Abstract
Hepatitis C virus (HCV)-induced end-stage liver disease is currently a major indication for liver transplantation. After transplantation the donor liver inevitably becomes infected with the circulating virus. Monoclonal antibodies (mAbs) against the HCV co-receptor scavenger receptor class B type I (SR-BI) inhibit HCV infection of different genotypes, both in cell culture and in humanized mice. Anti-SR-BI mAb therapy is successful even when initiated several days after HCV exposure, supporting its potential applicability to prevent HCV re-infection of liver allografts. However, HCV variants with reduced SR-BI dependency have been described in the literature, which could potentially limit the use of SR-BI targeting therapy.
In this study we show, both in a preventative and post-exposure setup, that humanized mice infected with HCV variants exhibiting increased in vitro resistance to SR-BI-targeting molecules remain responsive to anti-SR-BI mAb therapy in vivo. A two-week antibody therapy readily cleared HCV RNA from the circulation of infected humanized mice. We found no evidence supporting increased SR-BI-receptor dependency of viral particles isolated from humanized mice compared to cell culture-produced virus. However we observed that, unlike wild type virus, the in vitro infectivity of the resistant variants was inhibited by both human HDL and VLDL. The combination of mAb1671 with these lipoproteins further increased the antiviral effect.
Conclusion
HCV variants that are less dependent on SR-BI in vitro can still be efficiently blocked by an anti-SR-BI mAb in humanized mice. Since these variants are also more susceptible to neutralization by anti-HCV envelope antibodies their chance of emerging during anti-SR-BI therapy is severely reduced. Our data indicates that anti-SR-BI receptor therapy could be an effective way to prevent HCV infection in a liver transplant setting.
Keywords: Viral hepatitis, liver transplantation, HDL, VLDL, entry
Introduction
Approximately 3% of the world’s population is chronically infected with the hepatitis C virus (HCV). Depending on the genotype of the infecting virus, 50 to 80% of chronically infected patients can clear the virus upon treatment with pegylated interferon combined with ribavirin (1). Addition of one of the protease inhibitors, telaprevir or boceprevir, significantly increases the response rate in genotype 1 patients (2). Besides the existence and possible emergence of antiviral resistant mutants, side effects and drug-drug interactions severely complicate the use of double and triple therapy in chronically infected patients in need for liver transplantation (3, 4). Therefore safer and more effective cocktails of direct acting antivirals (DAA) without interferon or alternative novel antiviral strategies are highly needed to treat this expanding patient population. Chronic HCV infection can lead to liver fibrosis, cirrhosis, and hepatocellular carcinoma (50–76% of all liver cancers), which represent the major indications for liver transplantation. However, after transplantation graft re-infection occurs almost immediately and disease progression can be accelerated in these immune suppressed patients (5). This highlights the need for adequate measures to prevent re-infection after liver transplantation and for better and safer therapies to treat re-infection in case prevention fails.
Currently available data indicates that HCV entry into hepatocytes is a complex multistep process requiring an interplay between various host and viral factors, thereby offering multiple targets for antiviral intervention (reviewed in (6)). The virus probably first interacts with cellular membrane proteins that concentrate the virus at the cell surface of the host cell. While this initial contact occurs in a rather non-specific manner, it is followed by more specific interactions between the virus and the host thereby triggering viral entry. Besides CD81, claudin-1 and occludin, Scavenger Receptor Class B type I (SR-BI) is one of these important HCV (co-)receptors (7–10).
SR-BI is involved in HCV cell entry based on both its physiological lipid transfer function and its ability to interact with the HCV glycoprotein E2 (8, 11). Molecules targeting this host factor may offer an innovating and promising strategy to prevent and/or treat HCV infections. Indeed, small-molecule inhibitors of SR-BI-mediated cholesteryl ester lipid uptake with anti-HCV activity in vitro have been described (12, 13). In addition, monoclonal antibodies (mAbs) against SR-BI are able to inhibit HCV infection of Huh7.5 cells in a dose-dependent manner (14). Moreover, prophylactic administration of anti-SR-BI mAb1671, protects chimeric mice from infection by HCV of different genotypes (15); and from a viral variant that became dominant after liver transplantation (16). In some of these mice HCV RNA levels remained undetectable even when therapy was initiated three days after viral challenge, indicating an inhibitory effect on intrahepatic viral transmission. Therefore, this antibody may represent a novel therapeutic tool to prevent HCV re-infection of liver allografts.
However, different HCV variants have been described that carry changes in their envelope glycoproteins, which render them more resistant to SR-BI-blocking anti-HCV therapy in cell culture (17–21). Here, we investigate how these variants respond to an anti-SR-BI mAb therapy in humanized uPA-SCID mice.
Material and methods
A detailed description of all materials and Methods can be found in an online supplement.
In vitro HCV neutralization assay
Genotype 2a HCVcc (Jc1wt, Jc1ΔHVR1, Jc1mtCD81, Jc1G451R and J6/JFH1 Clone2) were generated as previously described (18, 22, 23). The receptor-targeting neutralization assay and the cell-to-cell spread assay were performed as described in (15, 16, 24, 25). To investigate the effect of human HDL and human VLDL on HCVcc infectivity, cells were pre-incubated with approximately 230 μg HDL and 180 μg VLDL cholesterol/ml (BTI Biomedical Technologies, Stoughton, USA) either alone or in combination with 20 μg/ml mAb1671, JS81 (0.2 μg/ml) or ITX-5061 (2μM).
In vivo HCV neutralization experiments
Human liver-uPA-SCID mice (chimeric mice) were produced as previously described (26, 27). All mice were transplanted with primary human hepatocytes obtained from a single donor (donor HH223; BD Biosciences, Belgium). The effectiveness of mAb1671 was evaluated in a preventive and post-exposure setting (15, 16). Infections for all the Jc1 variants were done with an equivalent virus inoculum. HCV RNA in plasma was quantified using the COBAS Ampliprep/COBAS TaqMan HCV test (Roche Diagnostics, Belgium).
Statistics
Statistical significance of experimental results was assessed by the Kruskal-Wallis test (Nonparametric ANOVA) with Dunn’s Multiple Comparisons post-test using GraphPad InStat v3.06 (GraphPad Software Inc.).
