Summary
Haploinsufficiency of ribosomal proteins (RPs) and upregulation of the tumour suppressor TP53 have been shown to be the common basis for the anaemia observed in Diamond Blackfan anaemia and 5q- myelodysplastic syndrome. We previously demonstrated that treatment with L-Leucine resulted in a marked improvement in anaemia in disease models. To determine if the L-Leucine effect was Tp53-dependent, we used antisense MOs to rps19 and rps14 in zebrafish; expression of tp53 and its downstream target cdkn1a remained elevated following L-leucine treatment. We confirmed this observation in human CD34+ cells. L-Leucine thus alleviates anaemia in RP-deficient cells in a TP53-independent manner.
Keywords: Diamond-Blackfan anaemia, leucine, haematopoiesis, TP53
Introduction
Diamond Blackfan anaemia (DBA) is a congenital bone marrow failure syndrome characterized by a severe macrocytic anaemia. More than half of patients with DBA have been shown to have a heterozygous loss of a ribosomal protein (RP) gene, with RPS19 being the most frequently mutated (reviewed in Khanna-Gupta 2013, Narla and Ebert 2010)). The 5q- syndrome is a subtype of myelodysplastic syndrome (MDS), also characterized by a severe anaemia that is caused by heterozygous loss of RPS14 on chromosome 5q (Ebert, et al 2008). TP53 has been implicated as a central mediator in a variety of in vivo and in vitro models, linking defects in ribosome biogenesis to aberrant cell proliferation and survival (reviewed in Khanna-Gupta 2013). Despite our increasing understanding of the pathophysiology, few therapeutic advances have been made and there is an understandable reluctance to manipulate the TP53 pathway given that RP-deficient patients are already at increased risk for cancer (Vlachos, et al 2012). The amino acid L-leucine is known to modulate protein synthesis by enhancing mRNA translation, (Boultwood, et al 2013, Payne, et al 2012) and was used to treat a DBA patient who responded with improvement in haemoglobin levels and transfusion independence (Pospisilova, et al 2007). This clinical observation has since been validated in several in vivo and in vitro models (Jakko, et al 2012, Payne, et al 2012) and forms the basis of a clinical trial that is open in the US. Our current study aimed to determine if the beneficial effects of L-leucine on erythropoiesis involved modulation of the TP53 pathway in models of DBA and 5q-MDS and to evaluate possible toxicities of L-leucine therapy.
Methods
Zebrafish husbandry and microinjections
Wild-type AB, tp53m/m (harbouring a Tp53 DNA-binding domain mutation (Berghmans, et al 2005)) and Tg(GATA1:dsRed) (Traver, et al 2003) transgenic lines of Danio rerio were maintained according to standard protocols. Antisense morpholinos (MOs) targeting zebrafish rps19 and rps14 were designed and injected into one-cell stage zebrafish embryos along with standard control MOs. In our previously described zebrafish model designed to simulate haploinsufficient levels of RPs observed in patients, we titrated the dose of MO to achieve 50% knockdown at the protein level as determined by western blotting. This corresponded to 0.02 ng MO for Rps19 and 0.8 ng MO for Rps14 (see Supplemental data in Payne, et al (2012)). Staining with o-dianisidine for haemoglobin was performed at 48-hs post-fertilization, as previously described (Payne, et al 2012).
Culture and lentiviral infection of haematopoietic progenitor cells
CD34+ cells were purified from cord blood and then infected the following day with lentivirus expressing shRNAs for control, RPS19 or RPS14 (previously validated; data available in Dutt, et al (2011)). Erythroid differentiation was induced in vitro using a liquid culture system as previously described (Dutt, et al 2011). L-Leucine was added to the culture (10 or 100 μM 24 h after thawing and with medium changes on day 4 of culture.
Real time polymerase chain reaction (PCR) (zebrafish)
Control, rps19-, or rps14-MO injected zebrafish embryos were either treated with L-Leucine (100 mM) or allowed to incubate in egg-water. At 48-h post-treatment, total RNA was isolated from the embryos (20-30 per condition). cDNA was derived and subjected to real time PCR using default cycling parameters in an iQ5 Cycler (Biorad, Hercules, CA).
