Abstract
Although androgen receptor (AR) signaling is the main molecular tool regulating growth and function of the prostate gland, estrogen receptor β (ERβ) is involved in the differentiation of prostatic epithelial cells and numerous antiproliferative actions on prostate cancer cells. However, ERβ splice variants have been associated with prostate cancer initiation and progression mechanisms. ERβ is promising as an anticancer therapy and in the prevention of prostate cancer. Herein, we review the recent experimental findings of ERβ signaling in the prostate.
INTRODUCTION
More than half century ago, Nobelists Huggins and Hodges provided clinical evidence that hormones can influence the development of prostate tumors, suggesting that androgens promote tumor growth and estrogens inhibit it (1). Since this innovative work, medical or surgical castration with antiestrogens remains the basic treatment for advanced prostate cancer (PCa). However, castrate-resistant prostate cancer cells (CRPC) can drive further disease progression (2). In this context, estrogen’s ability to decrease hypothalamic pituitary stimulation of luteinizing hormone (LH) and follicle-stimulating hormone (FSH) production and consequently reduce androgen synthesis made them suitable to be used as a PCa therapy. Unfortunately, estrogen therapy had numerous cardiovascular and thrombotic side effects hindering its clinical use as an alternative to castration (3).
The direct estrogens actions are mediated by estrogen receptors. Estrogen receptors α (ERα) and β (ERβ) are members of the nuclear receptor superfamily. ERβ is suggested to have a growth inhibitory role in prostate tissue and it was proposed as a new therapeutic target for prostate cancer (4,5). However, biological significance of ERβ signaling remains unclear (6).
THE ROLE OF ERβ IN PROSTATE GLAND
Expression and Mechanism of Action
ERβ is encoded by chromosome locus 14q22–24 (7) and it is expressed in both stromal and luminal epithelial cells of the human prostate (5). ERα is expressed mainly in prostate stroma (8,9).
As a member of the nuclear receptor family, ERβ acts individually, forming homodimers (ERβ/β) or heterodimers (ERβ/α). Ligand-induced dimerization leads to translocation of dimer to the nucleus, binding with coregulatory proteins and interaction with responsive elements (binding sites) in the promoter regions including nuclear factor-κB (NF-κB) and activator protein 1 (AP-1). ERβ binds indirectly to these alternative binding sites through the recruitment of cofactors to the receptor. However, less is known about the interactions between ERβ and transcriptional cofactors (10). ERβ/β and ERα/β homo- and hetero dimers, respectively, exhibit antiproliferative effects as they activate different target genes (11). Interestingly, ERα/β heterodimer is more stable than the ERβ/β homodimer (12). Overall, ER dimerization is a crucial step in defining ER signaling (11).
ERβ Isoforms in Prostate Gland
ERβ may play a significant role in human PCa affecting progression as indicated by the distinct expression of its spliced variants during the phases of progression (13). In humans, there are at least five identified isoforms of ERβ. ERβ1, ERβ2, ERβ4, and ERβ5 isoforms can be found in various cell types in the normal prostate and are differentially expressed during the prostate cell cycle (14,15). Recent studies suggested that ERβ1 is the only fully functional isoform of the ERβ family. Other ERβ isoforms have no intrinsic activity, since they neither form homodimers or recruit coregulator proteins and are characterized as variable dimer partners of the ERβ complex altering its activity (16). Thus, ERβ activity may depend on ERβ1 expression and the ERβ isoforms ratio.