Results
Comparison of in vitro cell free and cell-to-cell transmission of wild type and variant viruses
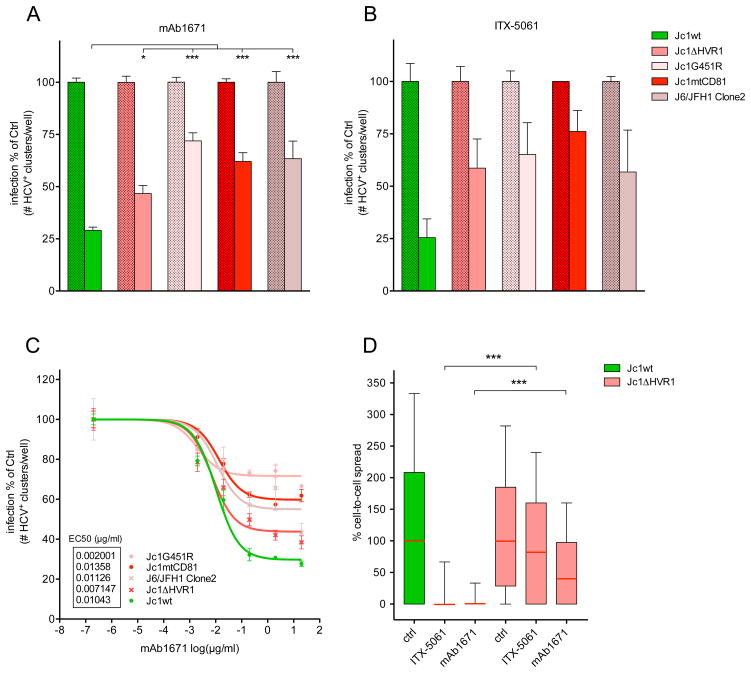
To confirm that the variants used in this study (Jc1ΔHVR1, Jc1G451R, Jc1mtCD81 and J6/JFH1 Clone2) are more resistant to anti-SR-BI therapy in vitro, their infectivity in the presence of SR-BI inhibitors was assessed and compared to that of the wild type virus. As shown in Figure 1A, a significantly less pronounced inhibition of the different variants was observed compared to the inhibition of the wild type virus by mAb1671. A similar inhibition pattern was observed when using the SR-BI-blocking small molecule ITX-5061, but given the limited sample size in this experiment a statistical analysis could not be performed (Figure 1B). A dose-response study showed that the EC50 values were comparable between all the viruses but the maximal inhibitory effect of the antibody was considerably lower against the variants compared to the wild type virus (Figure 1C). In addition, the effects of SR-BI inhibition (mAb1671 and ITX-5061) on cell-to-cell spread of Jc1ΔHVR1 and Jc1wt viruses were compared. Figure 1D shows that viral transmission of Jc1ΔHVR1 by way of cell-to-cell spread was significantly less responsive to SR-BI blockade than that of Jc1wt (P<0.001). This indicates that in addition to cell-free infectivity also the direct cell-to-cell spread of Jc1ΔHVR1 is less dependent on SR-BI.
Figure 1. In vitro neutralization assay.
Huh7.5 cells were pre-treated with 20 μg/ml mAb1671 (A) and 2 μM ITX-5061 (small molecule SR-BI antagonist) (B) before infection with Jc1wt, Jc1ΔHVR1, Jc1G451R, Jc1mtCD81 and J6/JFH1 Clone2. After two days the number of HCV-positive clusters was counted and normalized to control. The effect of mAb1671 on the infectivity of Jc1wt, ΔHVR1 and mtCD81 was evaluated in ten separate wells over four different experiments, while the effect on Jc1G451R and J6/JFH1 Clone2 was assessed over eight separate wells in three different experiments. The data of these experiments was merged and the means are shown. The asterisks (*: P<0.05; and ***: P<0.001) indicate that the effect of mAb1671 on Jc1ΔHVR1, Jc1G451R, Jc1mtCD81 and J6/JFH1 Clone2 differs significantly from its effect on Jc1wt infectivity. The effect of ITX-5061 was assessed in one experiment and the means of duplicates are shown (this limited sample size did not allow statistical analysis). (C) HCVcc infectivity under increasing concentrations of mAb1671. All conditions were tested in quadruplicate and the mean values are shown. (D) Box-and-whisker presentation of cell-to-cell spread. While mAb1671 (20 μg/ml) and ITX-5061 (2 μM) efficiently inhibit direct cell-to-cell transmission of Jc1wt, only a minor effect can be observed against Jc1ΔHVR1 (***: P<0.001). For each condition, the amount of infected target cells per cluster was determined in at least 100 clusters and normalized to the median of the control. The box extends from the 25th and 75th percentile, while the whiskers indicate the 10th and 90th percentile. The red horizontal line indicates the median. Error bars in panel A, B and C represent the standard error of the mean.
In vivo HCV neutralization experiments
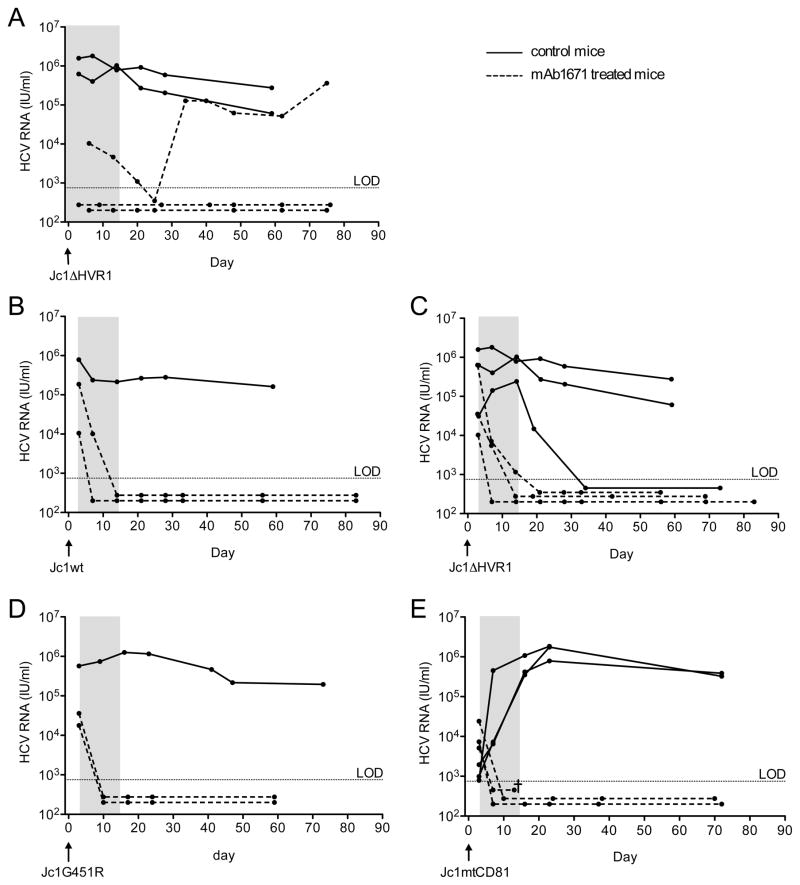
Next, the antiviral efficacy of the anti-SR-BI mAb1671 against infections with wild type and anti-SR-BI resistant variants was evaluated in humanized mice. Whereas viremia in non-treated mice rapidly increased to 106 IU/ml, prophylactic administration of the antibody was able to prevent infection with Jc1ΔHVR1 in two out of three mice (Figure 2A). Viremia was controlled in the third Jc1ΔHVR1-injected mouse but a rebound was observed after cessation of therapy. Figures 2B-E represent the effect on HCV viremia during and after post-exposure anti-SR-BI treatment of humanized mice injected with Jc1wt, Jc1ΔHVR1, Jc1mtCD81 and Jc1G45R. Already three days after injection of the virus, high levels of HCV RNA could be detected in the mouse plasma. In 7 out of 8 non-treated control mice HCV RNA remained detectable until at least 60 days after infection. One animal spontaneously cleared the virus five weeks after inoculation. In all ten treated mice the viremia declined steeply during antibody administration and remained below the limit of detection for at least two months after infection.
Figure 2. Efficacy of the SR-BI-specific antibody mAb1671 in blocking HCV dissemination in humanized mice.