Quantitative reverse transcription (qRT)-PCR (CD34)
RNA was purified from shRNA-infected cells with or without L-Leucine treatment 5 days post-infection using TRIzol reagent (Invitrogen, Grand Island, NY). First strand cDNA was reverse transcribed from total RNA using Invitrogen Superscript III. qRT-PCR was performed using Taqman 20× assay (Applied Biosystems, Grand Island, NY).
Reporter assays
A549 cells were transiently co-transfected with a TP53 reporter plasmid (TP53-GLB; a gift from Dr. Peter Schultz, The Salk Institute, La Jolla, CA) alone or with an expression plasmid for TP53 (2 μg), and treated with increasing concentrations of L-Leucine. The cells were harvested at 48-h post-transfection, and luciferase (Luc) reporter gene activity measured using a dual luciferase reporter gene assay kit (Promega Biotech, Madison, WI).
Flow cytometry
CD34+ cells were fixed 4 days post-infection with 2% paraformaldehyde for 15 mins at 37°C and permeabilized with methanol for a minimum of 30 min at −20°C. Cells were incubated with primary antibody for CDKN1A (#12D1; Cell Signaling Technology, Beverly, MA) followed by anti-rabbit antibody AlexaFluor 750 (Molecular Probes, Grand Island, NY). Flow cytometry was performed on a FACSCanto cytometer (BD Biosciences, San Jose, CA) and data were analysed using FlowJo software version 8.7.1 (TreeStar, Ashland, OR).
Statistical Analysis
The significance of experimental results was determined by Student t test unless otherwise noted. P values ≤0.05 were considered statistically significant.
Results and Discussion
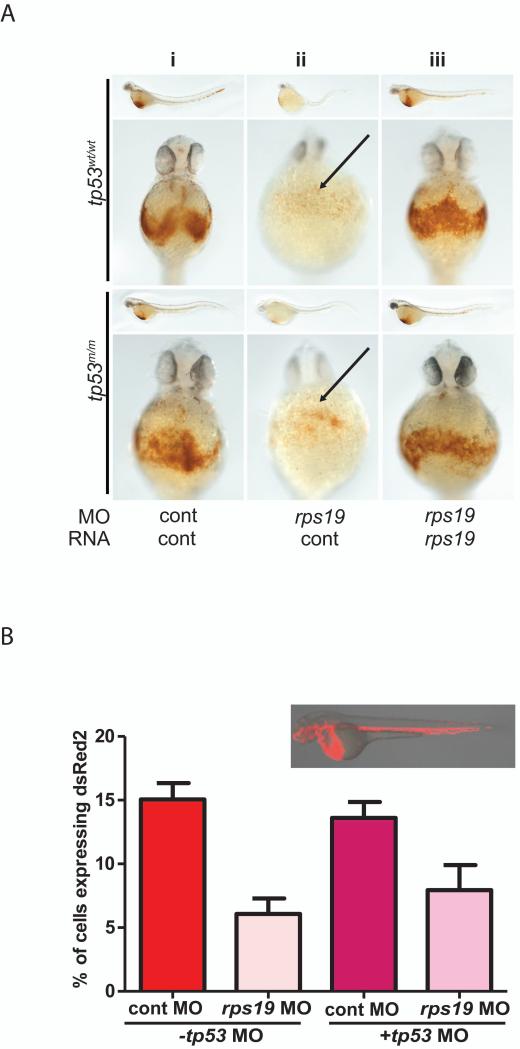
A key component of the molecular basis of anaemia in DBA and 5q-MDS involves upregulation of TP53. However, rescue of RP-deficient anaemia in a TP53-null setting is rarely complete (Singh, et al 2014, Yadav, et al 2014), leading to the hypothesis that RP-deficient anaemia has a TP53-independent component. Furthermore, TP53 activation fails to account for several aspects of disease pathophysiology. For example, it has recently been shown that depletion of RPL5 or RPL11 in primary lung fibroblasts does not induce TP53 expression despite reduced cell proliferation, suggesting the existence of a TP53-independent check point associated with cell cycle regulation in these cells (Teng, et al 2013). Studies in RP-depleted mouse ES cells have shown that primitive erythropoiesis is TP53 independent (Singh, et al 2014). Using Tp53 wildtype and mutant zebrafish embryos, we show that knockdown of Rps19 resulted in profound anaemia regardless of Tp53 status, supporting a TP53-independent component to the anaemia observed in DBA (Figure 1A)
Figure 1. The anaemia associated with Rps19 deficiency has a Tp53 independent component.
