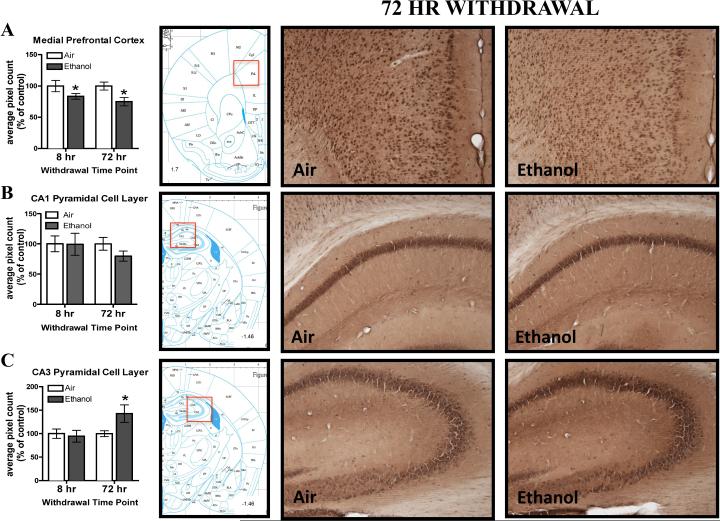
Following four cycles of CIE vapor exposure, we assessed the percent of control 3α,5α-THP immunoreactivity values in mPFC after 8 or 72 hr withdrawal. In general, ethanol-exposed mice showed lower 3α,5α-THP immunoreactivity compared to air-exposed mice (Figure 1A) as supported by a main effect of CIE Exposure [F(1,40)= 9.23, p<0.0005], but no effect of Withdrawal Time Point or CIE Exposure by Withdrawal Time Point interaction. Within experiment analyses of the raw pixel density indicate a significant reduction (−25.0 ± 9.3%) in 3α,5α-THP immunoreactivity was observed following 72 hr withdrawal (t(18)=2.70, p< 0.05) in ethanol-exposed mice, but no change following 8 hr withdrawal (Table 2). The overall reduction in 3α,5α-THP immunoreactivity appears to be driven by the 72 hr withdrawal time point.
Figure 1.
Effects of CIE exposure on 3α,5α-THP immunoreactivity in the A) mPFC, B) CA1 pyramidal cell layer and C) CA3 pyramidal cell layer 8 hr or 72 hr after withdrawal in air-exposed (clear bars) or ethanol-exposed mice (grey bars). Data depicted are mean positive pixels/mm2 ± SEM. Representative photomicrographs (10×) of 3α,5α-THP immunoreactivity following 72 hr withdrawal in air- (left photos; n=10-12) or ethanol-exposed (right photos; n=10-13) mice. Red box indicates coordinates relative to bregma depicted in photomicrographs. *p<0.05 compared to respective air-exposed control.
In the hippocampal CA3 pyramidal cell layer, there was a 42.8 ± 19.5% increase in 3α,5α-THP immunoreactivity 72 hr post-withdrawal (Figure 1C) in ethanol-exposed animals, but no significant difference between groups following 8 hr withdrawal. This result was supported by a significant CIE Exposure × Withdrawal Time Point interaction [F(1,39)= 5.69, p< 0.05; Fisher PLSD; p< 0.05]. The elevation of 3α,5α-THP immunoreactivity in the ethanol-exposed mice compared to the air-exposed mice was confirmed within the 72 hr withdrawal experiment analysis of raw pixel density [(t(20)=2.02, p= 0.05), Table 2]. There were no changes in 3α,5α-THP immunoreactivity in CA1 (Figure 1B; Table 2) or the polymorph cell layer of the dentate gyrus (Table 2) at either time point.
Regional reductions in 3α,5α-THP levels in VTA, NAcc and dorsal striatum 8-hr and 72-hr post-withdrawal following CIE exposure
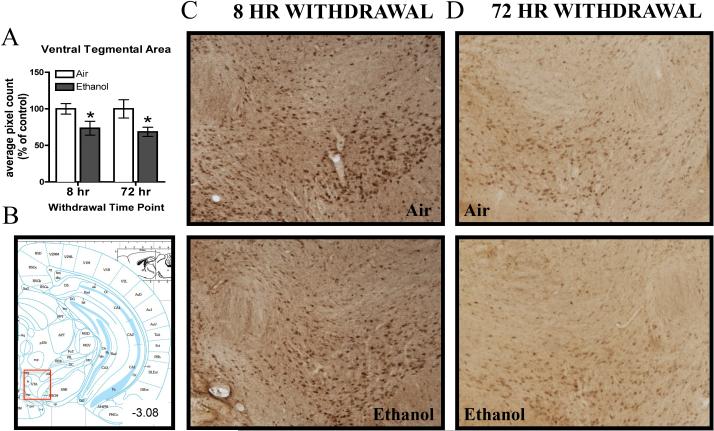
In the VTA, CIE exposure caused a persistent decrease in 3α,5α-THP immunoreactivity that was observed following both 8 hr and 72 hr withdrawal (Figure 2A, C, D). There was a main effect of CIE Exposure [F(1,42)= 10.02, p< 0.005), but no main effect of Withdrawal Time Point or CIE Exposure by Withdrawal Time Point interaction. Furthermore, within experiment analyses of raw pixel density indicate there was a 26.5 ± 9.9% reduction in 3α,5α-THP immunoreactivity in the VTA of ethanol-exposed mice following 8 hr withdrawal (t(22)=2.22, p< 0.05), and a 31.6 ± 13.8% reduction following 72 hr withdrawal [t(20)=2.25, p<0.05, Table 2].
