Abstract
Chemokine (C-X-C motif) receptor (CXCR) 4 and atypical chemokine receptor (ACKR) 3 ligands have been reported to modulate cardiovascular function in various disease models. The underlying mechanisms, however, remain unknown. Thus, it was the aim of the present study to determine how pharmacological modulation of CXCR4 and ACKR3 regulate cardiovascular function. In vivo administration of TC14012, a CXCR4 antagonist and ACKR3 agonist, caused cardiovascular collapse in normal animals. During the cardiovascular stress response to hemorrhagic shock, ubiquitin, a CXCR4 agonist, stabilized blood pressure, whereas coactivation of CXCR4 and ACKR3 with CXC chemokine ligand 12 (CXCL12), or blockade of CXCR4 with AMD3100 showed opposite effects. While CXCR4 and ACKR3 ligands did not affect myocardial function, they selectively altered vascular reactivity upon α1-adrenergic receptor (AR) activation in pressure myography experiments. CXCR4 activation with ubiquitin enhanced α1-AR-mediated vasoconstriction, whereas ACKR3 activation with various natural and synthetic ligands antagonized α1-AR-mediated vasoconstriction. The opposing effects of CXCR4 and ACKR3 activation by CXCL12 could be dissected pharmacologically. CXCR4 and ACKR3 ligands did not affect vasoconstriction upon activation of voltage-operated Ca2+ channels or endothelin receptors. Effects of CXCR4 and ACKR3 agonists on vascular α1-AR responsiveness were independent of the endothelium. These findings suggest that CXCR4 and ACKR3 modulate α1-AR reactivity in vascular smooth muscle and regulate hemodynamics in normal and pathological conditions. Our observations point toward CXCR4 and ACKR3 as new pharmacological targets to control vasoreactivity and blood pressure.
INTRODUCTION
The G protein-coupled receptors (GPCRs) chemokine (C-X-C motif) receptor 4 (CXCR4) and atypical chemokine receptor 3 (ACKR3, formerly known as RDC1 and CXCR7 [1]) play important roles during the development of the cardiovascular system. CXCR4 deficiency results in cardiac and vascular defects, whereas animals lacking ACKR3 show abnormal heart valve development (2–5). Both receptors share chemokine (C-X-C motif) ligand 12 (CXCL12, stromal cell-derived factor-1α) as a common cognate ligand (1,6,7). CXCR4 fulfills pleiotropic functions in the immune system and contributes to various pathophysiological processes, such as tissue repair, cancer metastases or human immunodeficiency virus infection (2,8,9). Functions of ACKR3 after birth are less well understood. Whereas ACKR3 was described initially as a scavenger receptor for CXCL12, recent evidence suggests that it is an active cell surface receptor, which induces G protein independent signaling (10–12).
CXCR4 and ACKR3 are expressed in many tissues after birth, including the heart and vasculature (10,13,14). Information on their possible roles in the regulation of cardiovascular function, however, is sparse. While interactions between CXCR4 and β2-adrenergic receptors (ARs) have been described in cardiomyocytes, the physiological relevance of this observation remains unclear (15–17).
Several lines of evidence, however, suggest that CXCR4 and ACKR3 may contribute to the regulation of cardiovascular function in pathological conditions. Ubiquitin, a noncognate CXCR4 agonist (1,18), improved hemodynamic stability in large animal models of endotoxic and traumatic-hemorrhagic shock, while blockade of CXCR4 with the selective antagonist AMD3100 (1,1′-[1,4-phenylenebis(methylene)]bis-1,4,8,11-tetraazacyclotetra-decane octahydrochloride, generic name: plerixafor) impaired hemodynamic stability (19–21). Furthermore, AMD3100 decreased chronic hypoxia-induced pulmonary hypertension and the selective CXCR4 antagonist AMD3465 (N-[(4-[1,4,8,11-tetraazacy-clotetradec-1-ylmethyl]phenyl)methyl]-2-pyridinemethanamine hexahydrobromide), which has a several-fold higher affinity for CXCR4 than AMD3100 (22), attenuated mineralocorticoid excess-induced hypertension in mice and rats (23,24). CCX771, an ACKR3 ligand (25), also reduced chronic hypoxia-induced pulmonary hypertension in a mouse model (26). The mechanisms underlying these cardiovascular effects of the CXCR4 and ACKR3 modulators, however, remain to be determined. Because a better understanding of the roles of CXCR4 and ACKR3 in cardiovascular physiology and pathology may identify new approaches to control hemodynamics and blood pressure, it was the aim the present study to assess how pharmacological modulation of CXCR4 and ACKR3 regulate cardiovascular function. Thus, we studied the effects of a panel of natural and synthetic CXCR4 and ACKR3 ligands on cardiovascular function in rats in vivo and on vascular reactivity of isolated mesenteric rat vessels in pressure myography experiments.
MATERIALS AND METHODS
Proteins and Reagents
Ubiquitin, anti-ACKR3 (CXCR7) 11G8 and IgG1 isotype control were purchased from R&D Systems, Minneapolis, MN, USA. TC14012 was from Tocris Bioscience, Minneapolis, MN, USA. Phenylephrine, endothelin-1 and AMD3100 were from Sigma-Aldrich, St. Louis, MO, USA. CCX771 and CCX704 (inactive analogue of CCX771) were kindly provided by Mark Penfold, ChemoCentryx, Mountain View, CA, USA. CXCL12 was produced as described previously (27).
Human CXCL11 was cloned into previously described pQE30 vectors that incorporate an N-terminal His6 and Saccharomyces cervisiae small ubiquitin-related modifier (SUMO) protein (Smt3) fusion tag (28–30) to be used for purification. The final, purified CXCL11 construct has a native N-terminal sequence vital for proper function. All expression vector inserts have been verified by DNA sequencing. His6Smt-CXCL11 vectors were transformed into E. coli strain BL21 (pREP4). Cells were then grown at 37°C in Terrific Broth medium. His6Smt-CXCL11 production was induced with 1 mmol/L isopropyl β-d-1-thiogalactopyranoside per liter upon reaching OD600nm of 0.6. After 5 h of incubation at 37°C cells were pelleted at 5,000g and then stored at −80°C until further processing. Cell pellets were resuspended in 20 mL of 50 mmol/L Na2PO4, 300 mmol/L NaCl, 10 mmol/L imidazole, 0.2% sodium azide, 1 mmol/L phenylmethylsulfonyl fluoride and 0.1% β-mercaptoethanol. Resuspended pellets were then lysed via three passages through a French press. Cell lysates were clarified by centrifugation at 15,000g. The supernatant was loaded onto 5 mL of Ni-NTA resin. After 30 min, the column was washed with 2 × 10 mL of buffer AD (6 mol/L guanidinium chloride, 50 mmol/L Na2PO4 [pH 8.0], 300 mmol/L NaCl, 10 mmol/L imidazole, 0.2% sodium azide). The insoluble inclusion body pellet was dissolved in 20 mL buffer AD and loaded onto the equilibrated resin. After 1 h, the column was washed with 4 × 10 mL of buffer AD plus 0.1% β-mercaptoethanol followed by elution with 6 mol/L guanidinium chloride, 50 mmol/L Na2PO4 [pH 7.4], 300 mmol/L NaCl, 500 mmol/L imidazole, 0.2% sodium azide, and 0.1% β-mercaptoethanol. The eluate was pooled and refolded via infinite dilution into a 100-mmol/L Tris (pH 8.0), 10 mmol/L cysteine and 0.5 mmol/L cystine solution. After refolding overnight, the solution was concentrated by ultrafiltration (molecular weight cutoff [MWCO] 10 kDa), and the tag was cleaved through incubation with 500 μg of Ulp1 protease supplemented with 10 mmol/L cysteine at 30°C for 12 h. The His6Smt was separated from the protein through cation-exchange chromatography. Samples were loaded onto SP Sepharose Fast Flow resin (GE Health-care, Chalfont St Giles, Buckinghamshire, UK) and washed with 100 mmol/L Tris (pH 8.0), 50 mmol/L NaCl to remove the His6Smt-tag. Protein was then eluted with a buffer containing 100 mmol/L Tris (pH 8.0) and 2 mol/L NaCl. Finally, samples were purified using reverse-phase high-performance liquid chromatography with a 30 min gradient from 30% to 60% acetonitrile in aqueous 0.1% trifluoroacetic acid. CXCL11 was frozen, lyophilized and stored at −20°C. Purification of CXCL11 was verified by sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS-PAGE), matrix-assisted laser desorption/ionization time-of-flight (MALDI-TOF) spectroscopy, and nuclear magnetic resonance (NMR) spectroscopy.
All reagents used in pressure myography experiments and in in vivo studies were tested for endotoxin contamination utilizing the ToxinSensor Chromogenic LAL Endotoxin Assay Kit (GenScript, Piscataway Township, NJ, USA), as described (21). The endotoxin concentrations in all solutions were less than 0.05 EU/mL, which would be suitable for parenteral use in humans (31 [Bacterial Endotoxins/Pyrogens]).