[A] rps19 knockdown was performed in both Tp53 wildtype (top panels) and Tp53 mutant (bottom panels) zebrafish lines. Staining for haemoglobin shows a profound reduction in circulating blood regardless of Tp53 status. Developmental defects associated with Rps19 deficiency were markedly improved in Tp53 mutant embryos (ii), however only modest improvements in haemoglobinization were observed. Co-injection of rps19 RNA rescued the anaemia in 50-80% of embryos regardless of Tp53 status (iii).
[B] rps19 morpholino (MO) was co-injected with tp53 MO or control MO into Tg(gata1:dsRed2) transgenic embryos (upper panel shows epiflourescent image of a control embryo) at 2 days post-fertilization. Embryos were dissociated and the percentage of erythroid cells (dsRed2 expressing) determined by fluorescence-activated cell sorting. A significant reduction in dsRed2 expressing cells in Rps19 morphants compared to controls was observed. The addition of Tp53 MO partially rescued red cell numbers but did not show an overall improvement in total red cell numbers compared to Rps19 knockdown in the presence of control MO, indicating a Tp53 independent component to the anaemia observed in the Rps19 morphants.
To determine whether the reduced haemoglobin levels observed were a result of abnormal haemoglobinization or reduced red cell numbers, we utilized a transgenic zebrafish line that expresses a red fluorescent protein (dsRed) from the gata1a promoter (see inset Figure 1B). The majority of the red fluorescent cells in these embryos are erythroid cells. Rps19-deficient embryos were dissociated into a single cell suspension and analysed by fluorescence-activated cell sorting (FACS). Representative FACS plots from 2 days post-fertilization embryos show a marked reduction in dsred+ cells in Rps19-injected embryos. Rps19 deficiency resulted in reduced red cell numbers regardless of Tp53 status (Figure 1B). Rps14-deficient Tp53 mutant zebrafish embryos were also severely anaemic (data not shown). This is consistent with recent studies that showed that a Tp53-independent but Rps19-dependent pathway could be responsible for defective erythropoiesis in zebrafish (Yadav, et al 2014).
L-Leucine treatment results in marked improvement in anaemia via activation of the mTOR pathway in both in vivo and in vitro models of DBA and 5q-MDS (Payne, et al 2012, Yip, et al 2012). Given the possibility of TP53-independent mechanisms associated with anaemia in these disorders and the clinical trial using L-Leucine, we wished to explore the effects of L-leucine on the TP53 pathway.
Using zebrafish models of DBA and 5q-MDS, we demonstrated by qPCR that, as expected, levels of tp53 and one of its major downstream targets, cdkn1a, are transcriptionally increased following knockdown of Rps19 or Rps14. We further demonstrated that these levels were not downregulated upon treatment with L-Leucine (Figure 2A). We corroborated these findings at the protein level in RPS19- and RPS14-deficient human CD34+ haematopoietic progenitor cells induced towards erythroid maturation. As we have previously reported, a small but significant increase in TP53 protein expression was observed in RPS14- (p=0.015) and RPS19- (p=0.012) deficient cells compared to control cells (Luc) (Dutt, et al 2011). TP53 expression levels remained unchanged upon L-Leucine treatment (Figure 2B top panel). Furthermore, expression of CDKN1A increased significantly upon knockdown of RPS14 (p=0.01) and RPS19 (p=0.0001) compared to controls (Figure 2B, bottom panel) and remained elevated following L-Leucine treatment, suggesting that the effects of L-Leucine are TP53-independent.
Figure 2. The activation of the TP53 pathway was not affected by L-leucine treatment in in vivo or in vitro models.
[A] rps19 and rps14 knockdown in zebrafish increased the expression of tp53 and its downstream target gene cdkn1a compared to controls. This increased expression was not downregulated by the addition of 100 mM of L-Leucine. Transcript levels of each mRNA were normalized to that of β-actin and expressed as a fold change over the signal observed in the control sample. MO, morpholino.