Figure 2.
Effects of CIE exposure on 3α,5α-THP immunoreactivity in the A) VTA 8 hr or 72 hr after withdrawal in air-exposed (clear bars) or ethanol-exposed mice (grey bars). Data depicted are mean positive pixels/mm2 ± SEM. B) Red box indicates coordinates relative to bregma depicted in photomicrographs. C-D) Representative photomicrographs (10×) 3α,5α-THP of immunoreactivity following 8 hr or 72 hr withdrawal in air- (n=11-12) and ethanol-exposed (n=11-13) mice. *p<0.05 compared to respective air-exposed control.
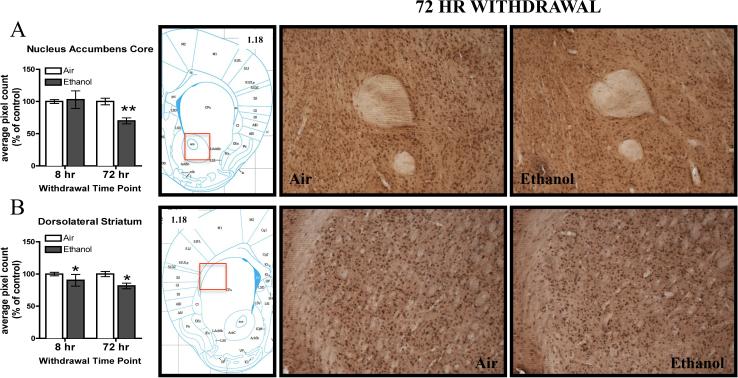
In the NAcc core, CIE exposure reduced 3α,5α-THP immunoreactivity by 29.9 ± 6.6% following 72 hr withdrawal (Figure 3A), but no significant difference between groups was observed following 8 hr withdrawal. The two-way ANOVA indicated a significant CIE Exposure x Withdrawal Time Point interaction [F(1,41)= 6.74, p< 0.05; , Fisher PLSD, p< 0.01] and a significant main effect of Withdrawal Time Point [F(1,41)= 6.74, p< 0.01]. The reduction in the ethanol-exposed mice compared to the air-exposed mice was confirmed within the 72 hr withdrawal experiment by assessment of raw pixel density [(t(20)= 4.35, p< 0.0005), Table 2]. There were no changes in the NAcc core following 8-hr withdrawal (Table 2). 3α,5α-THP immunoreactivity was not altered following CIE exposure in the NAcc shell at either time point (Table 2).
Figure 3.
Effects of CIE exposure on 3α,5α-THP immunoreactivity in the A) NAcc core and B) DLS following 8 hr or 72 hr withdrawal in air-exposed (clear bars) or ethanol-exposed mice (grey bars). Data depicted are mean positive pixels/mm2 ± SEM. Representative photomicrographs (10×) of 3α,5α-THP of immunoreactivity following air (n=11-12) or ethanol (n=11-13) exposure. Red box indicates coordinates relative to bregma depicted in photomicrographs. *p<0.05; **p<0.01 compared to respective air-exposed control.
Ethanol-exposed mice showed a decrease in 3α,5α-THP immunoreactivity in the DLS (Figure 3B) as supported by a main effect of CIE Exposure [F(1, 42)=4.62, p<0.05], but no main effect of Withdrawal Time Point or CIE Exposure by Withdrawal Time Point interaction. Within experiment analyses of raw pixel density indicate a 18.5 ± 6.0% reduction in 3α,5α-THP immunoreactivity in ethanol-exposed mice following 72 hr withdrawal [(t(20)= 3.12, p< 0.01)], Table 2]. There were no significant changes in 3α,5α-THP immunoreactivity after 8 hr withdrawal (Table 2). The overall reduction in 3α,5α-THP immunoreactivity appears to be driven by the 72 hr withdrawal time point. Additionally, there were no changes in 3α,5α-THP immunoreactivity in the DMS following 8-hr or 72-hr withdrawal (Table 2).
Alterations in 3α,5α-THP levels in amygdalar structures following 8 hr and 72 hr withdrawal following CIE exposure
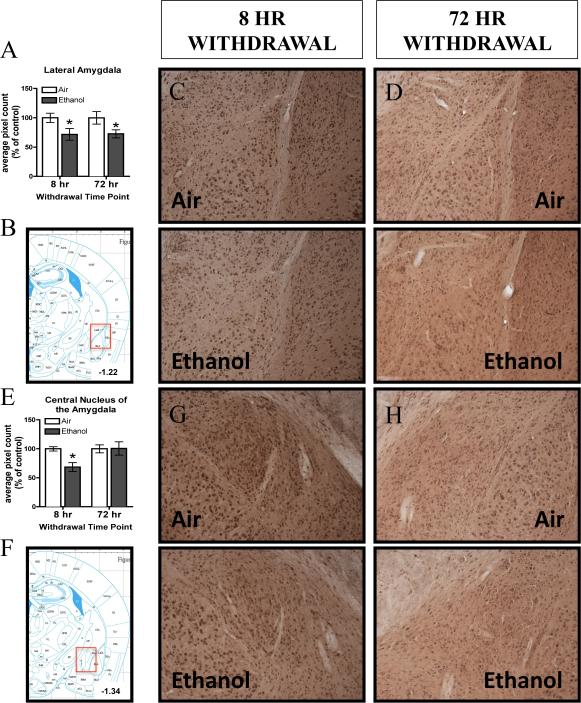
Ethanol exposure and withdrawal decreased 3α,5α-THP immunoreactivity in the LA following both 8 hr and 72 hr withdrawal (Figure 4A, C, D). There was a main effect of CIE Exposure [F(1,40)= 9.19, p< 0.005], but no main effect of Withdrawal Time Point or CIE Exposure x Withdrawal Time Point interaction. Furthermore, within experiment analyses of raw pixel density confirm that persistent reductions in 3α,5α-THP immunoreactivity were observed, with a 27.5 ± 12.4% reduction following 8 hr withdrawal (t(21)=2.20, p< 0.05) and a 28.3 ± 12.8% reduction following 72 hr withdrawal [(t(19)= 2.09, p< 0.05; Table 2).