Animals
All procedures were performed according to the National Institutes of Health guidelines for using laboratory animals (32) and were approved by the Institutional Animal Care and Use Committee of Loyola University Chicago and the US Army Medical Research and Materiel Command Animal Care and Use Review Office. Male Lewis rats (300 to 350 g) were purchased from Harlan (Indianapolis, IN, USA).
Pressure Myography
Pressure myography was performed as described in detail previously (33–36). In brief, animals were euthanized by cardiac extirpation under 4% isoflurane anesthesia. The mesentery was immediately removed, placed in 145 mmol/L NaCl, 4.7 mmol/L KCl, 1.2 mmol/L NaH2PO4, 1.2 mmol/L MgSO4, 2 mmol/L CaCl2, 2 mmol/L pyruvic acid, 0.02 mmol/L EDTA, 3 mmol/L 3-(N-morpholino)propanesulfonic acid (MOPS), and 5 mmol/L d-glucose with 1% BSA, pH 7.4 at 0°C, with the osmolarity adjusted to 300 ± 1 mOsm/L with d-glucose. Third or fourth order mesenteric vessels were then dissected free from the mesentery, mounted onto two glass cannulae with 11-0 sutures and pressurized as required in a DMT 110P Pressure Myograph System (DMT-USA Inc., Ann Arbor, MI, USA). The intraluminal solution contained 145 mmol/L NaCl, 4.7 mmol/L KCl, 1.2 mmol/L NaH2PO4, 1.2 mmol/L MgSO4, 2 mmol/L CaCl2, 2 mmol/L pyruvic acid, 0.02 mmol/L EDTA, 3 mmol/L MOPS, 5 mmol/L d-glucose and 1% BSA, pH 7.4, osmolarity adjusted to 300 ± 1 mOsm/L with d-glucose. The vessel bath solution contained the same solution without BSA. The inner diameter (i.d.) of the pressurized vessel was then continuously measured and recorded via digital video-edge detection. For each condition or drug treatment, the vessel was observed for at least 15 min or until the inner diameter remained stable. Mechanical endothelial denudation was performed in mesenteric arteries by rubbing the arterial lumen with a human hair, followed by air insufflation and washing the lumen with normal saline to remove the endothelium, as described (34).
Measurement of Sarcomere Length
Adult rat (Sprague Dawley) left ventricular cardiomyocytes, isolated using a standardized enzymatic dispersion technique (37), were kindly provided by X Ji and S Sadayappan, Loyola University Chicago. Left ventricular cardiomyocytes were superfused in 141.4 mmol/L NaCl, 4 mmol/L KCl, 0.33 mmol/L NaH2PO4, 1 mmol/L MgCl2, 10 mmol/L HEPES, 5.5 mmol/L glucose, 1.8 mmol/L CaCl2 and 14.5 mmol/L mannitol, pH 7.4, field-stimulated (20 V, 2 Hz, 6 ms pulse duration) and changes in sarcomere length during contractions after addition of vehicle or various concentrations of CXCR4/ACKR3 ligands (1 nmol/L to 10 μmol/L) measured using a video-sarcomere detection system (IonOptix Corp), as described (38,39). Measurements of 60 steady state contractions were averaged for each condition. All experiments were carried out at 36 ± 1°C.
Light Microscopy
Arteries with and without mechanical endothelial denudation were fixed in 10% formalin and embedded in paraffin. Sections were stained with hematoxylin and eosin (H&E). Slides were examined under light microscopy (magnification 40×, 100×, 200×, 400×), as described (40).
In Vivo Experiments
Animals were anesthetized with 100 mg/kg ketamine and 10 mg/kg xy-lazine intraperitoneally (IP). This dose allowed the animals to be deeply sedated but able to breathe spontaneously throughout the experiment. After a mid-line laparotomy, the aorta was instrumented with a 22-gauge angiocatheter for monitoring of arterial blood pressure, blood withdrawal and administration of the CXCR4/7 modulators. The abdomen was then closed with monofilament suture. Core body temperature was maintained using warming lamps. For left ventricular pressure-volume (PV) loop analyses, animals were instrumented with a 1.9 F PV catheter (FTS-1912B-8018; Scisense, London, ON, Canada) that was placed into the right carotid artery and advanced through the aortic valve into the left ventricle. Left ventricular function was then monitored using the Scisense ADVantage PV System with the iWorx data acquisition and analysis software. Left ventricular function indices were determined from 25 to 40 consecutive PV loops. CXCR4/7 modulators were injected in a total volume of 0.5 mL in 0.9% NaCl under normal conditions, in a hemorrhagic shock model with fluid resuscitation and in a lethal hemorrhagic shock model without fluid resuscitation, as described previously (41). In brief, in the hemorrhage and resuscitation model, animals were hemorrhaged to a mean arterial blood pressure (MAP) of 30 mmHg for 30 min. The hemorrhage blood volume to achieve MAP of 30 mmHg was similar in all groups (p > 0.05). At t = 30 min, CXCR4/7 modulators or vehicle (0.9% NaCl) were administered and the animals were then resuscitated with normal saline until MAP returned to 70 mmHg. MAP was then maintained at 70 mmHg by continuous fluid administration for a total of 45 min, as required. At the end of the resuscitation period, the animals were euthanized by rapid exsanguination, followed by thoracotomy resulting in bilateral pneumothorax. In the lethal hemorrhagic shock model, animals underwent withdrawal of 40% of the blood volume within 10 min. Blood was withdrawn at a rate of approximately 1 mL/min. At t = 15 min, CXCR4/7 modulators or vehicle were administered. Thereafter, 2% of the blood volume were withdrawn every 15 min until death, as defined by asystole or disappearance of a pulse pressure. Animals after administration of CXCR4/7 modulators under normal conditions were euthanized by exsanguination and bilateral pneumothorax at the end of the observation period while being under deep anesthesia.
Data Analyses
Data are described as mean ± SE. Data were analyzed with Student t-test, one-way analyses of variance (ANOVA) or two-way repeated measures (mixed model) analysis of variance and Bonferroni post hoc tests to correct for multiple testing, as appropriate. Best-fit values were compared with the extra sum-of-squares F test. Survival was plotted using the Kaplan-Meier method and survival between groups was compared with the log-rank test. All data were analyzed using the GraphPad Prism 5 software (GraphPad Software Inc., La Jolla, CA, USA). A two-tailed p < 0.05 was considered significant.
All supplementary materials are available online at www.molmed.org.
RESULTS
CXCR4 and ACKR3 Ligands Modulate Hemodynamics and Blood Pressure
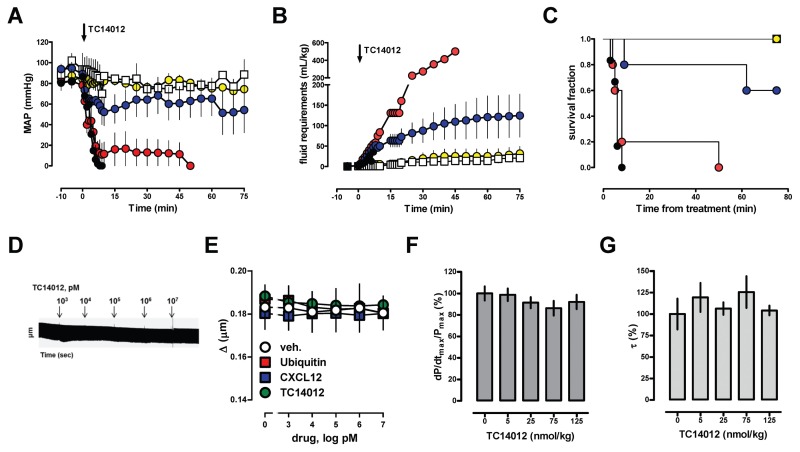
To assess whether modulation of CXCR4 and ACKR3 alters blood pressure in normal animals, we first studied the effects of CXCL12, the cognate CXCR4 and ACKR3 agonist (1), ubiquitin, a noncognate CXCR4 agonist without affinity for ACKR3 (1,18,42), AMD3100, a selective CXCR4 antagonist (43), and TC14012, a CXCR4 antagonist and ACKR3 agonist (44). As expected (19,21,45–47), CXCL12, AMD3100 and ubiquitin did not affect normal blood pressure or fluid requirements in rats when administered systemically in a dose of 350 nmol/kg (Supplementary Figure 1). Systemic administration of TC14012, however, resulted in cardiovascular collapse despite fluid resuscitation (Figures 1A–C). The deleterious effects of TC14012 on hemodynamics occurred dose dependently. TC14012 did not affect sarcomere length of isolated cardiomyocytes (Figures 1D, E) or left ventricular function in vivo (Figures 1F, G). The hemodynamic effects, however, severely compromised survival of normal animals and therefore precluded further in vivo testing of TC14012.
Figure 1.