Within a 2-week period (indicated by the gray area) the animals received 6 intraperitoneal injections, each containing 400 μg of the antibody. The antibody was tested in two different settings: (A) a prevention experiment where the first antibody dose was administered one day before viral challenge; and (B–D) a post-exposure setup where the anti-SR-BI therapy was initiated three days post-viral challenge. Antibody-treated mice are indicated with a dotted line, whereas non-treated control animals are represented by solid lines. Chimeric mice were challenged at day 0 with Jc1wt (B), Jc1ΔHVR1 (A and C), Jc1G451R (D) or Jc1mtCD81 (E). Each data point represents the plasma HCV RNA level (IU/ml) of an individual chimeric mouse at a given time point. The limit of detection (LOD) equals 750 IU/ml.
HCVcc mouse passaging and Iodixanol ultracentrifugation
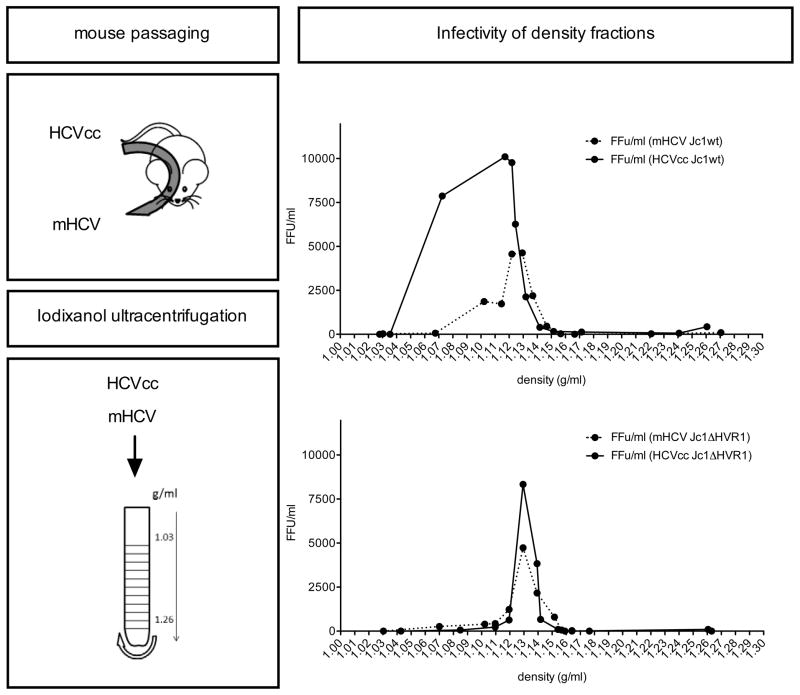
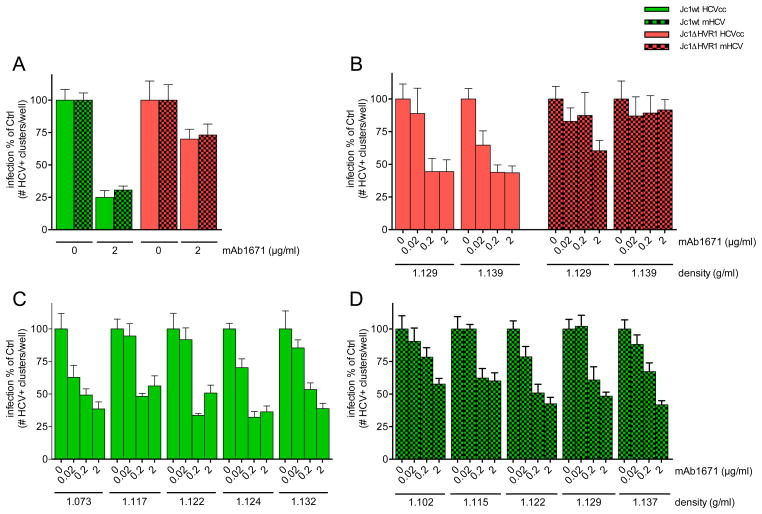
The in vivo antiviral efficacy of anti-SR-BI mAb therapy turned out to be considerably higher than what had been observed in cell culture. One major difference between both experimental settings may be the lipoprotein composition of the viral particles. For the in vitro experiments we have used HCVcc that were produced in hepatoma cells, which are known to have impaired VLDL biogenesis (28, 29). For the in vivo experiments the same HCVcc were used to initially infect the mice but rapidly new viral particles are produced, this time by fully functional primary hepatocytes. This is known to have an impact on the lipoprotein composition of these newly formed HCV and to change certain physico-chemical and biological characteristics (30, 31). It might well be that hence also their SR-BI receptor usage was modified. However, density fractionation analysis did not show an increased proportion of infectious viral particles at lower densities for mouse-derived (mHCV) Jc1wt or Jc1ΔHVR1 particles compared to the respective cell culture derived virions (Figure 3). Although based on our analyses we cannot exclude potential discrepancies in apolipoprotein composition (or other alterations) between mHCV and HCVcc virions, our in vitro infection prevention experiments did not reveal increased sensitivity to anti-SR-BI mAb therapy of mHCV compared to HCVcc (Figure 4A). Furthermore, prevention experiments using increasing concentrations of anti-SR-BI mAb did not show increased antiviral responses against specific mHCV subfractions (Figure 4B, C and D). This indicates that the sensitivity of HCVcc to mAb1671 treatment in cell culture did not change after mouse passaging of the virus and that the increased in vivo activity of mAb1671 is not directly caused by potential in vivo adaptations of the virus.
Figure 3. Buoyant density gradient analysis of HCV produced in cell culture and in humanized mice.
Serum was collected over a two month infection period from humanized mice inoculated with cell culture produced (HCVcc) Jc1wt and Jc1ΔHVR1. Pooled serum containing mouse-passaged HCV, designated mHCV, and culture supernatant containing HCVcc was ultracentrifuged over an iodixanol gradient. Twelve fractions were collected from the top of the gradient and analysed for cell culture infectivity (in triplicates) expressed as FFU/ml.
Figure 4. In vitro anti-SR-BI mAb1671 sensitivity determination.
(A) Huh7.5 cells were pre-treated with 2 μg/ml mAb1671 before infection with cell culture and mouse-passaged Jc1wt (green) and Jc1ΔHVR1 (red). Two days after infection, HCV-positive clusters were enumerated. (B–D) The different density fractions with in vitro detectable infectivity were incubated with mAb1671-pretreated Huh7.5 cells. Two days later the number of HCV-positive cell clusters was determined. All conditions were tested in quadruplicate and the means are shown. Error bars represent standard error of the mean.