Figure 4.
Effects of chronic intermittent ethanol (CIE) exposure on 3α,5α-THP immunoreactivity in the A) LA and E) CeA 8 hr or 72 hr after withdrawal in air-exposed (clear bars) or ethanol-exposed mice (grey bars). Data depicted are mean positive pixels/mm2 ± SEM. B and F) Red box indicates coordinates relative to bregma depicted in photomicrographs. Representative photomicrographs (10×) 3α,5α-THP of immunoreactivity in the LA (C, D) CeA (G,H) following 8 hr or 72 hr withdrawal in air- (n=11-12) and ethanol-exposed (n=11-13) mice. *p<0.05 compared to respective air-exposed control.
There was a transient 31.4 ± 9.3% decrease in 3α,5α-THP immunoreactivity in the CeA 8 hr after withdrawal (Figure 4E, G), as supported by a significant CIE Exposure x Withdrawal Time Point interaction [F(1,40)= 4.07, p= 0.05; Fisher PLSD, p< 0.05], but no main effect of CIE Exposure or Withdrawal Time Point. There were no differences in 3α,5α-THP immunoreactivity in the CeA following 72-hr withdrawal (Figure 4E, H). Within experiment analyses of raw pixel density confirmed the reduction in 3α,5α-THP immunoreactivity in the ethanol-exposed compared to air-exposed mice in the 8 hr withdrawal experiment [(t(22)= 2.27, p< 0.05; Table 2]. There were no changes in 3α,5α-THP immunoreactivity in the BLA at either time point [Table 2].
CIE exposure does not alter 3α,5α-THP immunoreactivity in hypothalamic and BNST structures
Other regions involved in stress reactivity and changes in ethanol consumption were assessed for changes in 3α,5α-THP immunoreactivity. No changes were observed in the PVN or the dorsal BNST 8-hr or 72-hr after withdrawal (Table 2). The ventral BNST showed a trend for decreased 3α,5α-THP immunoreactivity, but this effect did not reach statistical significance (main effect of CIE Exposure p= 0.07; Table 2).
Discussion
In general, the present set of experiments demonstrate decreased 3α,5α-THP immunoreactivity in several cortical and limbic structures, except in the CA3 pyramidal cell layer of the hippocampus where we observed an increase in 3α,5α-THP immunoreactivity. Increases in neurosteroidogenesis have been shown to alter functionality of neuronal circuitry following acute ethanol exposure (Follesa et al., 2006; Sanna et al., 2004; Tokuda et al., 2011). Acute ethanol administration increased plasma, cortical and hippocampal levels of 3α,5α-THP in Sprague Dawley rats (Morrow et al., 1999; Serra et al., 2003; VanDoren et al., 2000). However, chronic exposure to ethanol blunted the ethanol-induced increase in 3α,5α-THP and other neuroactive steroids in ethanol-withdrawn rats (Khisti et al., 2005; Morrow et al., 2001a). CIE exposure can be considered a chronic stressor, thereby, exposing animals to chronic stress and inducing lasting changes in 3α,5α-THP across the brain. In Swiss-Webster mice, chronic stress (social isolation) decreased 3α,5α-THP levels in frontal cortex, amygdala and hippocampus (Pibiri et al., 2008). CIE exposure produced similar lasting changes manifested as decreased 3α,5α-THP immunoreactivity in subregions of prefrontal cortex, amygdala, VTA, NAcc and dorsal striatum. These changes would be expected to blunt GABAergic tone by decreasing the amount of 3α,5α-THP present in these brain regions, and produce a state of hyperexcitability.
In the present set of experiments decreased 3α,5α-THP was observed in mPFC following 72 hr withdrawal from four cycles of CIE exposure. This is contrary to previous findings in our laboratory, where we observed elevated 3α,5α-THP levels in whole cerebral cortex using RIA methods following 72 hr withdrawal from CIE exposure in C57BL/6J mice (Morrow et al., 2009). In the previous work, the animals were given the opportunity to consume ethanol both prior to CIE exposure and during the days between ethanol exposure cycles (Morrow et al., 2009). Ethanol consumption using a similar limited access paradigm (2 hr/day), not involving CIE exposure, also resulted in elevated cerebral 3α,5α-THP levels in C57BL/6 mice (Finn et al., 2004). In the present work, animals were never exposed to a baseline-drinking paradigm and subsequent drinking between CIE exposure cycles, which would likely have increased 3α,5α-THP levels in the brain (Finn et al., 2004). The difference in 3α,5α-THP-induced changes in the brain following CIE with and without ethanol drinking between cycles are currently under investigation. Alternatively, the mPFC is a small subregion of the entire cerebral cortex, and therefore the mPFC may respond differently than other cortical subregions. Overall, the decrease in 3α,5α-THP immunoreactivity observed following 72 hr withdrawal from CIE exposure may indicate reduced GABAergic neuronal inhibition in the mPFC, which would be expected to increase output firing of projection neurons from this region. Furthermore, the decrease in 3α,5α-THP immunoreactivity following CIE exposure and 72 hr withdrawal may indicate alterations in biosynthesis/metabolism of precursors to produce corticosterone in the brain, instead of 3α,5α-THP (Porcu et al., 2014).