The CXCR4 antagonist and ACKR3 agonist TC14012 causes cardiovascular collapse in normal animals. Arrows indicate the time point of drug injection, n = 5/group. Open squares: vehicle. Yellow circles: 7 nmol/kg. Blue circles: 35 nmol/kg. Red circles: 175 nmol/kg. Black circles: 350 nmol/kg. (A) Mean arterial blood pressure (MAP, mmHg). (B) Cumulative fluid requirements to maintain hemodynamics (mL/kg). (C) Kaplan-Meier survival curve (p < 0.001 between groups). (D,E) Left ventricular cardiomyocytes were field-stimulated (20 V, 2 Hz, 6-ms pulse duration) and changes in sarcomere length during contractions measured using a video-sarcomere detection system. (D) Representative recording of the sarcomere length upon addition of increasing concentrations of TC14012 (1 nmol/L to 10 μmol/L). Concentrations of TC14012 were increased every 5 min. (E) Changes in sarcomere length (Δμm) upon addition of CXCR4/ACKR3 ligands. Measurements of 60 steady state contractions were averaged for each dose; n = 3–5. (F) Increasing doses of TC14012 (5 to 125 nmol/kg) were injected every 5 min and left ventricular contractility (dP/dtmax/Pmax) and (G) isovolumic relaxation constants (τ[Mirsky]) analyzed 2 min after injection. Data are expressed as % of control; n = 3 to 5/group.
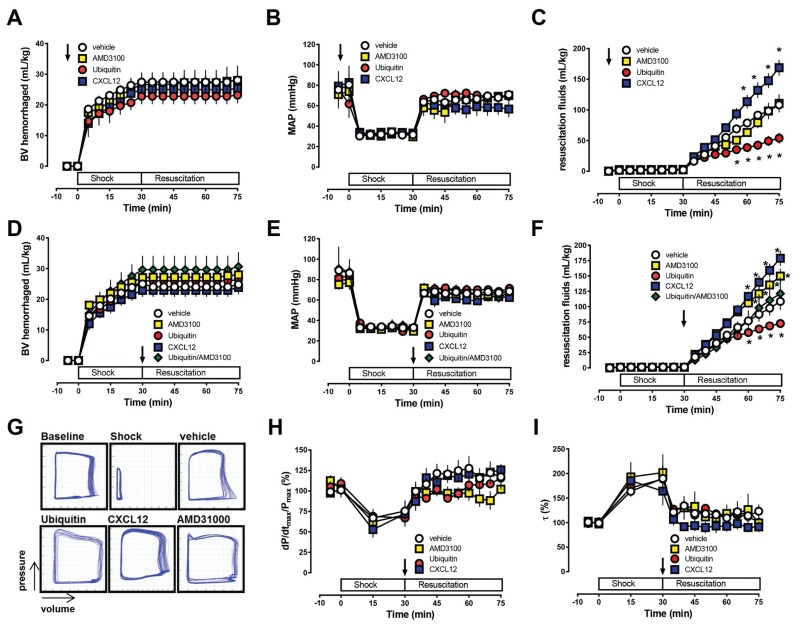
Next, we studied the effects of the CXCR4 and ACKR3 ligands that did not influence normal blood pressure in a hemorrhagic shock model. This model consisted of 30 min hemorrhage to a mean arterial blood pressure (MAP) of 30 mmHg followed by crystalloid fluid resuscitation to a MAP of 70 mmHg. When the same doses were administered 5 min before blood withdrawal, CXCR4 activation with ubiquitin reduced whereas coactivation of CXCR4 and ACKR3 with CXCL12 increased systemic fluid requirements to maintain MAP at the resuscitation target of 70 mmHg. AMD3100 pretreatment did not affect resuscitation fluid requirements in this model (Figures 2A–C). As pretreatment protocols lack translational relevance, we then tested the effects of the CXCR4 and ACKR3 ligands when administered at the end of the shock period, prior to fluid resuscitation (Figure 2D–F). Whereas CXCR4 activation with ubiquitin stabilized hemodynamics, coactivation of CXCR4 and ACKR3 with CXCL12 and blockade of CXCR4 with AMD3100 reduced hemodynamic stability, as estimated based on the systemic fluid requirements to maintain MAP. The effects of ubiquitin and AMD3100 were abolished when both CXCR4 ligands were coadministered. As observed for TC14012, none of the CXCR4/ACKR3 ligands affected sarcomere length of isolated cardiomyocytes (Figure 1E) or left-ventricular function in vivo (Figures 2G–I).
Figure 2.
CXCR4/ACKR3 ligands modulate hemodynamics during the cardiovascular stress response to hemorrhagic shock and resuscitation. Rats were hemorrhaged to a MAP of 30 mmHg for 30 min, followed by crystalloid resuscitation to maintain MAP of 70 mmHg. *: p < 0.05 versus vehicle. (A–C) CXCR4/ACKR3 ligands (350 nmol/kg) were injected 5 min before hemorrhage. Open circles: vehicle (n = 5); Red circles: ubiquitin (n = 5); Yellow squares: AMD3100 (n = 7). Blue squares: CXCL12 (n = 3). (A) Blood volume hemorrhaged (mL/kg). (B) MAP (mmHg). (C) Cumulative fluid requirements to maintain MAP at 70 mmHg (mL/kg). (D–F) CXCR4/ACKR3 ligands (350 nmol/kg) were injected at the end of the shock period (t = 30 min), prior to fluid resuscitation. Open circles: vehicle (n = 7); Red circles: ubiquitin (n = 7); Yellow squares: AMD3100 (n = 7). Blue squares: CXCL12 (n = 9). Green diamonds: ubiquitin plus AMD3100 (350 nmol/kg each; n = 6). (D) Blood volume hemorrhaged (mL/kg). (E) MAP (mmHg). (F) Cumulative fluid requirements to maintain MAP at 70 mmHg (mL/kg). (G) Representative left ventricular pressure-volume loops at baseline, during the shock period and 30 min after drug injection and fluid resuscitation. (H) Left ventricular contractility (dP/dtmax/Pmax, n = 3 to 5/group). (I) Isovolumic relaxation constants (τ[Mirsky], n = 3 to 5/group).
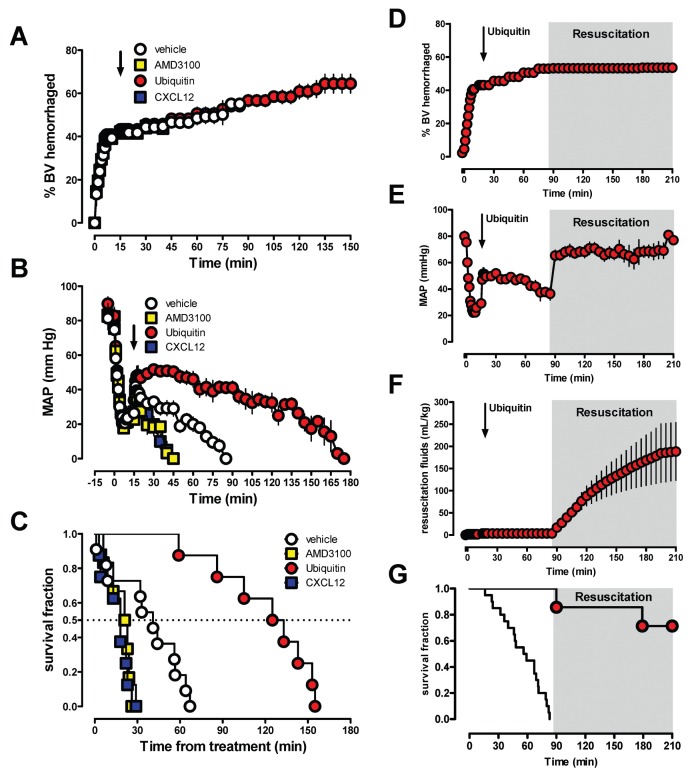
Because the resuscitation target of a MAP of 70 mmHg could be achieved in all animals, we then tested the effects of the CXCR4/ACKR3 ligands in a shock model without fluid resuscitation to be able to assess their direct effects on blood pressure regulation during the cardiovascular stress response to hemorrhage. In this fixed volume hemorrhage model, 40% of the blood volume were withdrawn within 10 min followed by an additional 2% blood volume hemorrhage every 15 min without intervention. The CXCR4/ACKR3 ligands or vehicle were administered at t = 15 min. As shown in Figures 3A–C, median survival time was 41 min with vehicle administration. CXCR4 activation with ubiquitin stabilized blood pressure and prolonged median survival time to 129 min (p < 0.001 versus vehicle, hazard ratio [95% confidence interval] 7.5 [2.9–18.8]). AMD3100 and CXCL12, however, reduced blood pressure and median survival time to 22 min (p = 0.0045 versus vehicle, hazard ratio [95% confidence interval] 0.12 [0.03–0.5]) and 18 min (p < 0.001 versus vehicle, hazard ratio [95% confidence interval] 0.11 [0.03–0.4]), respectively. To further assess whether the prolonged survival time after ubiquitin treatment could provide a therapeutic benefit, we then evaluated if a resuscitation target of MAP of 70 mmHg can be achieved when resuscitation is initiated at a time point when mortality was 100% in vehicle-treated animals. As shown in Figures 3D–G, in five out of seven animals with ubiquitin treatment, blood pressure could be restored to the resuscitation endpoint when crystalloid infusion was started at t = 85 min.