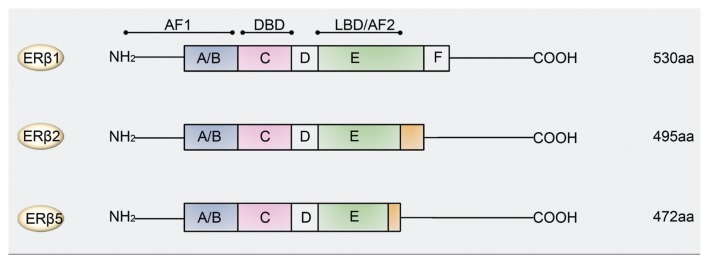
Two clinically important isoforms of ERβ are the ERβ2 (also known as ERβcx) and ERβ5 (17). ERβ2 and ERβ5 mRNA variants share identical sequences with ERβ1 from exon 1 to 7, but miss the sequences of exon 8 (18,19). However, they contain extra sequences which are distinct from each other, followed by sequences that are then identical (20). Consequently, ERβ2 and ERβ5 proteins have truncated c-terminal regions, resulting in loss of activation function 2 (AF-2) domains and have differences in ligand binding domains (LBDs) (17,20) (Figure 1). ERβ2 and ERβ5 isoforms cannot homodimerize, but they can form heterodimers with ERβ1 under the stimulation of estrogens (but not phytoestrogens) (16). Overall, much knowledge about the function and signaling of the ERβ isoforms family came from studies on ERβ1, while the distinct functions of other ERβ isoforms in the prostate remain unknown.
Figure 1.
Protein domains of estrogen receptor β1, β2 and β5 isoforms. All three isoforms contain an amino-terminal region (A/B), a DNA-binding region (C), a hinge region (D) and an LBD (E). ERβ1 also contains a carboxyl-terminal domain (F). Specific regions within C and E domains are important for receptor dimerization. Activation function 1 domain (AF-1) and AF-2 domains are required to transactivate gene expression. The N-terminal AF-1 domain and the C-terminal AF-2 domain correspond to ligand-independent and -dependent transactivation functions, respectively. The DNA-binding domain (DBD) shares a high degree of homology in the different ER isoforms. aa, Amino acids.
Steroidogenic Capacity and ERβ
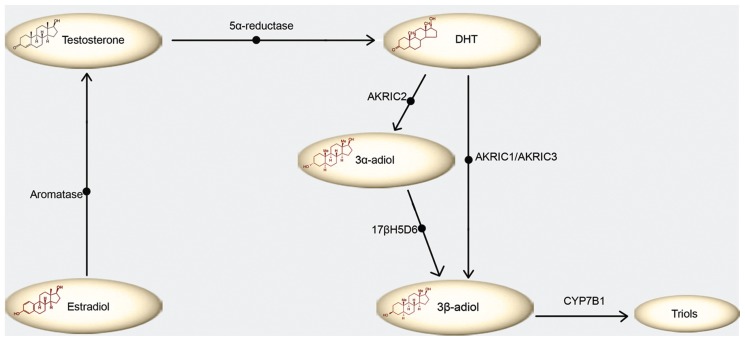
One level of regulation of ERβ function is on the local steroidogenesis of prostate cells. Various enzymes are essential for the transformation of steroid hormone precursors into ligands of the ERβ in prostate cancer (21). One of them, aromatase, catalyzes the estrogen biosynthesis from androgens (22). The aberrant expression of aromatase has an important role in the development of prostate malignancy (23). Also, highly expressed enzyme 5α-reductase converts testosterone (T) into dihydrotestosterone (DHT). Furthermore, DHT can be converted to 3β-adiol, directly by AKR1C1 or via 3α-adiol and 17β hydroxysteroid dehydrogenase (17βHSD6) (24). 3β-adiol is further metabolized to triols by CYP7B1 (25) (Figure 2). Remarkably, AKR1C1 and AKR1C3 enzymes can serve as drug development targets in PCa (26).
Figure 2.
Metabolism of estrogen receptor β physiological ligand, 3β-adiol. Aromatase converts estradiol into testosterone, which is converted downstream to dihydrotestosterone (DHT) by 5α-reductase. DHT is metabolized to 3β-adiol in two ways; 3-keto reduction of DHT to 3β-adiol by enzyme AKR1C1 (or AKR1C3) or alternatively 3-keto reduction of DHT to 3α-adiol by AKR1C2 followed by 3α- to 3β-hydroxysteroid epimerization by B17b hydroxysteroid dehydrogenase (17bHSD6). No matter the origin, 3β-adiol is further metabolized to triols by CYP7B1.