Human lipoproteins influence HCVcc infectivity and improve mAb1671 treatment outcome
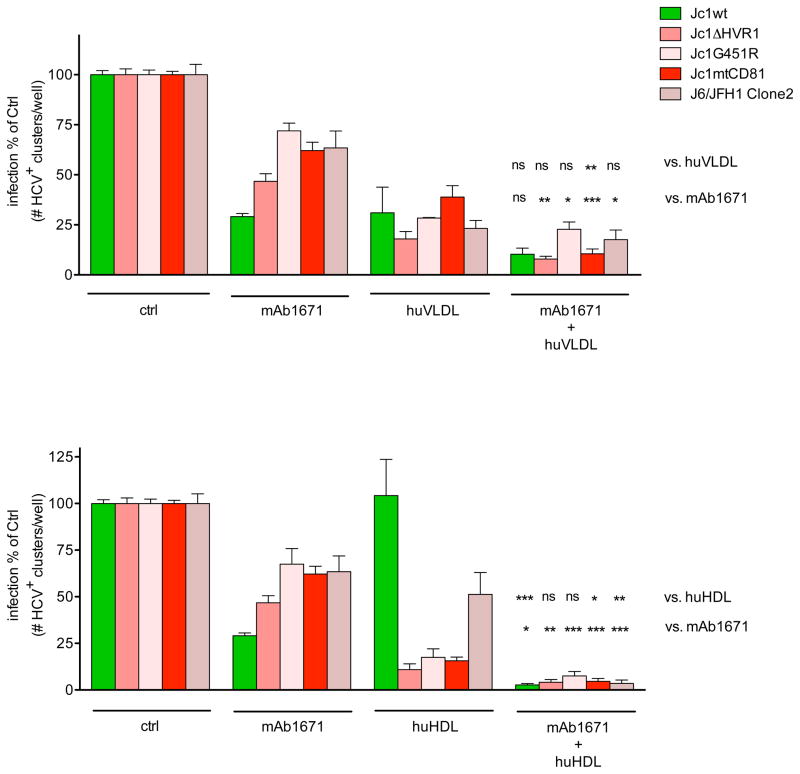
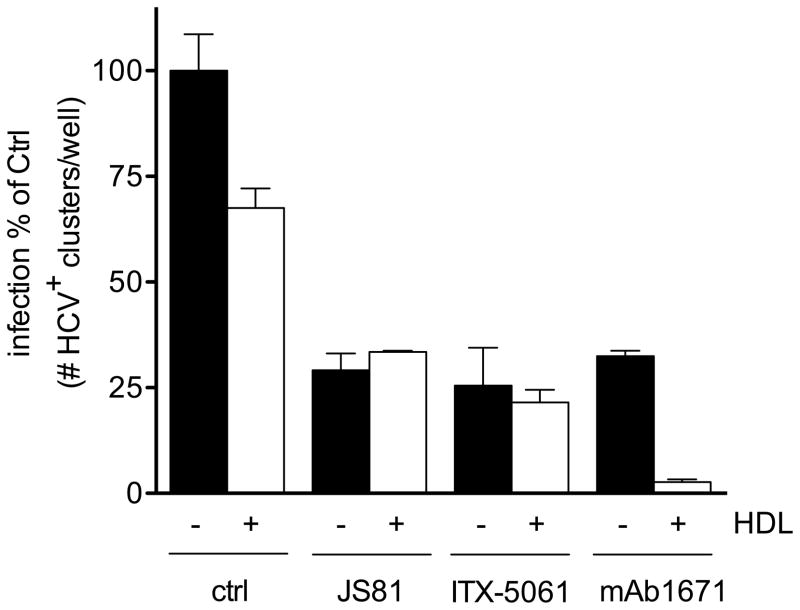
Human serum and especially its lipoprotein constituents are known to influence the infectivity of HCV and therefore also the efficacy of anti-HCV neutralizing antibodies. Cell culture media contains considerably less lipoproteins than serum in vivo (data not shown). Therefore, the effects of in vivo-like concentrations of human lipoproteins HDL and VLDL on HCV infectivity and their effect on the mAb1671 therapy efficacy were evaluated in cell culture. Huh7.5 cells were pre-treated with 230 μg human HDL cholesterol/ml or 180 μg human VLDL cholesterol/ml. These concentrations correspond with HDL and VLDL cholesterol levels detected in serum from humanized uPA-SCID mice. As shown in Figure 5, human VLDL inhibited infectivity of all viruses and, in combination with mAb1671, it showed an additive antiviral effect to the activity of mAb1671 alone. In contrast, HDL only inhibited the infectivity of the anti-SR-BI resistant variants, whereas wild type infectivity remained unchanged. Three different batches of human HDL were tested for their effect on Jc1wt infectivity; the first batch enhanced Jc1wt infectivity, the two other ones slightly inhibited infectivity of this virus. Merging of these data sets results in a seemingly unchanged infectivity of the wild type virus in the presence of human HDL. Nevertheless, the combination of HDL and mAb1671 nearly completely inhibited infection of all viruses tested. Although HDL alone did, on average, not change Jc1wt infectivity, it is able to significantly enhance the antiviral efficacy of the mAb1671 against this virus (P<0.05). While HDL had a clear synergistic effect on the activity of mAb1671 against the wild type virus, due to the pronounced activity by HDL as such it seemed to have a more additive effect on mAb1671’s activity against the resistant viruses. None of mAb1671-lipoprotein combinations decreased Huh7.5 cell growth or metabolic activity (data not shown). To test whether the enhanced antiviral activity against the wild type virus exerted by HDL is specific to mAb1671, we assessed the effect of HDL on the anti-CD81 mAb JS81 and the anti-SR-BI small molecule ITX-5061. Figure 6 shows that human HDL only improved the antiviral effect of mAb1671 and not that of JS81 or ITX-5061.
Figure 5. Effect of HDL and VLDL on in vitro HCVcc neutralization.
Huh7.5 cells were pre-treated with 230 μg human HDL cholesterol/ml or 180 μg human VLDL cholesterol/ml alone or in combination with 20 μg/ml mAb1671 (HDL and VLDL concentrations correspond with levels detected in serum from humanized uPA-SCID mice) before infection with Jc1wt, Jc1ΔHVR1, Jc1G451R, Jc1mtCD81 and J6/JFH1 Clone2. After two days, the number of HCV-positive clusters was enumerated. The data shown for Jc1wt, Jc1ΔHVR1 and Jc1mtCD81 originates from three (HDL and combination with mAb1671) and two (VLDL and combination with mAb1671) individual experiments, whereas the effect on Jc1G451R and J6/JFH1 Clone2 was assessed over two experiments (HDL and combination with mAb1671) and in one individual experiment (VLDL and combination with mAb1671). In each experiment all conditions were tested in duplicate and different batches of VLDL or HDL were used. Error bars represent standard error of the mean. The asterisks (*: P<0.05, **: P<0.01 and ***: P<0.001) indicate statistically significant differences, whereas “ns” stands for not significantly different.
Figure 6. In vitro effect of HDL in combination with different anti-receptor therapies.
Huh7.5 were pre-treated with 4 μM ITX-5061, 0.2 μg/ml JS81 and 20 μg/ml mAb1671 alone or in combination with 230 μg human HDL cholesterol/ml before infection with Jc1wt. After two days, the number of HCV-positive clusters was enumerated. All conditions were tested in duplicate and error bars represent standard error of the mean.