Figure 3.
CXCR4/ACKR3 ligands regulate blood pressure during the cardiovascular stress response to hemorrhage. Rats underwent 40% blood volume (BV) hemorrhage followed by 2% BV hemorrhage every 15 min without intervention. CXCR4/ACKR3 ligands (350 nmol/kg) were injected at t = 15 min (arrow). Open circles: vehicle (n = 19). Yellow squares: AMD3100 (n = 6). Red circles: ubiquitin (n = 8). Blue squares: CXCL12 (n = 8). (A) Blood volume (mL/kg) hemorrhaged. (B) MAP (mmHg). (C) Kaplan-Meier survival curve (dashed line: 50% survival; p < 0.0001 among groups). (D–G) Same hemorrhage model as in A–C. Animals were treated with 350 nmol/kg ubiquitin at t = 15 min (n = 7). At t = 85 min, crystalloid resuscitation to a MAP of 70 mmHg was started. (D) Blood volume (mL/kg) hemorrhaged. (E) MAP (mmHg). (F) Resuscitation fluid requirements (mL/kg). (G) Kaplan-Meier survival curve. For comparison, the line without symbols shows the survival curve from animals after vehicle treatment (from C).
CXCR4 and ACKR3 Agonists Have Opposing Effects on α1-AR-Mediated Vasoconstriction
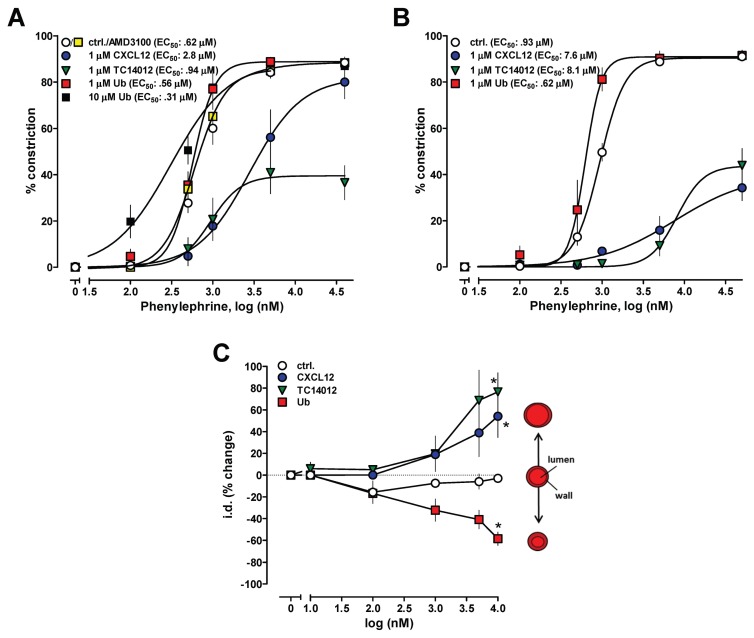
The observation that none of the CXCR4/ACKR3 ligands affected contractility of isolated cardiomyocytes or left ventricular function in vivo suggested that their effects on blood pressure are mediated through modulation of vascular function. Thus, we then utilized pressure myography to study the effects of various natural and synthetic CXCR4 and ACKR3 ligands on isolated mesenteric arteries and veins. The CXCR4/ACKR3 ligands did not affect the diameter of arteries in the absence of vasoconstrictors. Ubiquitin enhanced vascular reactivity to the selective α1-AR agonist phenylephrine, whereas CXCL12 and TC14012 reduced phenylephrine responsiveness (Figure 4A; p < 0.01 versus groups). AMD3100 did not affect phenylephrine responsiveness (see Figure 4A). In these experiments, CXCR4/ACKR3 ligands were administered to the organ bath; more robust effects of CXCL12 and TC14012 were observed when the CXCR4/ACKR3 modulators were administered intraluminally (Figure 4B; p < 0.01 versus groups). When arteries were constricted with the EC50 concentration of phenylephrine, addition of ubiquitin enhanced, whereas CXCL12 and TC14012 antagonized vasoconstriction in a dose-dependent manner (Figure 4C).
Figure 4.
(A,B) CXCR4/ACKR3 ligands regulate vasoconstriction upon α1-AR activation. Vasoconstriction was measured as change of the i.d. in pressure myography experiments. Mesenteric arteries were pressurized to 80 mmHg. *: p < 0.05 versus control (ctrl). Dose response to phenylephrine in the presence and absence of CXCR4/7 modulators (CXCL12, n = 4, ubiquitin [Ub], n = 5 [10 μmol/L] – 9 [1 μmol/L]; TC14012, n = 9; AMD3100, n = 6; vehicle, n = 15). Phenylephrine and CXCR4/7 modulators were (A) added to the organ bath or (B) administered intraluminally. Vasoconstriction is expressed as % of i.d. in the absence of phenylephrine. (C) Mesenteric arteries were constricted with the EC50 dose of phenylephrine (1 μmol/L) and the effects of the CXCR4/7 modulators tested. The effects of the CXCR4/7 modulators (n = 4 to 7) are expressed as % change of i.d.
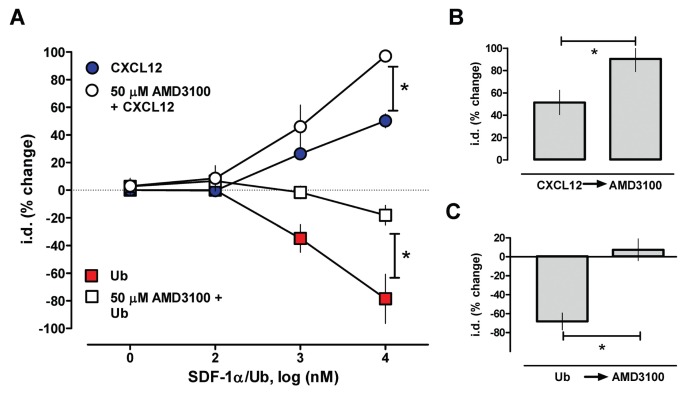
We next tested whether CXCR4- and ACKR3-mediated effects of CXCL12 can be differentiated by blockade of CXCR4 with AMD3100. Consistent with the opposing effects of CXCR4 and ACKR3, blockade of CXCR4 by AMD3100 enhanced the antagonistic effects of CXCL12 (Figures 5A, B) on phenylephrine-induced vasoconstriction. In contrast, AMD3100 antagonized the effects of ubiquitin on phenylephrine-induced vasoconstriction (Figures 5 A, C).
Figure 5.
Blockade of CXCR4 with AMD3100 antagonizes effects of ubiquitin (Ub) and enhances effects of CXCL12 on phenylephrine-induced vasoconstriction. Mesenteric arteries were constricted with the EC50 dose of phenylephrine (1 μmol/L) and the effects of the CXCR4/7 modulators tested. The effects of the CXCR4/7 modulators are expressed as % change of i.d. *: p < 0.05 versus control (ctrl). (A) Dose response of ubiquitin and CXCL12 in the presence and absence of 50 μmol/L AMD3100; n = 4. (B,C) Addition of AMD3100 (50 μmol/L) (B) enhances CXCL12-mediated effects and (C) antagonizes ubiquitin-mediated effects; n = 4.
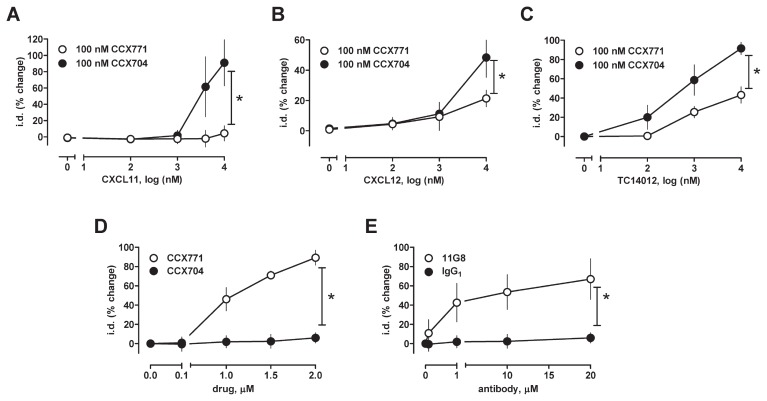
To further confirm the role of ACKR3, we then tested chemokine (C-X-C motif) ligand 11 (CXCL11, interferon-inducible T cell α chemoattractant), an alternative natural agonist of ACKR3 that does not bind or activate CXCR4 (48), and CCX771, a small molecule ACKR3 ligand, which has often been described as an antagonist at ACKR3 (25). As shown in Figure 6A, CXCL11 also antagonized phenylephrine-induced vasoconstriction and its effects could be antagonized with 100 nmol/L CCX771. CCX771 (100 nmol/L) also antagonized the effects CXCL12 (see Figure 4B) and TC14012 (Figure 4C) on phenylephrine-induced vasoconstriction. CCX704, an inactive analogue of CCX771, did not affect the effects of the ACKR3 agonists (Figures 6A–C). At higher concentrations (1 to 2 μmol/L), however, CCX771 alone antagonized vasoconstriction in response to phenylephrine (Figure 6D), whereas CCX704 was inactive. The specific ACKR3 antibody 11G8 (49) also antagonized phenylephrine-induced vasoconstriction, whereas a nonspecific IgG1 isotype control antibody was inactive (Figure 6E). The receptor selectivity of the various CXCR4 and ACKR3 ligands and their effects on phenylephrine-induced vasoconstriction are summarized in Table 1.