Indeed, 3β-adiol is considered as the physiological ligand for ERβ (27). This hypothesis is further supported by the fact that ERβ, activated by 3β-adiol, is involved in regulating the prostate AR content in wild-type mice, and in restraining epithelial growth (28). In addition, 3β-adiol is considered a powerful DHT metabolite since its intraprostatic protein level is 100-fold higher than that of estradiol (E2) (29). Notably, 3β-adiol has antiproliferative actions which are not reproduced by 17β-estradiol (30). Activation of ERβ by 3β-adiol induces apoptosis by upregulating the proapoptotic factor p53 and upregulating modulator of apoptosis (PUMA), an effect that implicates transcription factor FOXO3a (31).
ERβ in the Developing Prostate and Normal Function
Interestingly, prostate morphogenesis occurs under the control of androgens and is modulated by estrogens (32). However, ERβ is not required in early stages of prostate development, as it appears to be expressed in the prostate after 2 wks in the life of newborn mice following ERα expression (33). Moreover, in the developing rodent prostate gland, ERα-induced excessive estrogenic exposure leads to permanent alternation of the gland including squamous metaplasia, inflammation and epithelial dysplasia as reported by an in utero study (34). Notably, the developmental pattern for ERβ in the human prostate is different from the rodent. ERβ expression starts early in fetal wk 7 throughout the urogenital sinus epithelium and stroma, and this high expression is maintained during ductal morphogenesis. Apparently, ERβ is the only detectable ER in the developing human fetal prostate. However, by year 11 postnatally, expression of ERβ is restricted to the basal epithelial cells and prostate stromal compartments, similar to adult human prostate. (35). Thus, in the developing human prostate, ERβ is the predominant ER in both stromal and epithelial cells (36).
In the adult human prostate, ERβ is characterized as an important mediator of epithelial differentiation (37). The mechanisms through which ERβ maintain differentiation involve the degradation of hypoxia-inducible factor 1α (HIF-1α) (38). ERβ enhances transcription of prolyl hydroxylase domain-containing protein 2 (PHD2) that hydroxylates HIF-1α and marks HIF for destruction by the von Hippel-Lindau tumor suppressor (VHL) (39). Additionally, ERβ appears to have antiproliferative actions which are independent from the alternations of systemic androgen concentration and the activation of ERα, as documented in aromatase-knockout mice treated with ERβ-specific agonists (40). There, ERβ seemed to have a suppressive role in the proliferation process, stimulating the differentiation of adult prostate epithelial cells.
THE ROLE OF ERβ IN PROSTATE CARCINOGENESIS AND DISEASE PROGRESSION
ERβ and Isoforms Expression
The role of ERβ in the PCa initiation has been supported by studies using the ER-knockout (ERKO) mice. ERα-knockout mice do not develop prostate cancer after testosterone and/or estrogen treatment, whereas mice lacking ERβ receptor develop prostate cancer after the addition of sex hormones, similarly to wild-type mice (41). This antiproliferative role of ERβ also concurs with immunohistochemical findings in human PCa tissue samples, suggesting that ERβ expression is lost in high-grade tumors (42,43,44). Therefore, the loss of ERβ may be considered as a prognostic factor of prostate cancer.
ERβ isoforms in normal and cancerous prostate are differentially expressed. Transcriptional and posttranscriptional regulatory mechanisms may correlate with this phenomenon. In the transcriptional level, alternative promoter usage results in various amounts of ERβ transcripts. It has been proposed that promoters 0K and 0N upstream of exon1 (further described in [45]), are the regulatory promoters of ERβ expression in the prostate and that ERβ1 and ERβ2 are transcribed from both 0N and 0K promoters. On the contrary, only the 0K promoter is used for ERβ5 transcription. The control of 0N promoter methylation of CpG islands located in the 5′-flanking sequence of the 0N promoter results in loss of expression of ERβ during the development of PCa. AP-2 regulates the transcription of ERβ by acting through a methylation hotspot of the 0N promoter in prostate cancer cells. Loss of protein AP-2 allows methylation at the critical AP-2 binding domain in ERβ promoter (46). Interestingly, very recently, a regulation between ERβ2 and ERβ1 has been addressed; ERβ2 is proposed to repress ERβ1 transcription, thereby affecting its protective and favorable cellular responses (47).