[B] Increased levels of total TP53 and CDKN1A protein in primary human CD34+ cells as analysed by intracellular flow cytometry were observed upon RPS19 and RPS14 knockdown when compared to control shRNA (Luc). This increase was not affected by the addition of L-Leucine at either 10 μM or 100 μM. The experiments were performed in triplicate and the entire experiment was repeated with similar results. A 2-tailed Student t test was used to analyse the data *P≤0.05.
[C] A549 cells transfected with a TP53 reporter plasmid showed an 8-fold increase in luciferase reporter gene activity in the presence of a TP53 expression plasmid. Reporter gene activity was unaffected by the addition of increasing concentrations of L-Leucine. Normalized luciferase (to that of a co-transfected Renilla luciferase plasmid) values were used to calculate the fold change over that of the TP53 reporter plasmid alone (equal to 1). This experiment was performed in duplicate and repeated three times. Means +/− standard error from one representative experiment are shown.
These observations were further validated in a transient co-transfection assay in A549 (wild type for TP53) cells. TP53 (TP53 wt) transactivated a TP53 reporter gene plasmid (TP53-GLB) 8-fold above background. The addition of L-Leucine at 10 or 100 μM did not alter transactivation by TP53 (Figure 2C). We thus show that the TP53-mediated upregulation of a TP53-regulated reporter is not altered by the addition of increasing concentrations of L-Leucine.
Based on our observations we conclude that L-Leucine does not alleviate the anaemia in RP-deficient cells by downregulating TP53 activity. In keeping with our observations, Jaako et al (2012) recently demonstrated in their mouse model of DBA that elevated levels of CDKN1A were unresponsive to L-Leucine treatment. However, their study did report some TP53-target genes that were L-Leucine responsive. While it is possible that a subset of TP53-regulated genes may respond to L-Leucine levels, our data show that genes harbouring a TP53-binding site, that are therefore canonical targets of TP53, are unlikely to respond to L-Leucine (Figure 2C). The differential L-Leucine response to a subset of TP53-regulated genes in the Jaako study may thus reflect a complex indirect regulatory mechanism potentially involving putative L-Leucine regulatory elements within these genes.
Understanding the molecular events that mediate the improvement in the anaemia in DBA and 5q-MDS is critical. Our findings validate the use of L-Leucine for the clinical management of these patients because TP53 inactivation, which is associated with tumour growth, does not appear to be a confounding issue in the L-Leucine response of DBA and 5q-MDS. Our findings also provide a new avenue for investigation into the pathophysiology of these ribosomopathies that may lead to new therapeutic opportunities for these diseases.
Key Points.
The erythroid phenotype in ribosomopathies has a TP53-independent component
L-leucine does not alleviate the anaemia in ribosomal protein deficient cells by downregulating TP53 activity
Acknowledgements
The authors would like to thank Marie McConkey and Hong Sun for technical assistance. This work was funded by the National Institutes of Health (K08 DK090145-01A1) and the American Society of Hematology (Scholar Award) to A.N, Leukaemia and Lymphoma Research UK (GSK Greg Harper fellowship) to E.M.P, and by the Tomasso Family Hematology Fund to AK-G.
Footnotes
Authorship Contributions: A.N. and A.K-G. conceived the project; A.N., N.A., E.M.P., S.N.H., and D.M.R designed, performed and interpreted the experiments; B.L.E. and A.K-G. supervised the experimental work and interpretation of the data. A.N. and A.K-G. wrote the manuscript; and N.A., N.B., A.T.L., S.N.H., E.M.P., D.M.R., and B.L.E reviewed the manuscript.
Disclosure of Conflicts of Interest: The authors declare no competing financial interests.