Figure 6.
ACKR3 ligands function as agonists with varying efficacy. Mesenteric arteries were constricted with the EC50 dose of phenylephrine (1 μmol/L) and the effects of the CXCR4/7 modulators tested. The effects of the CXCR4/7 modulators are expressed as % change of i.d. *: p < 0.05 versus control (ctrl). (A–C) Dose-dependent effects of (A) CXCL11, (B) CXCL12 and (C)TC14012 in the presence and absence of 100 nmol/L of the ACKR3 ligand CCX771 and CCX704 (control, inactive analogue of CCX771); n = 3 to 6. (D) Dose-dependent effects of the ACKR3 ligand CXC771; CCX704 was used as a control; n = 3. (E) Dose-dependent effects of the ACKR3 antibody 11G8; IgG1 was used as a control. n = 4. Ub: ubiquitin.
Table 1.
Receptor selectivity of CXCR4 and ACKR3 ligands and their effects on α1-AR-mediated vasoconstriction of isolated mesenteric arteries.
| Ligand | Receptor selectivity | Effect on vascular α1-AR reactivity | ||
|---|---|---|---|---|
|
| ||||
| CXCR4 | ACKR3 | References | ||
| CXCL11 | NB | + | (1,48) | ↓ |
| CXCL12 | + | + | (1,6,7) | ↓ |
| TC14012 | − | + | (44) | ↓ |
| 11G8 | NB | + | (49) | ↓ |
| CCX771 | NB | + | (25) | ↓ |
| AMD3100 | − | (+) | (1,67,68) | ~ |
| Ubiquitin | + | NB | (1,18,42) | ↑ |
NB, not binding; +, agonist; (+), weak allosteric agonist; −, antagonist; ↓, antagonizes phenylephrine-induced vasoconstriction; ↑, enhances phenylephrine-induced vasoconstriction; ~, no effect on phenylephrine-induced vasoconstriction.
Effects of CXCR4 and ACKR3 Ligands Are Specific and Independent of the Vascular Endothelium
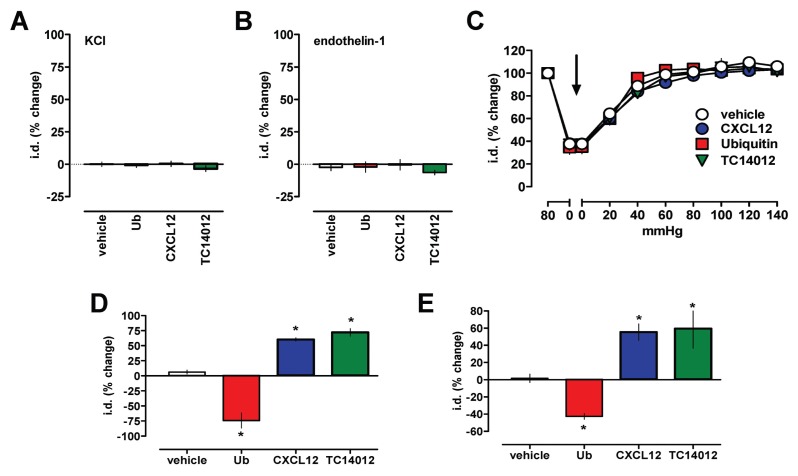
To assess the specificity of the observed effects of the CXCR4 and ACKR3 ligands on vascular reactivity, we next tested their effects on vasoconstriction induced via activation of voltage-operated Ca2+ channels by KCl or vasoconstriction mediated through the GPCR endothelin receptor. None of the CXCR4 and ACKR3 agonists, however, influenced vasoconstriction induced by KCl (Figure 7A) or endothelin-1 (Figure 7B).
Figure 7.
Effects of CXCR4/ACKR3 ligands on vascular α1-AR reactivity are specific. Vasoconstriction was measured as change of the i.d. in pressure myography experiments. *: p < 0.05 versus control. (A,B) Arteries were constricted to 40% to 60% of i.d. with (A) 60 mmol/L KCl (n = 3 to 4), (B) 800 pmol/L endothelin-1 (n = 3 to 6) and the effects of CXCR4/ACKR3 ligands (10 μmol/L) tested. The effects of the CXCR4/ACKR3 modulators are expressed as % change of i.d. (C) CXCR4/ACKR3 modulators do not affect the i.d. of mesenteric arteries at various transmural pressures; n = 4 to 8. Arrow: Addition of CXCR4/ACKR3 modulators, 10 μmol/L. (D) Mesenteric arteries pressurized to 30 mmHg were constricted with the EC50 dose of phenylephrine (1 μmol/L) and the effects of the CXCR4/ACKR3 modulators (10 μmol/L) tested; n = 3. (E) Mesenteric veins pressurized to 12 mmHg were constricted with the EC50 dose of phenylephrine (1 μmol/L) and the effects of the CXCR4/ACKR3 modulators (10 μmol/L) tested; n = 3 to 6.
To further characterize the vascular effects of the CXCR4 and ACKR3 ligands, we then tested the influence of the transmural vascular pressure in pressure myography experiments. The CXCR4 and ACKR3 ligands did not affect the diameter of arteries in the absence of vasoconstrictors at various transmural pressures (Figure 7C). As observed for arteries pressurized to 80 mmHg, the CXCR4/ACKR3 ligands also modulated phenylephrine-induced vasoconstriction in arteries maintained at a low transmural pressure (30 mmHg, Figure 7D) and in mesenteric veins pressurized to 12 mmHg (Figure 7E).
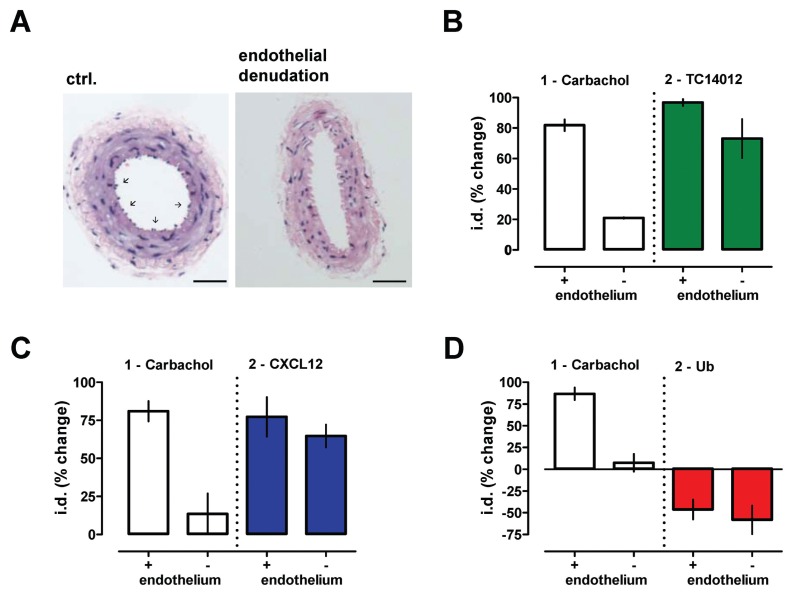
Because the endothelium plays important roles in the regulation of vascular function, we then evaluated the effects of the CXCR4/ACKR3 agonists on phenylephrine-mediated vasoconstriction in mesenteric arteries after mechanical endothelial denudation (Figure 8A). Endothelial denudation abolished the effects of the endothelium-dependent vasodilator carbachol on phenylephrine-induced vasoconstriction, but did not significantly influence the modulatory actions of TC14012 (Figure 8B), CXCL12 (Figure 8C) and ubiquitin (Figure 8D).
Figure 8.
Effects of CXCR4/ACKR3 ligands on vascular α1-AR reactivity do not depend on the endothelium. (A) Histology (H&E staining) of normal mesenteric arteries (left) and arteries after mechanical endothelial denudation (right). Arrows indicate endothelium. Scale bars: 100 μm. (B–D) Mesenteric arteries with (+) and without (−) endothelium were first constricted with the EC50 dose of phenylephrine (1 μmol/L), followed by addition of the endothelium-dependent vasodilator carbachol (10 μmol/L). Following washout of phenylephrine and carbachol, arteries were reexposed to 1 μmol/L phenylephrine and the effects of (B) TC14012, (C) CXCL12 and (D) ubiquitin (Ub) tested; 10 μmol/L each, n = 3. The effects of the CXCR4/ACKR3 modulators are expressed as % change of i.d.