The combinations of 5′ untranslated exons, known as exons 0Xs, are closely correlated with promoter 0K and are present in 5′ untranslated regions of ERβ2 and ERβ5 but not ERβ1. In the posttranscriptional level, upstream open reading frames (uORFs) can inhibit translation of transcripts composed of exons 0K and 0X. In PCa cells, there is a lower proportion of ERβ2 and ERβ5 transcripts containing exon 0Xs than in normal cells, suggesting that elevated protein expression in cancer cells promotes invasion and metastasis (48).
ERβ and Oxidative Stress
An important factor favoring PCa initiation is oxidative stress (OS), which is associated with inflammation, a possible precursor in neoplastic transformation of the prostate (49). Interestingly, oxidative stress is associated with aggressive phenotypes of prostate cancer (50) while antioxidants, meanwhile, have a positive role in prostate cancer chemoprevention (51). Moreover, cell lines with high amounts of ERβ and a low ratio of ERα/ERβ have a high expression of antioxidant enzymes and uncoupling proteins, resulting in lower oxidative stress (52). Origins of oxidative stress in prostate cancer include the mitochondrial hydrogen peroxide (H2O2) production through cytochrome c oxidation and the extramitochondrial origin of H2O2 via nicotinamide adenine dinucleotide phosphate-oxidase (NADPH) oxidases (53). PCa cells can produce increased levels of reactive oxygen species (ROS) and change the redox status of their microenvironment in response to locally produced transforming growth factor β1 (TGFβ1) prooxidant signals as revealed in DU145 PCa cells (54). Interestingly, oxidative stress inactivates ERβ by inhibiting the receptor’s dimerization by altering the second zinc-finger motif in the ERβ structure and therefore destabilizing its DNA-binding capacity (55). As a result, ERβ loses its ability to regulate various genes.
Switching Roles Theory
Recently, a theory of switching roles of ER during prostate carcinogenesis has been proposed, based on observations that elevated ERβ protein levels are detected in castration-resistant prostate cancer cells (CRPC), whereas these levels are related to lower survival in hormone-naive prostate cancer cells (HNPC). The theory suggests that in the early phases of PCa progression, ERβ presents a tumor-suppressing role and then is altered toward a tumor-promoting agent. It also proposes that ERβ signaling pathway in HNPC is mediated by Serin210-phosphorylated androgen receptor [pAR(S210)], but this observation disappears with the approach of CRPC, when AR gene stimulation takes over, perhaps in relevance to the lower levels of androgen in the body. The exact cause of this switch is not fully understood and the pathway that triggers the connection between pAR(S210) and ERβ in HNPC, if any exists, is currently unknown (56,57).
ERβ Isoforms during Prostate Cancer Progression
Multiple variants of ERβ exist, having possibly significant roles in PCa pathophysiology. Recent evidence suggests that apart from enhancing proliferation, ERβ2 promotes cancer cell migration and invasion, inducing the expression of factors involved in bone metastasis (58). In addition to elevated levels of ERβ2 associated with epithelial-to-mesenchymal transition (EMT), ERβ2 may have the ability to suppress the expression of ERβ1 (47,59), leading to EMT (59). After immunolocalization of gastrin-releasing peptide receptor (GRPR) in human PCa, the association of ERβ2 with PCa was first determined by Nagasaki et al., indicating a correlation between immunore-activity of GRPR, Gleason score and ERβ2, supporting the hypothesis that ERβ2 contributes to prostate carcinogenesis through GRPR expression in PCa cells (60). Apparently, ERβ2 and ERβ5 can act as cancer-enhancing molecules involving cell migration and invasion under specific circumstances (17).
ERα and Epithelial-to-Mesenchymal Transition (EMT)
It is well documented that high grade PCa cells lose their epithelial characteristics and exhibit mesenchymal features, including increased hypoxia-inducible factor-1α, vimentin and vascular endothelial growth factor (VEGF) expression (38) and loss of the E-cadherin, an epithelial cell adhesion protein, events typical of EMT phenomenon (61,62). 3β-adiol, the natural ligand of ERβ, promotes binding of dimerized ERβ to DNA promoter of E-cadherin (CDH1), stimulating its transcription (29). Hypoxic exposure and TGFβ1 signaling can induce EMT and decrease ERβ expression in both AR-dependent and AR-independent cells. Similarly, silencing of ERβ with short hairpin RNA techniques was adequate to promote EMT (38). The mechanism by which ERβ activation by 3β-adiol is associated to EMT inhibition in PCa cells involves the degradation of hypoxia inducible factor 1α (HIF-1α), a crucial EMT factor (38). HIF-1α-inducible genes, such as lysil oxidase (LOX), an enzyme that catalyzes cross-linking of extracellular matrix, and transcription factor TWIST, mediate epithelial dedifferentiation, leading to invasion and metastasis of the prostate cancer (63,64). Thus, ERβ acts as the “gatekeeper” of the epithelial phenotype in the prostate gland.