Discussion
HCV entry into the hepatocyte is a crucial event in the infection process, which can be targeted by anti-HCV antibodies (32–35). However, prevention of HCV recurrence after liver transplantation using antibodies that target HCV envelope proteins has not yet proven to be very successful (36, 37), probably due to the high heterogeneity of the viral envelope proteins. In addition, it was shown that HDL can reduce the neutralizing effect of anti-HCV antibodies (38, 39), raising additional concerns about the efficacy of anti-HCV antibodies for passive immunotherapy. Besides viral envelope proteins, different host factors are involved in the HCV life cycle. Mensa et al. recently reported that early reinfection kinetics of HCV after liver transplantation are modulated by HCV receptor levels, such as SR-BI, at the time of transplantation (40). This suggests that blockade of this receptor may delay or prevent HCV reinfection of the graft. Accordingly, we have shown in vivo efficacy of anti-SR-BI mAbs in chimeric mice and their potential applicability in the liver transplantation context (15, 16). Evidence for clinical safety of SR-BI inhibition has been established for the SR-BI small molecule inhibitor ITX-5061 (41, 42) and a monoclonal antibody similar to mAb1671 (43). Clinical efficacy of ITX-5061 in chronic HCV patients is lacking (44), but phase 1 efficacy studies in liver transplant patients are currently ongoing.
The emergence of HCV variants, resulting from the lack of proof-reading capacity of the viral polymerase, that are resistant to anti-envelope antibodies or DAA is a major cause of immunologic and therapeutic failure. The more conserved nature of host factors compared to viral proteins makes the former interesting therapeutic targets. However, HCV variants have been described that carry changes in their envelope glycoproteins which are less dependent on SR-BI for infection of hepatoma cells in vitro. Compared to wild type virus, cell culture infectivity of these variants is less efficiently blocked by anti-SR-BI therapy (17–20, 45). The existence of partially anti-SR-BI resistant HCV variants indicates that the efficacy of the SR-BI-targeting approaches might be compromised by reduced susceptibility of some (re-)infecting virus, raising questions about its applicability in vivo.
Since viral mutations that render the virus less dependent on a specific cell entry factor may also affect its in vivo fitness, we first determined whether humanized mice could be infected with HCV variants that are less dependent from SR-BI and examined next whether these variant-infected mice would still respond to anti-SR-BI mAb therapy. The mutants investigated in this study were: E2HVR1-deleted Jc1 (Jc1ΔHVR1) (18), E2 G451R substituted Jc1 (19), the mouse CD81-adapted Jc1 virus carrying amino acid changes in E1 (L216F) and E2 (V388G and M405T) (19), and the recently described J6/JFH1 Clone2 with mutations in E1 (I374L) and E2 (I411V) (20). Having confirmed the reduced sensitivity of these mutants to anti-SR-BI targeting agents (mAb1671 and ITX-5061) in vitro, we examined the effect of SR-BI-blockade in vivo. Administration of mAb1671 to mice not only inhibited infection with wild type Jc1, but also suppressed HCV RNA in mice infected with Jc1ΔHVR1, Jc1G451R and Jc1mtCD81 to undetectable levels. This suppression not only occurred in a prophylactic setting, as shown for Jc1ΔHVR1, but even when administration of the antibodies was initiated several days after the infection was established. Although a decrease in viremia was observed during mAb1671 administration in a mouse infected with J6/JFH1 Clone2, no clear-cut proof of its antiviral effect against this virus could be obtained due to unstable viremia in infected control animals (data not shown). This indicates that the J6/JFH1 Clone2 virus may have acquired in vitro adaptations that negatively affect its in vivo fitness.
Next, we addressed potential mechanisms that may contribute to the discrepancies in antiviral efficacy of mAb1671 observed in cell culture experiments (in vitro) versus studies performed in humanized mice (in vivo). Administration of mAb1671 to humanized mice completely prevented HCV infection with the wild type and mutant viruses whereas in vitro this was not the case. In addition, cell-to-cell spread of Jc1ΔHVR1 virus could not be completely inhibited in vitro, whereas in vivo even post-exposure therapy was highly effective against this virus.
We have previously observed that chimpanzee- and humanized mouse-derived J6/JFH1 viral particles have a higher specific infectivity than cell culture derived virus, correlating with a decreased average buoyant density (30). This suggests that differences in physical association of HCV with low-density factors in these HCV infection models influence viral infectivity. Hence, we hypothesized that humanized mouse-derived lower density viral particles, associated with lipoproteins, might be more dependent on the physiological HDL-binding and cholesterol transfer function of SR-BI and therefore more sensitive to anti-SR-BI therapy. However, the infectious virus particles used in this study did not show a shift to lower buoyant density fractions after passaging in the humanized mouse, in fact for Jc1wt rather the opposite was observed. Whether this relates to a difference between Jc1 and J6/JFH1 or to batch-to-batch differences is not clear. Additionally, no increased SR-BI-receptor usage could be observed in mouse-derived HCV as compared to culture-derived HCV. This is consistent with the observation that ex-vivo chimpanzee-derived HCVcc was equally sensitive to anti-SR-BI mAb than cell culture produced HCV (14).
Although HDL lowers the neutralization efficacy of anti-HCV antibodies by enhancing HCV entry (38, 39), it does not negatively affect the anti-HCV effect of anti-SR-BI mAb C167 in culture (14). Previously it was reported that SR-BI blocking agents are able to inhibit the enhancement of HCV infectivity mediated by HDL (46, 47). While in these studies low amounts of HDL were used (range 1.6 to 6 μg HDL cholesterol/ml), we describe here that the addition of in vivo-like concentrations of human HDL (230 μg cholesterol/ml) to mAb1671 is able to substantially enhance its in vitro efficacy against the wild type virus. While HDL and VLDL by themselves already have an inhibitory influence on the infectivity of the resistant viruses, addition of mAb1671 seemed to further increase this effect, although not always statistically significant. Both these observations may explain why in vivo higher protection rates can be achieved against wild type and resistant viruses compared to what was previously observed in vitro. While HDL seemed to have a synergistic effect on the antiviral efficacy of mAb1671 against wild type HCV, it did not alter the antiviral activity of JS81 or ITX-5061 indicating that this synergism might possibly be specific for mAb1671. Our study also confirms that human VLDL inhibits the infectivity of HCVcc and shows that mAb1671 further increases this inhibition.
In vivo-like concentrations of HDL clearly inhibited the anti-SR-BI resistant mutants and seemingly did not affect wild type infectivity. However, different HDL batches behaved differently in such a way that one batch had an enhancing effect on Jc1wt infectivity while the two others were inhibiting. The observation that some HDL batches decreased rather than enhanced Jc1wt infectivity is consistent with another study that used increasing concentrations of HDL and observed reduced wild type infectivity at the highest concentrations (14). Overall, even when HDL enhanced Jc1wt infectivity, addition of HDL to mAb1671 was able to almost completely suppress both variant and wild type infectivity.