The CA1 pyramidal cell layer of the hippocampus is sensitive to modulation of 3α,5α-THP in rodents (Belelli and Herd, 2003; Gililland-Kaufman et al., 2008). In vitro, ethanol increased 3α,5α-THP levels in Sprague Dawley rat hippocampal pyramidal cells (Sanna et al., 2004; Tokuda et al., 2011). In vivo, acute ethanol increased 3α,5α-THP immunoreactivity in the CA1 pyramidal cell layer in Wistar rats (Cook et al., 2014). During ethanol withdrawal, rats are sensitized to the anticonvulsant effects of 3α,5α-THP (Cagetti et al., 2004; Devaud et al., 1996), but Withdrawal-Seizure-Prone (WSP) mice exhibit tolerance to intra-hippocampal delivery of 3α,5α-THP in the CA1 pyramidal cell layer (Gililland-Kaufman et al., 2008). The CA1 pyramidal cell layer of the hippocampus has been shown to be insensitive to neuronal activation following ethanol vapor exposure and withdrawal in C57BL/6J mice, where no changes in c-fos immunoreactivity were observed (Chen et al., 2009). Following four cycles of CIE exposure and withdrawal, we did not observe changes in 3α,5α-THP immunoreactivity in the CA1 pyramidal cell layer following 8 hr or 72 hr withdrawal. We also did not observe alterations in the polymorph cell layer of the dentate gyrus. However, we did observe increased 3α,5α-THP immunoreactivity in the CA3 pyramidal cell layer of the hippocampus following 72 hr withdrawal in CIE-exposed mice. Greater neuronal activation was observed in the CA3 region of the hippocampus in ethanol-withdrawn C57BL/6J mice compared to air-exposed mice, as indexed by c-fos immunoreactivity (Chen et al., 2009). At physiologically relevant concentrations, 3α,5α-THP increases presynaptic glutamate release in the CA3 pyramidal cell layer in the hippocampus (Iwata et al., 2013; Park et al., 2011). This increase in 3α,5α-THP immunoreactivity may be related to altered pyramidal neuronal activation (Tokuda et al., 2011), which was manifested following 72 hr withdrawal. Since we observed an increase in 3α,5α-THP immunoreactivity 72 hr after withdrawal, this would be consistent with changes in function of pyramidal neurons in CA3 following CIE exposure and, specifically, contribute to a hyperexcitable state.
The amygdala is a brain region that exhibits changes in functionality and emotional responsivity following chronic ethanol exposure and withdrawal. Changes in 3α,5α-THP levels in the amygdala could be due to changes in activity of principal neurons through a disinhibition of GABAergic activity (Andreen et al., 2009). Decreased firing rate was observed in the LA following CIE exposure in Sprague-Dawley rats (Feng and Faingold, 2008), which would be functionally consistent with a decrease of 3α,5α-THP in the LA. The LA serves as a sensory information region that receives input from many brain regions, including the cortex and hippocampus, and projects to other amygdalar subregions and reciprocally back to input regions (Faber et al., 2001; Pitkanen et al., 1997). The role of the LA is to process this excitatory input information into output structures (Faber et al., 2001). Reduced GABAergic inhibition mediates hyperexcitability within the LA (Danober and Pape, 1998), which could be accomplished by prolonged chronic ethanol exposure. Compromised sensory processing in the LA could be produced by CIE-induced increases in excitatory transmission via decreased 3α,5α-THP levels, and thus, less GABAergic inhibition on excitatory pyramidal neurons within this important region.
Pyramidal neurons appear to be the primary cell type in the LA (Faber et al., 2001) and GABAergic interneurons form a smaller proportion of neurons within the LA, which receive afferents from thalamic and cortical regions (Szinyei et al., 2000). These GABAergic interneurons regulate excitation of pyramidal neurons within the LA, thus gating their excitation to efferent connections. Reductions in 3α,5α-THP in the LA would, in turn, decrease the inhibitory tone of these GABAergic interneurons onto the pyramidal neurons within the LA and, thus, increase excitation of these neurons to other subregions of the amygdala, including the CeA and associated output structures including the mPFC and hippocampus. To our knowledge, these are the first data that implicate the LA as a key structure that exhibits significant changes in 3α,5α-THP induced by CIE exposure and withdrawal. Using IHC, we were able to isolate the LA and observe changes in this substructure within the amygdala.
The CeA serves as a relay point for amygdalar input from the LA and serves as the major behavioral output subregion of the amygdala, while the BLA sends inhibitory and excitatory projections to the LA (Pitkanen et al., 1997). Similar to recent work (Cook et al., 2014), we observed a transient decrease in 3α,5α-THP immunoreactivity in the CeA 8 hr after withdrawal from CIE exposure and no change in the BLA. Lesioning the CeA did not change CIE-induced increased ethanol consumption following three cycles of CIE-exposure in C57BL/6J mice (Dhaher et al., 2008). This is consistent with the lack of change in 3α,5α-THP levels in the CeA following 72 hr withdrawal, a time point when changes in ethanol consumption are observed. Together, these data suggest that 3α,5α-THP modulation of CeA activity may only be transiently involved during ethanol withdrawal.