DISCUSSION
In the present study, we demonstrate that pharmacological modulation of CXCR4 and ACKR3 in vivo influences blood pressure in normal and pathological conditions. Furthermore, our findings suggest that activation of CXCR4 and ACKR3 have opposing effects on α1-AR-mediated vasoconstriction of isolated vessels, which are independent of the endothelium. While CXCR4 activation enhances vascular α1-AR responsiveness, ACKR3 activation results in opposite effects. In combination with the observation that the CXCR4 and ACKR3 ligands did not directly affect cardiac function, our findings imply that CXCR4 and ACKR3 regulate cardiovascular function through their modulatory actions on vascular smooth muscle reactivity.
The observed in vivo actions of TC14012 indicate that simultaneous blockade of CXCR4 and activation of ACKR3 causes cardiovascular collapse in normal animals. As the natural CXCR4 and ACKR3 ligands CXCL11, CXCL12 and ubiquitin are constitutively expressed in normal plasma (50–52), these data imply that CXCR4 and ACKR3 may act as an intricate control system that regulates vascular function and blood pressure under normal conditions.
The findings that blockade of CXCR4 with AMD3100 and coactivation of CXCR4 and ACKR3 with CXCL12 impaired blood pressure during the cardiovascular stress response to hemorrhage, whereas selective CXCR4 activation with ubiquitin-stabilized blood pressure is consistent with the effects of CXCR4 and ACKR3 activation on vascular phenylephrine responsiveness ex vivo. In combination with previously described effects of ubiquitin in various shock models (19–21,45), these data indicate that selective CXCR4 activation stabilizes blood pressure during the cardiovascular stress response to hemorrhagic shock, providing an extended therapeutic window during which blood pressure can be restored with fluid resuscitation.
Ubiquitin is known to be cosecreted with catecholamines from chromaffin cells of the adrenal gland (53). In connection with the findings from the present study, these data point toward a physiological linkage between α1-AR and CXCR4 activation during the fight-or-flight response.
The observation that AMD3100 did not affect blood pressure and fluid requirements when administered as a pretreatment in the hemorrhagic shock model can be explained by its pharmacological properties. After systemic administration, the volume of distribution of AMD3100 is small, its half-life in rats is approximately 60 min and binding of AMD3100 to CXCR4, unlike binding of CXCL12 and ubiquitin to CXCR4, does not result in ligand-induced receptor internalization (14,43,54). Thus, in our experiments, withdrawal of blood removed large proportions of AMD3100 from the systemic circulation and subsequent fluid resuscitation further reduced the remaining drug concentrations. In addition, the finding that AMD3100 did not affect phenylephrine-induced vasoconstriction of isolated arteries is consistent with the absence of natural agonists in this model system.
The biological properties that we observed for CXCL12 are in agreement with the much higher affinity of CXCL12 for ACKR3, as compared with its affinity for CXCR4 (7). Furthermore, pharmacological blockade of CXCR4 with AMD3100 and partial blockade of ACKR3 with CCX771 permitted differentiation of the opposing effects of CXCR4 and ACKR3 upon activation with CXCL12. The finding that AMD3100 was able to block the effects of ubiquitin is in agreement with the known receptor selectivity of ubiquitin (42) and the observed effects after in vivo coadministration of both CXCR4 ligands in the present study.
CXCL11 is known to be highly induced following endotoxin and interferon exposure (55). Therefore, it appears likely that CXCL11 may contribute to the development of vasodilatory shock and catecholamine refractoriness, which is characteristic for endotoxic and septic shock.
All natural, synthetic and antibody ACKR3 ligands that we tested attenuated phenylephrine responsiveness of arteries, which indicates that these ligands function as receptor agonists with varying efficacy. Nevertheless, the synthetic ACKR3 ligand CCX771 acted as a low efficacy agonist that partially antagonized the effects of the endogenous ligands CXCL11 and CXCL12. A more detailed pharmacological exploration of the role of ACKR3 in the regulation of cardiovascular function is currently not possible because ACKR3 ligands without intrinsic activity are not available. While genetic models may be useful to dissect the mechanistic basis for our observations, such studies, however, would lack translational relevance and would not provide preclinical evidence for possible new therapeutic opportunities. Thus, the development of ACKR3 antagonists without intrinsic activity will be important to provide a more detailed evaluation of the role of ACKR3 as a therapeutic target.
The finding that the CXCR4 and ACKR3 ligands did not affect the diameter of arteries in the absence of vasoconstrictors at various transmural pressures suggests that they do not affect myogenic tone. The observation that endothelin-1 or KCl-induced vasoconstriction was not affected by CXCR4/ACKR3 ligands documents specificity for α1-AR-mediated functions. As CXCR4 and ACKR3 ligands also modulated vascular reactivity at low transmural pressures and in mesenteric veins, these data indicate that CXCR4 and ACKR3 ligands modulate effects of α1-AR on vascular resistance and capacitance over a wide range of vascular pressures.
CONCLUSION
Our findings identify CXCR4 and ACKR3 as regulators of vascular α1-AR function and provide evidence for a new physiological role of chemokines beyond their known biological properties, the regulation of vascular function. We currently cannot exclude that CXCR4 or ACKR3 activation affects α1-AR receptor expression. Such effects could explain our observations on phenylephrine reactivity of isolated arteries when arteries were pretreated with CXCR4 and ACKR3 ligands, but are unlikely to account for the enhancing and antagonizing effects of the CXCR4 and ACKR3 agonists on vasoconstriction in response to the EC50 dose of phenylephrine, respectively. Regulation of GPCR function is complex and cross-talk between GPCR-signaling pathways, whereby activation of one receptor system positively or negatively affects the signaling of another signaling receptor system in the same cell, is a well-recognized principle that permits fine tuning of GPCR functions (56–61). Receptor cross-talk has been attributed to various molecular mechanisms, such as interactions between intracellular signal transduction pathways, receptor transactivation or receptor hetero-oligomerization (56–63). The formation of homo-and/or heteromers between GPCRs is known to be important for many aspects of GPCR function (64). α1a-AR and α1b-AR have been reported to exist as homo- and hetero-oligomers, which is thought to provide a mechanism that regulates their physiological responses (65). Interestingly, α1a-AR have recently been described to colocalize with CXCR2 in the smooth muscle layer of prostate tissue and to form heteromeric complexes with CXCR2 in a recombinant system, resulting in signaling events distinct from the mono-/homomers (66). Thus, it appears possible that heteromeric complexes between α1a-AR and CXCR2, CXCR4, ACKR3 or other chemokine receptors also exist in vascular smooth muscle, which may modulate vascular α1-AR function.
Although the exact molecular events underlying the regulatory functions of CXCR4 and ACKR3 on vascular α1-AR reactivity remain to be determined, our findings provide initial mechanistic insights, which explain pharmacological effects of CXCR4 and ACKR3 ligands on cardiovascular function and point toward CXCR4 and ACKR3 as new therapeutic targets to regulate vascular function and blood pressure. This may enable development of new classes of drugs for the treatment of a broad variety of diseases that are associated with impaired vascular function, such as hypertension, pulmonary arterial hypertension, vasodilatory shock or catecholamine refractoriness in critical illness.
Supplemental Data
ACKNOWLEDGMENTS
The authors thank Heather M La Porte for technical help and P de Tombe, X Ji, S Sadayappan, and R. Tiniakov, Loyola University Chicago, for help with myocardial function analyses. This research was made possible, in part, by a grant that was awarded and administered by the U.S. Army Medical Research & Materiel Command (USAMRMC) and the Telemedicine and Advanced Technology Research Center (TATRC), at Fort Detrick, MD, USA, under contract number W81XWH1020122. The views, opinions and/or findings contained in this research are those of the author(s) and do not necessarily reflect the views of the Department of Defense and should not be construed as an official DoD/Army position, policy or decision unless so designated by other documentation. No official endorsement should be made. This research was also supported, in part, by grants from the American Heart Association (13GRNT17230072), the NIH (T32GM008750) and the Dr. Ralph and Marian Falk Medical Research Trust.
Footnotes
Online address: http://www.molmed.org
DISCLOSURES
The therapeutic use of ubiquitin has been patented (US patent #7,262,162). M Majetschak is an inventor and has not received any income related to the patent.
