Abstract
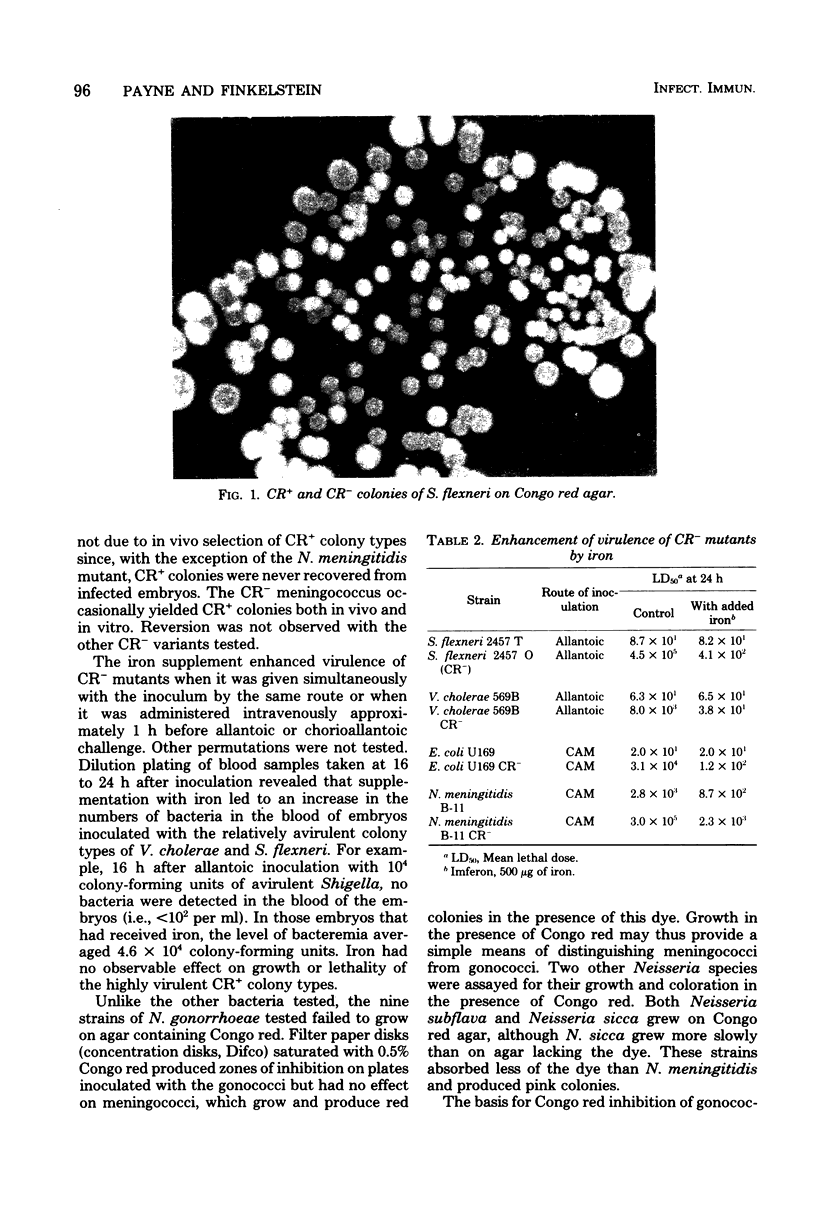
Agar medium containing Congo red dye differentiates virulent and avirulent colonies of Shigella, Vibrio cholerae, Escherichia coli, and Neisseria meningitidis. Like virulent plague bacilli, wild-type cells of these species absorb the dye and produce red colonies. Mutants or colonial variants have been isolated that fail to absorb the dye and produce colorless colonies. These mutants exhibit reduced virulence in the chicken embryo model, but their virulence is enhanced by supplementation with iron. Of those species tested, only Neisseria gonorrhoeae isolates failed to grow in the presence of this dye. Inhibition of growth by Congo red may thus provide a simple means for differentiating gonococci from other Neisseria.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BURROWS T. W., JACKSON S. The pigmentation of Pasteurella pestis on a defined medium containing haemin. Br J Exp Pathol. 1956 Dec;37(6):570–576. [PMC free article] [PubMed] [Google Scholar]
- BURROWS T. W., JACKSON S. The virulence-enhancing effect of iron on nonpigmented mutants of virulent strains of Pasteurella pestis. Br J Exp Pathol. 1956 Dec;37(6):577–583. [PMC free article] [PubMed] [Google Scholar]
- Bullen J. J., Leigh L. C., Rogers H. J. The effect of iron compounds on the virulence of Escherichia coli for guinea-pigs. Immunology. 1968 Oct;15(4):581–588. [PMC free article] [PubMed] [Google Scholar]
- Bullen J. J., Ward C. G., Wallis S. N. Virulence and the role of iron in Pseudomonas aeruginosa infection. Infect Immun. 1974 Sep;10(3):443–450. doi: 10.1128/iai.10.3.443-450.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bumgarner L. R., Finkelstein R. A. Pathogenesis and immunology of experimental gonococcal infection: virulence of colony types of Neisseria gonorrhoeae for chicken embryos. Infect Immun. 1973 Dec;8(6):919–924. doi: 10.1128/iai.8.6.919-924.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FINKELSTEIN R. A., RANSOM J. P. Non-specific resistance to experimental cholera in embryonated eggs. J Exp Med. 1960 Aug 1;112:315–328. doi: 10.1084/jem.112.2.315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finkelstein R. A., Vasil M. L., Holmes R. K. Studies on toxinogenesis in Vibrio cholerae. I. Isolation of mutants with altered toxinogenicity. J Infect Dis. 1974 Feb;129(2):117–123. doi: 10.1093/infdis/129.2.117. [DOI] [PubMed] [Google Scholar]