GABAergic neurons within the VTA have interconnectivity with cortical and limbic regions (Diana et al., 2003), and the VTA appears to be sensitive to endogenous modulation of 3α,5α-THP levels (Frye and Rhodes, 2006). Recently it was shown that microinjection of 3α,5α-THP directly into the VTA produced an anticonvulsant effect in air-exposed WSP mice (Tanchuck et al., 2013). Following CIE exposure, GABAergic modulation of dopamine VTA neurons was decreased during acute withdrawal (Diana et al., 2003). During ethanol withdrawal, a down-regulation in GABAA alpha 1 receptor subunit protein levels in the VTA was observed in C57BL/6J mice (Arora et al., 2013). These findings are consistent with the persistent decrease in 3α,5α-THP immunoreactivity we observed in ethanol-withdrawn mice following four cycles of CIE exposure. The persistent decrease observed in the VTA may indicate increased neuronal excitability within the mesocorticolimbic circuitry that promotes drug-seeking behavior following CIE exposure.
Animals from each experiment were exposed to similar ethanol concentrations across the first three ethanol-exposure cycles. However, during the final ethanol cycle mice from the 8 hr withdrawal experiment showed lower BECs compared to those in the 72 hr withdrawal experiment. This difference in ethanol exposure could contribute to the fewer number of regions that showed changes in 3α,5α-THP immunolabeling following 8 hr withdrawal compared to 72 hr withdrawal. Alternatively, animals may experience different physiological effects 8 hr post-withdrawal when they would be in a hyperexcitable state and experience greater seizure susceptibility (Becker et al., 1997) compared to 72 hr post-withdrawal. Given we did not assess changes in 3α,5α-THP immunolabeling immediately upon removal from the vapor chamber (i.e., 0-hr withdrawal), the changes observed following 8 hr withdrawal may be due to repeated ethanol exposure and withdrawal that occurs during the first 8 hr upon removal from the vapor inhalation chamber. It is also possible that the decreases in 3α,5α-THP immunolabeling observed following 8 hr withdrawal could be due to incomplete elimination/metabolism of ethanol and/or pyrazole, or due to alterations in stress reactivity at each withdrawal time point.
Maintaining a reduction in 3α,5α-THP levels may decrease GABAergic neurotransmission during ethanol withdrawal (Finn et al., 2006). Changes in neurosteroid levels are related to changes in sensitivity to ethanol's effects, which in turn alter GABAergic tone (Finn et al., 2010). These data suggest that specific neuroadaptations in GABAergic neuroactive steroid levels may be present in regions of brain that mediate anxiety, stress, and reinforcement relevant to ethanol dependence. It has been proposed that an up-regulation of α4β2δ containing GABAA receptors, in areas where there were few of these receptors, would reverse the chloride gradient in pyramidal neurons, and thus decrease tonic inhibitory tone in such regions (Smith et al., 2009). When the chloride gradient is reversed to an inward flow, 3α,5α-THP inhibits GABAergic tone (Smith et al., 2009). Exposure to stress was shown to reverse the chloride flux, which resulted in excitation of HPA neurons (Hewitt et al., 2009; Sarkar et al., 2011). Collectively, these results indicate regions of the brain in which decreases in 3α,5α-THP levels would likely produce a hyperexcitable state during ethanol withdrawal. These effects may be especially relevant in the LA and VTA, which showed persistent reductions in 3α,5α-THP immunoreactivity during ethanol withdrawal.
Acknowledgements
We would like to thank Drs. Darin Knapp and Joyce Besheer for helpful advice and valuable scientific discussions and Dr. Clyde Hodge for providing microscopy equipment for image capture and analysis.
Funding and Disclosures This research was supported by the NIAAA INIA U01-AA016672, U01-AA020935 (ALM), U01-AA014095, AA010761 (HCB), and U01-AA020929 (MFL), and the Department of Veterans Affairs Medical Research (HCB). AMD was supported by the UNC Curriculum in Toxicology NIEHS Training Grant T32 ES007126.
References
- Andreen L, Nyberg S, Turkmen S, van Wingen G, Fernandez G, Backstrom T. Sex steroid induced negative mood may be explained by the paradoxical effect mediated by GABAA modulators. Psychoneuroendocrinology. 2009;34(8):1121–32. doi: 10.1016/j.psyneuen.2009.02.003. [DOI] [PubMed] [Google Scholar]
- Arora DS, Nimitvilai S, Teppen TL, McElvain MA, Sakharkar AJ, You C, Pandey SC, Brodie MS. Hyposensitivity to gamma-aminobutyric acid in the ventral tegmental area during alcohol withdrawal: reversal by histone deacetylase inhibitors. Neuropsychopharmacology : official publication of the American College of Neuropsychopharmacology. 2013;38(9):1674–84. doi: 10.1038/npp.2013.65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Becker HC. Effects of alcohol dependence and withdrawal on stress responsiveness and alcohol consumption. Alcohol research : current reviews. 2012;34(4):448–58. doi: 10.35946/arcr.v34.4.09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Becker HC, Diaz-Granados JL, Weathersby RT. Repeated ethanol withdrawal experience increases the severity and duration of subsequent withdrawal seizures in mice. Alcohol. 1997;14(4):319–26. doi: 10.1016/s0741-8329(97)87949-9. [DOI] [PubMed] [Google Scholar]