ERβ AS A USEFUL THERAPEUTIC TARGET FOR PCA TREATMENT
In addition, many reports have shown the antiproliferative role of ERβ in PCa cells, suggesting that ERβ is a promising therapeutic target for PCa therapy and prevention. Indeed, Walton and partners have induced apoptosis of PCa cells by using histone deacetylase inhibitor and DNA dimethylating agents to release ERβ expression from epigenetic silencing (65). Moreover, restoration of ERβ expression using adenoviruses has resulted in the suppression of cellular invasion and proliferation of DU145 PCa cells (66). Furthermore, high levels of ERβ1 did result in cell cycle arrest in early G1 phase in LNCaP cells (14). Recently, ERβ has been proposed to affect PCa acting as a cell cycle regulator, controlling the expression of cyclin D1 (CCND1) and affecting its downstream pathway (67), supporting further the notion that restoring ERβ expression may provide a new promising therapeutic approach for PCa.
Indeed, immunohistochemical studies have suggested that high grade tumors express ERβ (68) and that human PCa DU145 cells have the ability to activate ERβ by generating specific ligands by the transformation of androgen precursors produced by stromal cells (54). However, local paracrine signals and ROS of stromal cells can limit the antitumor activity of ERβ. Enzymes responsible for de novo steroidogenesis are not highly expressed in prostate gland, thus androgen metabolites may be the main ligand sources for ER-dependent signaling (69). In addition, in obese patients, aromatase enzyme is downregulated in prostate stroma suggesting that obesity can alter sex steroid production in stromal cells (70). Therefore, any treatment aiming toward the regulation of ERβ activity also should address the regulation of steroidogenesis in prostate tissue, thus targeting tumor microenvironment.
ERβ Agonists
Having in mind the ERβ tumor-suppressing functions, researchers have been interested in the development of specific agonists (71). In a study that investigates the therapeutic potential of 8β-VE2, a potent synthetic selective agonist for ERβ, using AR-knockout (ARKO) mice, researchers suggested that ERβ is responsible for androgen-independent apoptosis in prostatic stroma and epithelia, an effect that requires tumor necrosis factor-α (TNFα) signaling. Moreover, the same study showed that 8β-VE2-activated ERβ induces apoptosis in androgen-independent PCa cells including PC3 and DU145, as well as in primary human PCa xenografts. The pathway that mediates the link between ERβ and TNFα, if any exists, is not currently known, although the authors documented caspase-8 and −3 activation (72). A selective estrogen-receptor modulator (SERM) named ICI 182,780 exerted a dose-dependent growth inhibition action on DU145 cells, which was mediated by ERβ (73) through binding to NF-κB and enhancement of transcription factor FOXO1. Additionally, another SERM, raloxifene, induced the apoptosis and inhibited proliferation of both androgen-dependent and -independent cell lines via the activation of ERβ, lowering Bcl-2 expression, and increasing caspase-3 and Par-4 levels (74,75).
Although preclinical studies described above point out the protective effect of ERβ, the clinical utility of ERβ-selective agents in the treatment of men with PCa has never been proved. A possible reason for the inconsistency between in vitro and in vivo findings is that the prostate cancer cell lines (LNcAP, PC-3, DU145) used in in vitro studies exhibit differential expression profiles of the nuclear receptors (AR, ERα and ERβ and cannot represent the human tissue. Furthermore, these model cell lines are not reflecting the complex cross-talk between AR, ERα and ERβ and other stromal:epithelial interactions known to occur in vivo (76).