References
- Berghmans S, Murphey RD, Wienholds E, Neuberg D, Kutok JL, Fletcher CD, Morris JP, Liu TX, Schulte-Merker S, Kanki JP, Plasterk R, Zon LI, AT L. tp53 mutant zebrafish develop malignant peripheral nerve sheath tumors. Proc. Natl. Acad. Sci. USA. 2005;102:407–412. doi: 10.1073/pnas.0406252102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boultwood J, Yip B, Vuppusetty C, Pellagatti A, Wainscoat J. Activation of the mTOR pathway by amino acid L-Leuicne in 5q-syndrome and other ribosomopathies. Adv. Biol. Regulation. 2013;53:8–17. doi: 10.1016/j.jbior.2012.09.002. [DOI] [PubMed] [Google Scholar]
- Dutt S, Narla A, Lin K, Mullally A, Abayasekara N, Megerdichian C, Wilson FH, Currie T, Khanna-Gupta A,N,B, Kutok JL, BL E. Haploinsufficiency for ribosomal protein genes causes selective activation of p53 in human erythroid progenitor cells. Blood. 2011;117:2567–2576. doi: 10.1182/blood-2010-07-295238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ebert B, Pretz J, Bosco J, Chang C, Tamayo P, Galili N, Raza A,, Root DE, Attar E, Ellis SR, Golub TR. Identification of RPS14 as a 5q-syndrome gene by RNA interference screen. Nature. 2008;451:335–339. doi: 10.1038/nature06494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jakko P, Debnath S, Olsson K, Bryder D, Flygare J, S K. Dietary L-Leucine improves the anemia in a mouse model for Diamond-Blackfan anemia. Blood. 2012;120:2225–2228. doi: 10.1182/blood-2012-05-431437. [DOI] [PubMed] [Google Scholar]
- Khanna-Gupta A. Bone marrow failure syndromes: the Ribosomopathies. J Bone Marrow Res. 2013;1:106–109. doi: 10.4172/jbmr.1000106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Narla A, Ebert B. Ribosomopathies: human disorders of ribosome dysfunction. Blood. 2010;115:3196–3205. doi: 10.1182/blood-2009-10-178129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Payne E, Virgilio M, Narla A, Sun H, Levine M, Paw BH, Berliner N, Look AT, Ebert B, A K-G. L-Leucine improves anemia and developmental defects associated with Diamond-Blackfan anemia and del(5q)MDS by activating the mTOR pathway. Blood. 2012;120:2214–2224. doi: 10.1182/blood-2011-10-382986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pospisilova D, J C, Hale J, Adam R, Cmejla R. Successful treatment of a Diamond Balckfan anemia patient with the amino acid leucine. Hematologica. 2007;92:e66–e67. doi: 10.3324/haematol.11498. [DOI] [PubMed] [Google Scholar]
- Singh S, Goldberg T, Henson A, Husain-Krautter A, Nihrane A, Blanc L, Ellis S, Lipton J, Liu J. p53 independent cell cycle and erythroid differentiation defects in murine embryonic stem cells haploinsufficient for Diamond Blackfan Anemia-proteins: RPS19 versus RPL5. PloS One. 2014;9:e89098. doi: 10.1371/journal.pone.0089098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teng T, Mercer C, Hexley P, Thomas G, Fumagalli S. Loss of tumor suppresor RPL5/RPL11 does not induce cell cycle arrest but impedes proliferation due to reduced ribosome content and translation capacity. Mol Cell Biol. 2013;33:4660–4671. doi: 10.1128/MCB.01174-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Traver D, Paw B, Poss K, Penberthy W, Lin S, Zon L. Transplantation aand invivo imaging of multilineage engraftmant in zebrafish bloodless mutants. Nat Immunol. 2003;4:1238–1246. doi: 10.1038/ni1007. [DOI] [PubMed] [Google Scholar]
- Vlachos A, Rosenberg P, Atsidaftos E, Alter B, Lipton J. Incidence of neoplasia in Diamond Blackfan Anemia: a report from the Diamond Blackfan Anemia Registry. Blood. 2012;119:3815–3819. doi: 10.1182/blood-2011-08-375972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yadav G, Chakraborty A, Uechi T, Kenmochi N. Ribosomal protein deficiency causes Tp53-independent erythropoiesis failure in zebrafish. Int. J. Biochem. Cell. Biol. 2014;49:1–7. doi: 10.1016/j.biocel.2014.01.006. [DOI] [PubMed] [Google Scholar]
- Yip B, Pellagatti A, Vuppusetty C, Giagounidis A, Germing U, Lamikanra A, Roberts DJ, Fernandez-Mercado M, McDonald EJ, Killick S, Wainscoat JS, Boultwood J. Effects of L-leucine in 5q-syndrome and other RPS14-deficient erythroblasts. Leukemia. 2012:2154–2158. doi: 10.1038/leu.2012.82. [DOI] [PubMed] [Google Scholar]