- Becker HC, Lopez MF. Increased ethanol drinking after repeated chronic ethanol exposure and withdrawal experience in C57BL/6 mice. Alcohol Clin Exp Res. 2004;28(12):1829–38. doi: 10.1097/01.alc.0000149977.95306.3a. [DOI] [PubMed] [Google Scholar]
- Belelli D, Herd MB. The contraceptive agent Provera enhances GABA(A) receptor-mediated inhibitory neurotransmission in the rat hippocampus: evidence for endogenous neurosteroids? J Neurosci. 2003;23(31):10013–20. doi: 10.1523/JNEUROSCI.23-31-10013.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Besheer J, Lindsay TG, O'Buckley TK, Hodge CW, Morrow AL. Pregnenolone and ganaxolone reduce operant ethanol self-administration in alcohol-preferring P rats. Alcohol Clin Exp Res. 2010;34(12):2044–2052. doi: 10.1111/j.1530-0277.2010.01300.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cagetti E, Pinna G, Guidotti A, Baicy K, Olsen RW. Chronic intermittent ethanol (CIE) administration in rats decreases levels of neurosteroids in hippocampus, accompanied by altered behavioral responses to neurosteroids and memory function. Neuropharmacology. 2004;46(4):570–9. doi: 10.1016/j.neuropharm.2003.10.001. [DOI] [PubMed] [Google Scholar]
- Chen G, Reilly MT, Kozell LB, Hitzemann R, Buck KJ. Differential activation of limbic circuitry associated with chronic ethanol withdrawal in DBA/2J and C57BL/6J mice. Alcohol. 2009;43(6):411–20. doi: 10.1016/j.alcohol.2009.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cook JB, Dumitru AM, O'Buckley TK, Morrow AL. Ethanol Administration Produces Divergent Changes in GABAergic Neuroactive Steroid Immunohistochemistry in the Rat Brain. Alcohol Clin Exp Res. 2014;38(1):90–99. doi: 10.1111/acer.12223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Danober L, Pape HC. Mechanisms and functional significance of a slow inhibitory potential in neurons of the lateral amygdala. Eur J Neurosci. 1998;10(3):853–67. doi: 10.1046/j.1460-9568.1998.00092.x. [DOI] [PubMed] [Google Scholar]
- Devaud LL, Purdy RH, Finn DA, Morrow AL. Sensitization of γ-aminobutyric acidA receptors to neuroactive steroids in rats during ethanol withdrawal. J. Pharmacol. Exp. Ther. 1996;278:510–517. [PubMed] [Google Scholar]
- Dhaher R, Finn D, Snelling C, Hitzemann R. Lesions of the extended amygdala in C57BL/6J mice do not block the intermittent ethanol vapor-induced increase in ethanol consumption. Alcohol. Clin. Exp. Res. 2008;32(2):197–208. doi: 10.1111/j.1530-0277.2007.00566.x. [DOI] [PubMed] [Google Scholar]
- Diana M, Brodie M, Muntoni A, Puddu MC, Pillolla G, Steffensen S, Spiga S, Little HJ. Enduring effects of chronic ethanol in the CNS: basis for alcoholism. Alcohol Clin Exp Res. 2003;27(2):354–61. doi: 10.1097/01.ALC.0000057121.36127.19. [DOI] [PubMed] [Google Scholar]
- Faber ES, Callister RJ, Sah P. Morphological and electrophysiological properties of principal neurons in the rat lateral amygdala in vitro. Journal of Neurophysiology. 2001;85(2):714–23. doi: 10.1152/jn.2001.85.2.714. [DOI] [PubMed] [Google Scholar]
- Feng HJ, Faingold CL. The effects of chronic ethanol administration on amygdala neuronal firing and ethanol withdrawal seizures. Neuropharmacology. 2008;55(5):648–53. doi: 10.1016/j.neuropharm.2008.05.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finn DA, Beckley EH, Kaufman KR, Ford MM. Manipulation of GABAergic steroids: Sex differences in the effects on alcohol drinking- and withdrawal-related behaviors. Hormones and Behavior. 2010;57(1):12–22. doi: 10.1016/j.yhbeh.2009.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finn DA, Douglass AD, Beadles-Bohling AS, Tanchuck MA, Long SL, Crabbe JC. Selected line difference in sensitivity to a GABAergic neurosteroid during ethanol withdrawal. Genes, brain, and behavior. 2006;5(1):53–63. doi: 10.1111/j.1601-183X.2005.00137.x. [DOI] [PubMed] [Google Scholar]
- Finn DA, Sinnott RS, Ford MM, Long SL, Tanchuck MA, Phillips TJ. Sex differences in the effect of ethanol injection and consumption on brain allopregnanolone levels in C57BL/6 mice. Neuroscience. 2004;123(4):813–9. doi: 10.1016/j.neuroscience.2003.11.017. [DOI] [PubMed] [Google Scholar]
- Follesa P, Biggio F, Talani G, Murru L, Serra M, Sanna E, Biggio G. Neurosteroids, GABAA receptors, and ethanol dependence. Psychopharmacology. 2006;186:267–280. doi: 10.1007/s00213-005-0126-0. [DOI] [PubMed] [Google Scholar]