REFERENCES
- 1.Bachelerie F, et al. International Union of Basic and Clinical Pharmacology. [corrected]. LXXXIX. Update on the extended family of chemokine receptors and introducing a new nomenclature for atypical chemokine receptors. Pharmacol Rev. 2013;66:1–79. doi: 10.1124/pr.113.007724. erratum 66:467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Tachibana K, et al. The chemokine receptor CXCR4 is essential for vascularization of the gastrointestinal tract. Nature. 1998;393:591–4. doi: 10.1038/31261. [DOI] [PubMed] [Google Scholar]
- 3.Gerrits H, et al. Early postnatal lethality and cardiovascular defects in CXCR7-deficient mice. Genesis. 2008;46:235–45. doi: 10.1002/dvg.20387. [DOI] [PubMed] [Google Scholar]
- 4.Nagasawa T, et al. Defects of B-cell lymphopoiesis and bone-marrow myelopoiesis in mice lacking the CXC chemokine PBSF/SDF-1. Nature. 1996;382:635–8. doi: 10.1038/382635a0. [DOI] [PubMed] [Google Scholar]
- 5.Sierro F, et al. Disrupted cardiac development but normal hematopoiesis in mice deficient in the second CXCL12/SDF-1 receptor, CXCR7. Proc. Natl. Acad. Sci. U. S. A. 2007;104:14759–64. doi: 10.1073/pnas.0702229104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bleul CC, et al. The lymphocyte chemoattractant SDF-1 is a ligand for LESTR/fusin and blocks HIV-1 entry. Nature. 1996;382:829–33. doi: 10.1038/382829a0. [DOI] [PubMed] [Google Scholar]
- 7.Balabanian K, et al. The chemokine SDF-1/CXCL12 binds to and signals through the orphan receptor RDC1 in T lymphocytes. J. Biol. Chem. 2005;280:35760–6. doi: 10.1074/jbc.M508234200. [DOI] [PubMed] [Google Scholar]
- 8.Teicher BA, Fricker SP. CXCL12 (SDF-1)/CXCR4 pathway in cancer. Clin Cancer Res. 2010;16:2927–31. doi: 10.1158/1078-0432.CCR-09-2329. [DOI] [PubMed] [Google Scholar]
- 9.Nagasawa T, Tachibana K, Kishimoto T. A novel CXC chemokine PBSF/SDF-1 and its receptor CXCR4: their functions in development, hematopoiesis and HIV infection. Semin Immunol. 1998;10:179–85. doi: 10.1006/smim.1998.0128. [DOI] [PubMed] [Google Scholar]
- 10.Rajagopal S, et al. Beta-arrestin- but not G protein-mediated signaling by the “decoy” receptor CXCR7. Proc. Natl. Acad. Sci. U. S. A. 2010;107:628–32. doi: 10.1073/pnas.0912852107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Boldajipour B, et al. Control of chemokine-guided cell migration by ligand sequestration. Cell. 2008;132:463–73. doi: 10.1016/j.cell.2007.12.034. [DOI] [PubMed] [Google Scholar]
- 12.Kumar R, et al. CXCR7 mediated Giα independent activation of ERK and Akt promotes cell survival and chemotaxis in T cells. Cell Immunol. 2012;272:230–41. doi: 10.1016/j.cellimm.2011.09.015. [DOI] [PubMed] [Google Scholar]
- 13.Regard JB, Sato IT, Coughlin SR. Anatomical profiling of G protein-coupled receptor expression. Cell. 2008;135:561–71. doi: 10.1016/j.cell.2008.08.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tripathi A, Davis JD, Staren DM, Volkman BF, Majetschak M. CXC chemokine receptor 4 signaling upon co-activation with stromal cell-derived factor-1alpha and ubiquitin. Cytokine. 2013;65:121–5. doi: 10.1016/j.cyto.2013.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.LaRocca TJ, et al. β2-Adrenergic receptor signaling in the cardiac myocyte is modulated by interactions with CXCR4. J. Cardiovasc. Pharmacol. 2010;56:548–59. doi: 10.1097/FJC.0b013e3181f713fe. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Agarwal U, et al. Role of cardiac myocyte CXCR4 expression in development and left ventricular remodeling after acute myocardial infarction. Circ Res. 2010;107:667–76. doi: 10.1161/CIRCRESAHA.110.223289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Levoye A, Balabanian K, Baleux F, Bachelerie F, Lagane B. CXCR7 heterodimerizes with CXCR4 and regulates CXCL12-mediated G protein signaling. Blood. 2009;113:6085–93. doi: 10.1182/blood-2008-12-196618. [DOI] [PubMed] [Google Scholar]
- 18.Saini V, Marchese A, Majetschak M. CXC chemokine receptor 4 is a cell surface receptor for extracellular ubiquitin. J. Biol. Chem. 2010;285:15566–76. doi: 10.1074/jbc.M110.103408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Majetschak M, Cohn SM, Nelson JA, Burton EH, Obertacke U, Proctor KG. Effects of exogenous ubiquitin in lethal endotoxemia. Surgery. 2004;135:536–43. doi: 10.1016/j.surg.2003.09.006. [DOI] [PubMed] [Google Scholar]
- 20.Baker TA, Romero J, Bach HHt, Strom JA, Gamelli RL, Majetschak M. Effects of exogenous ubiquitin in a polytrauma model with blunt chest trauma. Crit Care Med. 2012;40:2376–84. doi: 10.1097/CCM.0b013e3182514ed9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bach HH, Iv, Saini V, Baker TA, Tripathi A, Gamelli RL, Majetschak M. Initial assessment of the role of CXC chemokine receptor 4 after polytrauma. Mol Med. 2012;18:1056–66. doi: 10.2119/molmed.2011.00497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bodart V, et al. Pharmacology of AMD3465: a small molecule antagonist of the chemokine receptor CXCR4. Biochem Pharmacol. 2009;78:993–1000. doi: 10.1016/j.bcp.2009.06.010. [DOI] [PubMed] [Google Scholar]
- 23.Chu PY, et al. CXCR4 antagonism attenuates the cardiorenal consequences of mineralocorticoid excess. Circ Heart Fail. 2011;4:651–8. doi: 10.1161/CIRCHEARTFAILURE.110.960831. [DOI] [PubMed] [Google Scholar]
- 24.Yu L, Hales CA. Effect of chemokine receptor CXCR4 on hypoxia-induced pulmonary hypertension and vascular remodeling in rats. Respir. Res. 2011;12:21. doi: 10.1186/1465-9921-12-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zabel BA, et al. Elucidation of CXCR7-mediated signaling events and inhibition of CXCR4-mediated tumor cell transendothelial migration by CXCR7 ligands. J Immunol. 2009;183:3204–11. doi: 10.4049/jimmunol.0900269. [DOI] [PubMed] [Google Scholar]
- 26.Sartina E, et al. Antagonism of CXCR7 attenuates chronic hypoxia-induced pulmonary hypertension. Pediatr Res. 2012;71:682–8. doi: 10.1038/pr.2012.30. [DOI] [PubMed] [Google Scholar]
- 27.Veldkamp CT, Seibert C, Peterson FC, Sakmar TP, Volkman BF. Recognition of a CXCR4 sulfotyrosine by the chemokine stromal cell-derived factor-1alpha (SDF-1alpha/CXCL12) J. Mol. Biol. 2006;359:1400–9. doi: 10.1016/j.jmb.2006.04.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Veldkamp CT, et al. Structural basis of CXCR4 sulfotyrosine recognition by the chemokine SDF-1/CXCL12. Sci. Signal. 2008;1:ra4. doi: 10.1126/scisignal.1160755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Veldkamp CT, et al. Monomeric structure of the cardioprotective chemokine SDF-1/CXCL12. Protein Sci. 2009;18:1359–69. doi: 10.1002/pro.167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Takekoshi T, Ziarek JJ, Volkman BF, Hwang ST. A locked, dimeric CXCL12 variant effectively inhibits pulmonary metastasis of CXCR4-expressing melanoma cells due to enhanced serum stability. Mol Cancer Ther. 2012;11:2516–25. doi: 10.1158/1535-7163.MCT-12-0494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.(1985) Bacterial endotoxins/pyrogens [Internet] Silver Spring (MD): FDA; [cited 2014 Aug 29]. (Inspection technical guide). Available from: http://www.fda.gov/ICECI/Inspections/InspectionGuides/InspectionTechnicalGuides/ucm072918.htm (web page last updated 2009 Feb 17) [Google Scholar]
- 32.Committee for the Update of the Guide for the Care and Use of Laboratory Animals, Institute for Laboratory Animal Research, Division on Earth and Life Studies, National Research Council of the National Academies. Guide for the Care and Use of Laboratory Animals. 8th edition. Washington (DC): National Academies Press; 2011. [Google Scholar]