HDL-mediated enhancement of infection depends both on the lipid transfer function of SR-BI and its ability to bind HCV E2 (11, 48). The fact that mAb1671 interferes with both functions (data not shown), should enable this therapeutic approach to at least prevent the enhancement of infection as was shown for other SR-BI inhibitors (12, 39, 46). Moreover, we observe here that inhibitory effects of HDL start to dominate when it fails to support HCV infectivity, as in the case for viruses with particular envelope mutations that alter the SR-BI receptor usage or in case of antibody-, but not ITX-5061-, mediated SR-BI blockade. These particular factors that preclude the virus from using the SR-BI-mediated HCV entry route, seem to convert HDL into an inhibitory particle. We hypothesize that HDL’s effect on HCV infectivity is double-edged. In case the SR-BI-mediated entry pathway is fully operational, the HDL-mediated infection enhancement either dominates or at least conceals the HDL inhibitory effects. However, in situations leading to a partial redundancy of the SR-BI-mediated entry pathway, HDL’s inhibitory effects prevail. Accordingly, while ApoCI, an exchangeable apolipoprotein that resides in HDL, was shown to be a key mediator of the HDL-mediated infection enhancement process, increasing its concentration resulted in decreased HCV infectivity by specifically disrupting the viral membrane (47). Although ITX-5061 also inhibits the SR-BI receptor, it is interesting to note that HDL did not enhance the therapeutic efficacy of ITX-5061 in vitro. Possibly, this phenomenon may be specific for the antibody used in this study. Other signs for the inhibitory activity of HDL can be found in the work of Bartosch et al. and Dreux et al. Their studies show that HCVpp containing the E2 point mutation L399R do not profit from HDL-mediated infection enhancement and that the presence of HDL (6μg/ml) even reduced infectivity (46, 47). In addition, Dao Thi and colleagues mention that “the absence of HDL-mediated infection enhancement uncovered an inhibitory activity of lipoproteins”, which they attribute to the possible presence of oxidized lipids (48).
The results from our study address possible concerns arising from the fact that antiviral pressure may select for therapy-resistant HCV variants, resulting in an increased likelihood of virologic failure during anti-SR-BI therapy. However, compared to the wild type virus, all the investigated variants are more vulnerable to inhibition by HDL and envelope-targeting neutralizing antibodies (18–20). Because of these characteristics, in addition to remaining sensitive in vivo to anti-SR-BI mAb therapy, it is unlikely that such variants would emerge and propagate during the course of an anti-SR-BI therapy. In fact, to maximize virologic response, a therapy that combines anti-SR-BI with anti-envelope agents might be worth considering.
We demonstrate for the first time that, except for J6/JFH1 Clone2, hepatitis C viruses with altered SR-BI usage in vitro are fit in humanized uPA-SCID mice but can be successfully blocked by the SR-BI-targeting antibody mAb1671. The differences between in vitro and in vivo mAb1671 therapy outcomes may be explained by the presence in vivo of human lipoproteins. We show that VLDL by itself inhibits both wild type and resistant virus infectivity, whereas HDL only has a direct negative impact on the infectivity of the resistant viruses but also potentiates mAb1671’s antiviral effect against the wild type virus. This study also highlights that the humanized mouse is a more appropriate HCV infection model than the Huh7.5 cell culture system, presumably by more physiologically relevant location and function of the receptors on polarized primary hepatocytes in the liver. Our findings implicate that novel (host-targeting) therapeutics should preferentially be evaluated in this model.
Supplementary Material
Acknowledgments
Financial Support
This project was funded by the Ghent University (Concerted Action Grant 01G01712), The Research Foundation – Flanders (Project G.0521.12N to P.M.), the Belgian state (IUAP P7/47-HEPRO-2), and the European Union (FP7, HepaMab). AAM is a recipient of a PhD Fellowship provided by the Egyptian Government. S.B. was supported by a Marie Curie International Reintegration Grant (PIRG- GA-2009-256300). C.M.R. and M.T.C. were supported by National Institutes of Health Grant (R01 AI072613 to C.M.R.), The Greenberg Medical Research Institute, The Starr Foundation and a Women & Science Fellowship from The Rockefeller University (M.T.C.). T.P. was supported by grants from the DFG (PI 734/2-1 and CRC 900 project A6) and the Helmholtz Association SO-024.
Abbreviations
- HCV
hepatitis C virus
- mAbs
monoclonal antibodies
- SR-BI/Cla1
scavenger receptor class B type I
- uPA
urokinase-type plasminogen activator
- SCID
severe combined immunodeficient
- LT
liver transplantation
- E1/E2
envelope glycoprotein 1 and 2
- HDL
high density lipoprotein
- VLDL
Very Low Density Lipoprotein
- HCVcc
cell culture derived HCV
- NLS
nuclear localization signal
- IPS
IFN-β promoter stimulator protein
- RFP
Red Fluorescent Protein
- EGFP
Enhance Green Fluorescent Protein
- mHCV
mouse passaged HCV
- EC50
half maximal effective concentration
- CD81
cluster of differentiation 81
- DAA
Directly acting antivirals
- anti-SR-BI mAb
anti-SR-BI mAb
- HVR1
Hyper Variable Region 1
- DMSO
Dimethyl Sulfoxide
- MID100
100% mouse infectious dose
- PFA
paraformaldehyde
Footnotes
Conflict of interest
Nothing to disclose.
Contributor Information
Koen Vercauteren, Email: Koen.Vercauteren@ugent.be.
Naomi Van Den Eede, Email: Naomi.VandenEede@UGent.be.
Ahmed Atef Mesalam, Email: Ahmed.Mesalam@UGent.be.
Sandrine Belouzard, Email: sandrine.belouzard@ibl.fr.
Maria Teresa Catanese, Email: maria.catanese@kcl.ac.uk.
Dorothea Bankwitz, Email: dorothea.bankwitz@twincore.de.
Flossie Wong-Staal, Email: fwongstaal@itxpharma.com.
Riccardo Cortese, Email: cortese@ceinge.unina.it.
Jean Dubuisson, Email: jean.dubuisson@ibl.fr.
Charles M. Rice, Email: ricec@mail.rockefeller.edu.
Thomas Pietschmann, Email: thomas.pietschmann@twincore.de.
Geert Leroux-Roels, Email: Geert.LerouxRoels@UGent.be.
Alfredo Nicosia, Email: nicosia@ceinge.unina.it.
Philip Meuleman, Email: Philip.Meuleman@UGent.be.