- Ford MM, Mark GP, Nickel JD, Phillips TJ, Finn DA. Allopregnanolone influences the consummatory processes that govern ethanol drinking in C57BL/6J mice. Behav Brain Res. 2007;179:265–272. doi: 10.1016/j.bbr.2007.02.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ford MM, Nickel JD, Phillips TJ, Finn DA. Neurosteroid modulators of GABAA receptors differentially modulate ethanol intake patterns in male C57BL/6J mice. Alcohol Clin Exp Res. 2005;29(9):1630–1640. doi: 10.1097/01.alc.0000179413.82308.6b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frye CA, Rhodes ME. Infusions of 5alpha-pregnan-3alpha-ol-20-one (3alpha,5alpha-THP) to the ventral tegmental area, but not the substantia nigra, enhance exploratory, anti-anxiety, social and sexual behaviours and concomitantly increase 3alpha,5alpha-THP concentrations in the hippocampus, diencephalon and cortex of ovariectomised oestrogen-primed rats. Journal of Neuroendocrinology. 2006;18(12):960–75. doi: 10.1111/j.1365-2826.2006.01494.x. [DOI] [PubMed] [Google Scholar]
- Gililland-Kaufman KR, Tanchuck MA, Ford MM, Crabbe JC, Beadles-Bohling AS, Snelling C, Mark GP, Finn DA. The neurosteroid environment in the hippocampus exerts bidirectional effects on seizure susceptibility in mice. Brain Res. 2008;1243:113–23. doi: 10.1016/j.brainres.2008.09.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffin WC., 3rd Alcohol dependence and free-choice drinking in mice. Alcohol. 2014;48(3):287–293. doi: 10.1016/j.alcohol.2013.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffin WC, 3rd, Lopez MF, Yanke AB, Middaugh LD, Becker HC. Repeated cycles of chronic intermittent ethanol exposure in mice increases voluntary ethanol drinking and ethanol concentrations in the nucleus accumbens. Psychopharmacology. 2009;201(4):569–80. doi: 10.1007/s00213-008-1324-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hewitt SA, Wamsteeker JI, Kurz EU, Bains JS. Altered chloride homeostasis removes synaptic inhibitory constraint of the stress axis. Nature Neuroscience. 2009;12(4):438–43. doi: 10.1038/nn.2274. [DOI] [PubMed] [Google Scholar]
- Hosie AM, Wilkins ME, da Silva HM, Smart TG. Endogenous neurosteroids regulate GABAA receptors through two discrete transmembrane sites. Nature. 2006;444:486–489. doi: 10.1038/nature05324. [DOI] [PubMed] [Google Scholar]
- Iwata S, Wakita M, Shin MC, Fukuda A, Akaike N. Modulation of allopregnanolone on excitatory transmitters release from single glutamatergic terminal. Brain Research Bulletin. 2013;93:39–46. doi: 10.1016/j.brainresbull.2012.11.002. [DOI] [PubMed] [Google Scholar]
- Janak PH, Redfern JEM, Samson HH. The reinforcing effects of ethanol are altered by the endogenous neurosteroid, allopregnanolone. Alcohol Clin Exp Res. 1998;22:1106–1112. [PubMed] [Google Scholar]
- Janis GC, Devaud LL, Mitsuyama H, Morrow AL. Effects of chronic ethanol consumption and withdrawal on the neuroactive steroid 3α-hydroxy-5α-pregnan-20-one in male and female rats. Alcohol Clin Exp Res. 1998;22:2055–2061. [PubMed] [Google Scholar]
- Khisti RT, Boyd KN, Kumar S, Morrow AL. Systemic ethanol administration elevates deoxycorticosterone levels and chronic ethanol exposure attenuates this response. Brain Res. 2005;1049(1):104–11. doi: 10.1016/j.brainres.2005.05.007. [DOI] [PubMed] [Google Scholar]
- Kumar S, Porcu P, Werner DF, Matthews DB, Diaz-Granados JL, Helfand RS, Morrow AL. The role of GABAA receptors in the acute and chronic effects of ethanol: a decade of progress. Psychopharmacology (Berl) 2009;205:529–564. doi: 10.1007/s00213-009-1562-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopez MF, Griffin WC, 3rd, Melendez RI, Becker HC. Repeated cycles of chronic intermittent ethanol exposure leads to the development of tolerance to aversive effects of ethanol in C57BL/6J mice. Alcoholism, Clinical and Experimental Research. 2012;36(7):1180–7. doi: 10.1111/j.1530-0277.2011.01717.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melendez RI, McGinty JF, Kalivas PW, Becker HC. Brain region-specific gene expression changes after chronic intermittent ethanol exposure and early withdrawal in C57BL/6J mice. Addiction Biology. 2012;17(2):351–64. doi: 10.1111/j.1369-1600.2011.00357.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morrow AL, Biggio G, Serra M, Becker HC, Lopez MF, Porcu P, Alward SE, O'Buckley TK. The role of neuroactive steroids in ethanol/stress interactions: proceedings of symposium VII at the Volterra conference on alcohol and stress, May 2008. Alcohol. 2009;43:521–530. doi: 10.1016/j.alcohol.2009.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morrow AL, Janis GC, VanDoren MJ, Matthews DB, Samson HH, Janak PH, Grant KA. Neurosteroids mediate pharmacological effects of ethanol: A new mechanism of ethanol action? Alcohol Clin Exp Res. 1999;23(12):1933–1940. doi: 10.1111/j.1530-0277.1999.tb04094.x. [DOI] [PubMed] [Google Scholar]
- Morrow AL, Suzdak PD, Paul SM. Steroid hormone metabolites potentiate GABA receptor-mediated chloride ion flux with nanomolar potency. Eur J Pharmacol. 1987;142:483–485. doi: 10.1016/0014-2999(87)90094-x. [DOI] [PubMed] [Google Scholar]
- Morrow AL, VanDoren MJ, Fleming R, Penland S. In: Ethanol and neurosteroid interactions in the brain, in Int. Rev. Neurobiol. Biggio G, Purdy RH, editors. Vol. 46. Academic Press; San Diego: 2001a. pp. 349–377. [DOI] [PubMed] [Google Scholar]