- Formal S. B., Labrec E. H., Palmer A., Falkow S. Protection of Monkeys Against Experimental Shigellosis with Attenuated Vaccines. J Bacteriol. 1965 Jul;90(1):63–68. doi: 10.1128/jb.90.1.63-68.1965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldschneider I., Gotschlich E. C., Artenstein M. S. Human immunity to the meningococcus. II. Development of natural immunity. J Exp Med. 1969 Jun 1;129(6):1327–1348. doi: 10.1084/jem.129.6.1327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kochan I., Golden C. A., Bukovic J. A. Mechanism of tuberculostasis in mammalian serum. II. Induction of serum tuberculostasis in guinea pigs. J Bacteriol. 1969 Oct;100(1):64–70. doi: 10.1128/jb.100.1.64-70.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kochan I., Kvach J. T., Wiles T. I. Virulence-associated acquisition of iron in mammalian serum by Escherichia coli. J Infect Dis. 1977 Apr;135(4):623–632. doi: 10.1093/infdis/135.4.623. [DOI] [PubMed] [Google Scholar]
- Labrec E. H., Schneider H., Magnani T. J., Formal S. B. EPITHELIAL CELL PENETRATION AS AN ESSENTIAL STEP IN THE PATHOGENESIS OF BACILLARY DYSENTERY. J Bacteriol. 1964 Nov;88(5):1503–1518. doi: 10.1128/jb.88.5.1503-1518.1964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Payne S. M., Finkelstein R. A. Imferon agar: improved medium for isolation of pathogenic Neisseria. J Clin Microbiol. 1977 Sep;6(3):293–297. doi: 10.1128/jcm.6.3.293-297.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Payne S. M., Finkelstein R. A. Pathogenesis and immunology of experimental gonococcal infection: role of iron in virulence. Infect Immun. 1975 Dec;12(6):1313–1318. doi: 10.1128/iai.12.6.1313-1318.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Surgalla M. J., Beesley E. D. Congo red-agar plating medium for detecting pigmentation in Pasteurella pestis. Appl Microbiol. 1969 Nov;18(5):834–837. doi: 10.1128/am.18.5.834-837.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]