References
- 1.Zeuzem S. Interferon-based therapy for chronic hepatitis C: current and future perspectives. Nat Clin Pract Gastroenterol Hepatol. 2008;5:610–622. doi: 10.1038/ncpgasthep1274. [DOI] [PubMed] [Google Scholar]
- 2.Sarrazin C, Hezode C, Zeuzem S, Pawlotsky JM. Antiviral strategies in hepatitis C virus infection. J Hepatol. 2012;56 (Suppl 1):S88–100. doi: 10.1016/S0168-8278(12)60010-5. [DOI] [PubMed] [Google Scholar]
- 3.Peveling-Oberhag J, Zeuzem S, Hofmann WP. Antiviral therapy of chronic hepatitis C in patients with advanced liver disease and after liver transplantation. Med Microbiol Immunol. 2010;199:1–10. doi: 10.1007/s00430-009-0131-8. [DOI] [PubMed] [Google Scholar]
- 4.Charlton M. Telaprevir, boceprevir, cytochrome P450 and immunosuppressive agents - A potentially lethal cocktail. Hepatology. 2011;54:3–5. doi: 10.1002/hep.24470. [DOI] [PubMed] [Google Scholar]
- 5.Rubin A, Aguilera V, Berenguer M. Liver transplantation and hepatitis C. Clin Res Hepatol Gastroenterol. 2011;35:805–812. doi: 10.1016/j.clinre.2011.04.009. [DOI] [PubMed] [Google Scholar]
- 6.Vercauteren K, Leroux-Roels G, Meuleman P. Blocking HCV entry as potential antiviral therapy. Future Virology. 2012;7:547–561. [Google Scholar]
- 7.Pileri P, Uematsu Y, Campagnoli S, Galli G, Falugi F, Petracca R, Weiner AJ, et al. Binding of hepatitis C virus to CD81. Science. 1998;282:938–941. doi: 10.1126/science.282.5390.938. [DOI] [PubMed] [Google Scholar]
- 8.Scarselli E, Ansuini H, Cerino R, Roccasecca RM, Acali S, Filocamo G, Traboni C, et al. The human scavenger receptor class B type I is a novel candidate receptor for the hepatitis C virus. EMBO J. 2002;21:5017–5025. doi: 10.1093/emboj/cdf529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Evans MJ, von Hahn T, Tscherne DM, Syder AJ, Panis M, Wolk B, Hatziioannou T, et al. Claudin-1 is a hepatitis C virus co-receptor required for a late step in entry. Nature. 2007;446:801–805. doi: 10.1038/nature05654. [DOI] [PubMed] [Google Scholar]
- 10.Ploss A, Evans MJ, Gaysinskaya VA, Panis M, You HN, de Jong YP, Rice CM. Human occludin is a hepatitis C virus entry factor required for infection of mouse cells. Nature. 2009;457:882–886. doi: 10.1038/nature07684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dreux M, Dao Thi VL, Fresquet J, Guerin M, Julia Z, Verney G, Durantel D, et al. Receptor complementation and mutagenesis reveal SR-BI as an essential HCV entry factor and functionally imply its intra- and extra-cellular domains. PLoS Pathog. 2009;5:e1000310. doi: 10.1371/journal.ppat.1000310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Voisset C, Callens N, Blanchard E, Op De Beeck A, Dubuisson J, Vu-Dac N. High density lipoproteins facilitate hepatitis C virus entry through the scavenger receptor class B type I. J Biol Chem. 2005;280:7793–7799. doi: 10.1074/jbc.M411600200. [DOI] [PubMed] [Google Scholar]
- 13.Syder AJ, Lee H, Zeisel MB, Grove J, Soulier E, Macdonald J, Chow S, et al. Small molecule scavenger receptor BI antagonists are potent HCV entry inhibitors. J Hepatol. 2011;54:48–55. doi: 10.1016/j.jhep.2010.06.024. [DOI] [PubMed] [Google Scholar]
- 14.Catanese MT, Graziani R, von Hahn T, Moreau M, Huby T, Paonessa G, Santini C, et al. High-avidity monoclonal antibodies against the human scavenger class B type I receptor efficiently block hepatitis C virus infection in the presence of high-density lipoprotein. J Virol. 2007;81:8063–8071. doi: 10.1128/JVI.00193-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Meuleman P, Teresa Catanese M, Verhoye L, Desombere I, Farhoudi A, Jones CT, Sheahan T, et al. A Human monoclonal antibody targeting scavenger receptor class B type I precludes hepatitis C virus infection and viral spread in vitro and in vivo. Hepatology. 2012;55:364–372. doi: 10.1002/hep.24692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lacek K, Vercauteren K, Grzyb K, Naddeo M, Verhoye L, Slowikowski MP, Fafi-Kremer S, et al. Novel human SR-BI antibodies prevent infection and dissemination of HCV in vitro and in humanized mice. J Hepatol. 2012;57:17–23. doi: 10.1016/j.jhep.2012.02.018. [DOI] [PubMed] [Google Scholar]
- 17.Grove J, Nielsen S, Zhong J, Bassendine MF, Drummer HE, Balfe P, McKeating JA. Identification of a residue in hepatitis C virus E2 glycoprotein that determines scavenger receptor BI and CD81 receptor dependency and sensitivity to neutralizing antibodies. J Virol. 2008;82:12020–12029. doi: 10.1128/JVI.01569-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bankwitz D, Steinmann E, Bitzegeio J, Ciesek S, Friesland M, Herrmann E, Zeisel MB, et al. Hepatitis C virus hypervariable region 1 modulates receptor interactions, conceals the CD81 binding site, and protects conserved neutralizing epitopes. J Virol. 2010;84:5751–5763. doi: 10.1128/JVI.02200-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bitzegeio J, Bankwitz D, Hueging K, Haid S, Brohm C, Zeisel MB, Herrmann E, et al. Adaptation of hepatitis C virus to mouse CD81 permits infection of mouse cells in the absence of human entry factors. PLoS Pathog. 2010;6:e1000978. doi: 10.1371/journal.ppat.1000978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Catanese MT, Loureiro J, Jones CT, Dorner M, von Hahn T, Rice CM. Different requirements for scavenger receptor class B type I in hepatitis C virus cell-free versus cell-to-cell transmission. Journal of virology. 2013;87:8282–8293. doi: 10.1128/JVI.01102-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Prentoe J, Serre SB, Ramirez S, Nicosia A, Gottwein JM, Bukh J. Hypervariable region 1 deletion and required adaptive envelope mutations confer decreased dependency on scavenger receptor class B type I and low-density lipoprotein receptor for hepatitis C virus. Journal of virology. 2014;88:1725–1739. doi: 10.1128/JVI.02017-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lindenbach BD, Evans MJ, Syder AJ, Wölk B, Tellinghuisen TL, Liu CC, Maruyama T, et al. Complete replication of hepatitis C virus in cell culture. Science. 2005;309:623. doi: 10.1126/science.1114016. [DOI] [PubMed] [Google Scholar]
- 23.Wakita T, Pietschmann T, Kato T, Date T, Miyamoto M, Zhao Z, Murthy K, et al. Production of infectious hepatitis C virus in tissue culture from a cloned viral genome. Nat Med. 2005;11:791–796. doi: 10.1038/nm1268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jones CT, Catanese MT, Law LM, Khetani SR, Syder AJ, Ploss A, Oh TS, et al. Real-time imaging of hepatitis C virus infection using a fluorescent cell-based reporter system. Nat Biotechnol. 2010;28:167–171. doi: 10.1038/nbt.1604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Meuleman P, Albecka A, Belouzard S, Vercauteren K, Verhoye L, Wychowski C, Leroux-Roels G, et al. Griffithsin Has Antiviral Activity against Hepatitis C Virus. Antimicrob Agents Chemother. 2011;55:5159–5167. doi: 10.1128/AAC.00633-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Meuleman P, Vanlandschoot P, Leroux-Roels G. A simple and rapid method to determine the zygosity of uPA-transgenic SCID mice. Biochem Biophys Res Commun. 2003;308:375–378. doi: 10.1016/s0006-291x(03)01388-3. [DOI] [PubMed] [Google Scholar]