- Morrow AL, VanDoren MJ, Penland SN, Matthews DB. The role of GABAergic neuroactive steroids in ethanol action, tolerance and dependence. Brain Res Brain Res Rev. 2001b;37:98–109. doi: 10.1016/s0165-0173(01)00127-8. [DOI] [PubMed] [Google Scholar]
- Olsen RW, Spigelman I. GABAA Receptor Plasticity in Alcohol Withdrawal. In: Noebels JL, Avoli M, Rogawski MA, Olsen RW, Delgado-Escueta AV, editors. Jasper's Basic Mechanisms of the Epilepsies. 4th ed. Bethesda (MD): 2012. [Google Scholar]
- Park HM, Choi IS, Nakamura M, Cho JH, Lee MG, Jang IS. Multiple effects of allopregnanolone on GABAergic responses in single hippocampal CA3 pyramidal neurons. European Journal of Pharmacology. 2011;652(1-3):46–54. doi: 10.1016/j.ejphar.2010.10.097. [DOI] [PubMed] [Google Scholar]
- Pibiri F, Nelson M, Guidotti A, Costa E, Pinna G. Decreased corticolimbic allopregnanolone expression during social isolation enhances contextual fear: A model relevant for posttraumatic stress disorder. Proceedings of the National Academy of Sciences of the United States of America. 2008;105(14):5567–72. doi: 10.1073/pnas.0801853105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pitkanen A, Savander V, LeDoux JE. Organization of intra-amygdaloid circuitries in the rat: an emerging framework for understanding functions of the amygdala. Trends in Neurosciences. 1997;20(11):517–23. doi: 10.1016/s0166-2236(97)01125-9. [DOI] [PubMed] [Google Scholar]
- Porcu P, Locci A, Santoru F, Beretti R, Morrow AL, Concas A. Failure of acute ethanol administration to alter cerebrocortical and hippocampal allopregnanolone levels in C57BL/6J and DBA/2J mice. Alcohol Clin Exp Res. 2014 doi: 10.1111/acer.12329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porcu P, O'Buckley TK, Alward SE, Song SC, Grant KA, de Wit H, Morrow AL. Differential effects of ethanol on serum GABAergic 3α,5α/3α,5β neuroactive steroids in mice, rats, cynomolgus monkeys and humans. Alcohol Clin Exp Res. 2010;34(3):432–442. doi: 10.1111/j.1530-0277.2009.01123.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romeo E, Brancati A, De Lorenzo A, Fucci P, Furnari C, Pompili E, Sasso GF, Spalletta G, Troisi A, Pasini A. Marked decrease of plasma neuroactive steroids during alcohol withdrawal. Clinical Neuropharmacology. 1996;19(4):366–9. doi: 10.1097/00002826-199619040-00011. [DOI] [PubMed] [Google Scholar]
- Sanna E, Talani G, Busonero F, Pisu MG, Purdy RH, Serra M, Biggio G. Brain steroidogenesis mediates ethanol modulation of GABAA receptor activity in rat hippocampus. J Neurosci. 2004;24(29):6521–30. doi: 10.1523/JNEUROSCI.0075-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarkar J, Wakefield S, MacKenzie G, Moss SJ, Maguire J. Neurosteroidogenesis is required for the physiological response to stress: role of neurosteroid-sensitive GABAA receptors. J Neurosci. 2011;31(50):18198–210. doi: 10.1523/JNEUROSCI.2560-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serra M, Pisu MG, Floris I, Cara V, Purdy RH, Biggio G. Social isolation-induced increase in the sensitivity of rats to the steroidogenic effect of ethanol. J Neurochem. 2003;85(1):257–63. doi: 10.1046/j.1471-4159.2003.01680.x. [DOI] [PubMed] [Google Scholar]
- Sinnott RS, Phillips TJ, Finn DA. Alteration of voluntary ethanol and saccharin consumption by the neurosteroid allopregnanolone in mice. Psychopharmacology. 2002;162(4):438–47. doi: 10.1007/s00213-002-1123-1. [DOI] [PubMed] [Google Scholar]
- Smith SS, Aoki C, Shen H. Puberty, steroids and GABA(A) receptor plasticity. Psychoneuroendocrinology 34 Suppl. 2009;1:S91–S103. doi: 10.1016/j.psyneuen.2009.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szinyei C, Heinbockel T, Montagne J, Pape HC. Putative cortical and thalamic inputs elicit convergent excitation in a population of GABAergic interneurons of the lateral amygdala. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2000;20(23):8909–15. doi: 10.1523/JNEUROSCI.20-23-08909.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanchuck MA, Cozzoli DK, He I, Kaufman KR, Snelling C, Crabbe JC, Mark GP, Finn DA. Local changes in neurosteroid levels in the substantia nigra reticulata and the ventral tegmental area alter chronic ethanol withdrawal severity in male withdrawal seizure-prone mice. Alcohol Clin Exp Res. 2013;37(5):784–93. doi: 10.1111/acer.12027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tokuda K, Izumi Y, Zorumski CF. Ethanol enhances neurosteroidogenesis in hippocampal pyramidal neurons by paradoxical NMDA receptor activation. J Neurosci. 2011;31(27):9905–9. doi: 10.1523/JNEUROSCI.1660-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- VanDoren MJ, Matthews DB, Janis GC, Grobin AC, Devaud LL, Morrow AL. Neuroactive steroid 3α-hydroxy-5α-pregnan-20-one modulates electrophysiological and behavioral actions of ethanol. J Neurosci. 2000;20(5):1982–1989. doi: 10.1523/JNEUROSCI.20-05-01982.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]