- 33.Henderson KK, Byron KL. Vasopressin-induced vasoconstriction: two concentration-dependent signaling pathways. J. Appl. Physiol. 2007;102:1402–9. doi: 10.1152/japplphysiol.00825.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Brueggemann LI, Mackie AR, Mani BK, Cribbs LL, Byron KL. Differential effects of selective cyclooxygenase-2 inhibitors on vascular smooth muscle ion channels may account for differences in cardiovascular risk profiles. Mol Pharmacol. 2009;76:1053–61. doi: 10.1124/mol.109.057844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Brueggemann LI, Mani BK, Haick J, Byron KL. Exploring arterial smooth muscle Kv7 potassium channel function using patch clamp electrophysiology and pressure myography. J. Vis. Exp. 2012;14:e4263. doi: 10.3791/4263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Mani BK, Brueggemann LI, Cribbs LL, Byron KL. Activation of vascular KCNQ (Kv7) potassium channels reverses spasmogen-induced constrictor responses in rat basilar artery. Br. J. Pharmacol. 2011;164:237–49. doi: 10.1111/j.1476-5381.2011.01273.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Dow JW, Harding NG, Powell T. Isolated cardiac myocytes. I. Preparation of adult myocytes and their homology with the intact tissue. Cardiovasc Res. 1981;15:483–514. doi: 10.1093/cvr/15.9.483. [DOI] [PubMed] [Google Scholar]
- 38.Govindan S, et al. Pathogenic properties of the N-terminal region of cardiac myosin binding protein-C in vitro. J. Muscle Res. Cell Motil. 2012;33:17–30. doi: 10.1007/s10974-012-9292-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Jin CZ, et al. Myofilament Ca desensitization mediates positive lusitropic effect of neuronal nitric oxide synthase in left ventricular myocytes from murine hypertensive heart. J. Mol. Cell. Cardiol. 2013;60:107–15. doi: 10.1016/j.yjmcc.2013.04.017. [DOI] [PubMed] [Google Scholar]
- 40.Geng Q, et al. A subset of 26S proteasomes is activated at critically low ATP concentrations and contributes to myocardial injury during cold ischemia. Biochem. Biophys. Res. Commun. 2009;390:1136–41. doi: 10.1016/j.bbrc.2009.10.067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Bach HHt, Laporte HM, Wong YM, Gamelli RL, Majetschak M. Proteasome inhibition prolongs survival during lethal hemorrhagic shock in rats. J Trauma Acute Care Surg. 2013;74:499–507. doi: 10.1097/TA.0b013e31827d5db2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Saini V, et al. The CXC chemokine receptor 4 ligands ubiquitin and stromal cell-derived factor-1α function through distinct receptor interactions. J. Biol. Chem. 2011;286:33466–77. doi: 10.1074/jbc.M111.233742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hatse S, Princen K, Bridger G, De Clercq E, Schols D. Chemokine receptor inhibition by AMD3100 is strictly confined to CXCR4. FEBS Lett. 2002;527:255–62. doi: 10.1016/s0014-5793(02)03143-5. [DOI] [PubMed] [Google Scholar]
- 44.Gravel S, et al. The peptidomimetic CXCR4 antagonist TC14012 recruits beta-arrestin to CXCR7: roles of receptor domains. J. Biol. Chem. 2010;285:37939–43. doi: 10.1074/jbc.C110.147470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Majetschak M, Cohn SM, Obertacke U, Proctor KG. Therapeutic potential of exogenous ubiquitin during resuscitation from severe trauma. J Trauma. 2004;56:991–9. doi: 10.1097/01.ta.0000127770.29009.5a. [DOI] [PubMed] [Google Scholar]
- 46.O’Boyle G, Mellor P, Kirby JA, Ali S. Anti-inflammatory therapy by intravenous delivery of non-heparan sulfate-binding CXCL12. FASEB J. 2009;23:3906–16. doi: 10.1096/fj.09-134643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Misra P, et al. Quantitation of CXCR4 expression in myocardial infarction using 99mTc-labeled SDF-1alpha. J. Nucl. Med. 2008;49:963–9. doi: 10.2967/jnumed.107.050054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Burns JM, et al. A novel chemokine receptor for SDF-1 and I-TAC involved in cell survival, cell adhesion, and tumor development. J. Exp. Med. 2006;203:2201–13. doi: 10.1084/jem.20052144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Berahovich RD, Penfold ME, Schall TJ. Nonspecific CXCR7 antibodies. Immunol Lett. 2010;133:112–4. doi: 10.1016/j.imlet.2010.06.010. [DOI] [PubMed] [Google Scholar]
- 50.Majetschak M. Extracellular ubiquitin: immune modulator and endogenous opponent of damage-associated molecular pattern molecules. J. Leukoc. Biol. 2011;89:205–19. doi: 10.1189/jlb.0510316. [DOI] [PubMed] [Google Scholar]
- 51.Butera D, et al. Plasma chemokine levels correlate with the outcome of antiviral therapy in patients with hepatitis C. Blood. 2005;106:1175–82. doi: 10.1182/blood-2005-01-0126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Derdeyn CA, et al. Correlation between circulating stromal cell-derived factor 1 levels and CD4+ cell count in human immunodeficiency virus type 1-infected individuals. AIDS Res. Hum. Retroviruses. 1999;15:1063–71. doi: 10.1089/088922299310359. [DOI] [PubMed] [Google Scholar]
- 53.Kieffer AE, et al. The N- and C-terminal fragments of ubiquitin are important for the antimicrobial activities. Faseb J. 2003;17:776–8. doi: 10.1096/fj.02-0699fje. [DOI] [PubMed] [Google Scholar]
- 54.Hendrix CW, et al. Pharmacokinetics and safety of AMD-3100, a novel antagonist of the CXCR-4 chemokine receptor, in human volunteers. Antimicrob Agents Chemother. 2000;44:1667–73. doi: 10.1128/aac.44.6.1667-1673.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Widney DP, Xia YR, Lusis AJ, Smith JB. The murine chemokine CXCL11 (IFN-inducible T cell alpha chemoattractant) is an IFN-gamma-and lipopolysaccharide-inducible glucocorticoid-attenuated response gene expressed in lung and other tissues during endotoxemia. J Immunol. 2000;164:6322–31. doi: 10.4049/jimmunol.164.12.6322. [DOI] [PubMed] [Google Scholar]
- 56.Moore CA, Milano SK, Benovic JL. Regulation of receptor trafficking by GRKs and arrestins. Annu. Rev. Physiol. 2007;69:451–82. doi: 10.1146/annurev.physiol.69.022405.154712. [DOI] [PubMed] [Google Scholar]
- 57.Busillo JM, Benovic JL. Regulation of CXCR4 signaling. Biochim. Biophys. Acta. 2007;1768:952–63. doi: 10.1016/j.bbamem.2006.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Marchese A, Chen C, Kim YM, Benovic JL. The ins and outs of G protein-coupled receptor trafficking. Trends Biochem Sci. 2003;28:369–76. doi: 10.1016/S0968-0004(03)00134-8. [DOI] [PubMed] [Google Scholar]
- 59.Rockman HA, Koch WJ, Lefkowitz RJ. Seven-transmembrane-spanning receptors and heart function. Nature. 2002;415:206–12. doi: 10.1038/415206a. [DOI] [PubMed] [Google Scholar]
- 60.Diviani D, et al. Effect of different G protein-coupled receptor kinases on phosphorylation and desensitization of the alpha1B-adrenergic receptor. J. Biol. Chem. 1996;271:5049–58. doi: 10.1074/jbc.271.9.5049. [DOI] [PubMed] [Google Scholar]
- 61.Collins S, Bouvier M, Lohse MJ, Benovic JL, Caron MG, Lefkowitz RJ. Mechanisms involved in adrenergic receptor desensitization. Biochem. Soc. Trans. 1990;18:541–4. doi: 10.1042/bst0180541. [DOI] [PubMed] [Google Scholar]
- 62.McGraw DW, et al. Airway smooth muscle prostaglandin-EP1 receptors directly modulate beta2-adrenergic receptors within a unique heterodimeric complex. J. Clin. Invest. 2006;116:1400–9. doi: 10.1172/JCI25840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Dzimiri N. Receptor crosstalk. Implications for cardiovascular function, disease and therapy. Eur. J. Biochem. 2002;269:4713–30. doi: 10.1046/j.1432-1033.2002.03181.x. [DOI] [PubMed] [Google Scholar]
- 64.Milligan G, Canals M, Pediani JD, Ellis J, Lopez-Gimenez JF. The role of GPCR dimerisation/oligomerisation in receptor signalling. Ernst Schering Found. Symp. Proc. 2006;2:145–61. doi: 10.1007/2789_2006_007. [DOI] [PubMed] [Google Scholar]
- 65.Stanasila L, Perez JB, Vogel H, Cotecchia S. Oligomerization of the alpha 1a- and alpha 1b-adrenergic receptor subtypes. Potential implications in receptor internalization. J. Biol. Chem. 2003;278:40239–51. doi: 10.1074/jbc.M306085200. [DOI] [PubMed] [Google Scholar]
- 66.Mustafa S, et al. Identification and profiling of novel alpha1A-adrenoceptor-CXC chemokine receptor 2 heteromer. J. Biol. Chem. 2012;287:12952–65. doi: 10.1074/jbc.M111.322834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Schols D, Struyf S, Van Damme J, Este JA, Henson G, De Clercq E. Inhibition of T-tropic HIV strains by selective antagonization of the chemokine receptor CXCR4. J. Exp. Med. 1997;186:1383–8. doi: 10.1084/jem.186.8.1383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Kalatskaya I, Berchiche YA, Gravel S, Limberg BJ, Rosenbaum JS, Heveker N. AMD3100 is a CXCR7 ligand with allosteric agonist properties. Mol Pharmacol. 2009;75:1240–7. doi: 10.1124/mol.108.053389. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.