- 27.Meuleman P, Libbrecht L, De Vos R, de Hemptinne B, Gevaert K, Vandekerckhove J, Roskams T, et al. Morphological and biochemical characterization of a human liver in a uPA-SCID mouse chimera. Hepatology. 2005;41:847–856. doi: 10.1002/hep.20657. [DOI] [PubMed] [Google Scholar]
- 28.Icard V, Diaz O, Scholtes C, Perrin-Cocon L, Ramiere C, Bartenschlager R, Penin F, et al. Secretion of hepatitis C virus envelope glycoproteins depends on assembly of apolipoprotein B positive lipoproteins. PLoS One. 2009;4:e4233. doi: 10.1371/journal.pone.0004233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Meex SJ, Andreo U, Sparks JD, Fisher EA. Huh-7 or HepG2 cells: which is the better model for studying human apolipoprotein-B100 assembly and secretion? J Lipid Res. 2011;52:152–158. doi: 10.1194/jlr.D008888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lindenbach BD, Meuleman P, Ploss A, Vanwolleghem T, Syder AJ, McKeating JA, Lanford RE, et al. Cell culture-grown hepatitis C virus is infectious in vivo and can be recultured in vitro. Proceedings of the National Academy of Sciences of the United States of America. 2006;103:3805. doi: 10.1073/pnas.0511218103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Podevin P, Carpentier A, Pene V, Aoudjehane L, Carriere M, Zaidi S, Hernandez C, et al. Production of infectious hepatitis C virus in primary cultures of human adult hepatocytes. Gastroenterology. 139:1355–1364. doi: 10.1053/j.gastro.2010.06.058. [DOI] [PubMed] [Google Scholar]
- 32.Vanwolleghem T, Bukh J, Meuleman P, Desombere I, Meunier JC, Alter H, Purcell RH, et al. Polyclonal immunoglobulins from a chronic hepatitis C virus patient protect human liver-chimeric mice from infection with a homologous hepatitis C virus strain. Hepatology. 2008;47:1846–1855. doi: 10.1002/hep.22244. [DOI] [PubMed] [Google Scholar]
- 33.Law M, Maruyama T, Lewis J, Giang E, Tarr AW, Stamataki Z, Gastaminza P, et al. Broadly neutralizing antibodies protect against hepatitis C virus quasispecies challenge. Nat Med. 2008;14:25–27. doi: 10.1038/nm1698. [DOI] [PubMed] [Google Scholar]
- 34.Meuleman P, Bukh J, Verhoye L, Farhoudi A, Vanwolleghem T, Wang RY, Desombere I, et al. In vivo evaluation of the cross-genotype neutralizing activity of polyclonal antibodies against hepatitis C virus. Hepatology. 2011;53:755–762. doi: 10.1002/hep.24171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Giang E, Dorner M, Prentoe JC, Dreux M, Evans MJ, Bukh J, Rice CM, et al. Human broadly neutralizing antibodies to the envelope glycoprotein complex of hepatitis C virus. Proc Natl Acad Sci U S A. 2012;109:6205–6210. doi: 10.1073/pnas.1114927109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Davis GL, Nelson DR, Terrault N, Pruett TL, Schiano TD, Fletcher CV, Sapan CV, et al. A randomized, open-label study to evaluate the safety and pharmacokinetics of human hepatitis C immune globulin (Civacir) in liver transplant recipients. Liver Transpl. 2005;11:941–949. doi: 10.1002/lt.20405. [DOI] [PubMed] [Google Scholar]
- 37.Schiano TD, Charlton M, Younossi Z, Galun E, Pruett T, Tur-Kaspa R, Eren R, et al. Monoclonal antibody HCV-AbXTL68 in patients undergoing liver transplantation for HCV: results of a phase 2 randomized study. Liver Transpl. 2006;12:1381–1389. doi: 10.1002/lt.20876. [DOI] [PubMed] [Google Scholar]
- 38.Voisset C, de Beeck AO, Horellou P, Dreux M, Gustot T, Duverlie G, Cosset FL, et al. High-density lipoproteins reduce the neutralizing effect of hepatitis C virus (HCV)-infected patient antibodies by promoting HCV entry. Journal of General Virology. 2006;87:2577–2581. doi: 10.1099/vir.0.81932-0. [DOI] [PubMed] [Google Scholar]
- 39.Dreux M, Pietschmann T, Granier C, Voisset C, Ricard-Blum S, Mangeot PE, Keck Z, et al. High density lipoprotein inhibits hepatitis C virus-neutralizing antibodies by stimulating cell entry via activation of the scavenger receptor BI. J Biol Chem. 2006;281:18285–18295. doi: 10.1074/jbc.M602706200. [DOI] [PubMed] [Google Scholar]
- 40.Mensa L, Crespo G, Gastinger MJ, Kabat J, Perez-del-Pulgar S, Miquel R, Emerson SU, et al. Hepatitis C virus receptors claudin-1 and occludin after liver transplantation and influence on early viral kinetics. Hepatology. 2011;53:1436–1445. doi: 10.1002/hep.24110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Masson D, Koseki M, Ishibashi M, Larson CJ, Miller SG, King BD, Tall AR. Increased HDL cholesterol and apoA-I in humans and mice treated with a novel SR-BI inhibitor. Arterioscler Thromb Vasc Biol. 2009;29:2054–2060. doi: 10.1161/ATVBAHA.109.191320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wong-Staal F, Syder AJ, McKelvy JF. Targeting HCV entry for development of therapeutics. Viruses. 2010;2:1718–1733. doi: 10.3390/v2081718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Flores MV, Corbau RG, Guionaud S. Pharmacokinetics and safety in cynomolgus monkeys of a monoclonal antibody targeting human scavenger receptor class B type-1 for hepatitis C treatment. Antiviral therapy. 2013;18:775–784. doi: 10.3851/IMP2570. [DOI] [PubMed] [Google Scholar]
- 44.Sulkowski MS, Kang M, Matining R, Wyles D, Johnson VA, Morse GD, Amorosa V, et al. Safety and Antiviral Activity of the HCV Entry Inhibitor ITX5061 in Treatment-Naive HCV-Infected Adults: A Randomized, Double-Blind, Phase 1b Study. The Journal of infectious diseases. 2013 doi: 10.1093/infdis/jit503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Prentoe J, Serre SB, Ramirez S, Nicosia A, Gottwein JM, Bukh J. Hypervariable region 1 deletion and required adaptive envelope mutations confer decreased dependency on scavenger receptor class B type I and low-density lipoprotein receptor for hepatitis C virus. J Virol. 2014;88:1725–1739. doi: 10.1128/JVI.02017-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Bartosch B, Verney G, Dreux M, Donot P, Morice Y, Penin F, Pawlotsky JM, et al. An interplay between hypervariable region 1 of the hepatitis C virus E2 glycoprotein, the scavenger receptor BI, and high-density lipoprotein promotes both enhancement of infection and protection against neutralizing antibodies. J Virol. 2005;79:8217–8229. doi: 10.1128/JVI.79.13.8217-8229.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Dreux M, Boson B, Ricard-Blum S, Molle J, Lavillette D, Bartosch B, Pecheur EI, et al. The exchangeable apolipoprotein ApoC-I promotes membrane fusion of hepatitis C virus. J Biol Chem. 2007;282:32357–32369. doi: 10.1074/jbc.M705358200. [DOI] [PubMed] [Google Scholar]
- 48.Dao Thi VL, Granier C, Zeisel MB, Guerin M, Mancip J, Granio O, Penin F, et al. Characterization of hepatitis C virus particle subpopulations reveals multiple usage of the scavenger receptor BI for entry steps. J Biol Chem. 2012;287:31242–31257. doi: 10.1074/jbc.M112.365924. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.