Phytoestrogens
Phytoestrogens are natural compounds that mimic the biological activity of estrogens with a binding preference for ERβ (77) and have the ability to upregulate ERβ, which is lost during PCa progression. Mice with PCa lacking ERα or ERβ treated with phytoestrogens showed that cancer did not progress in ERαKO mice whereas ERβKO mice presented an increasing incidence of poorly differentiated carcinoma (Gleason scores 4 and 5) (78). Re-expression of ERβ via phytoestrogens elicited antiandrogenic effects, including downregulation of AR and its coactivators (79). In addition, many phytoestrogens were found to stimulate the expression of p21 (cyclin-dependent kinase inhibitor 1) through ER, including genistein and silymarin (a polyphenolic flavonoid extracted from plant Silybum marianum) (80,81). Ongoing studies suggest that phytoestrogens can be used in treatment and/or prevention of PCa, as supported by findings that men who receive a rich phytoestrogen diet present a lower incidence of PCa (82).
CONCLUSION
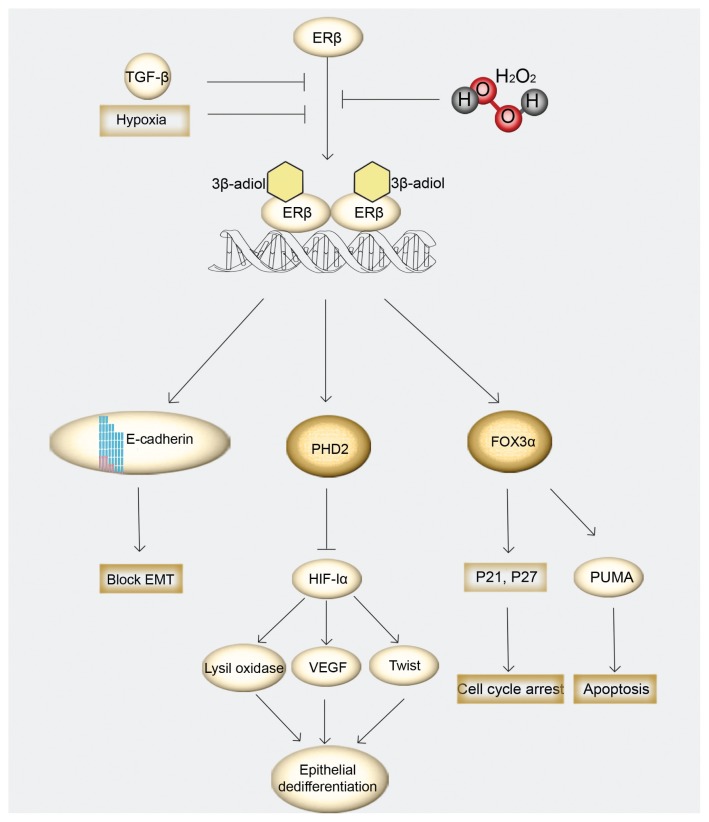
ERβ has been proposed as a mediator of epithelial differentiation and as an antiproliferative molecule, mediating many molecular pathways on PCa. ERβ protects epithelial integrity and block EMT by upregulating transcription of E-cadherin (CDH1), an epithelial adhesion protein. Furthermore, ERβ upregulates the expression of PHD2, that hydroxylates the tumor enhancer HIF-1α and marks HIF for destruction by the von Hippel-Lindau tumor suppressor (VHL). ERβ signaling has antiproliferative effects on the prostate, enhancing the expression of FOXO3α, and consequently upregulating apoptotic genes including PUMA, a proapoptotic protein, and p21, a regulator of cell cycle progression. However, ERβ is sensitive to putative changes in tumor microenvironment, thus hypoxic conditions and TGFβ1 signaling diminish ERβ levels and alter its action, favoring PCa progression (Figure 3).
Figure 3.
Schematic representation of ERβ-mediated antitumor pathways. After receptor dimerization, ERβ enhances expression of E-cadherin, a protein that maintains epithelial integrity by blocking EMT. In addition, ERβ upregulates transcription of PHD2 and FOXO3a. In turn, PHD2 marks HIF-1α for destruction, resulting in the suppression of the oncogenic genes LOX, VEGF and TWIST and therefore preventing epithelial dedifferentiation, invasion and metastasis. ERβ through FOX3α, induce apoptosis by upregulating the proapoptotic factor PUMA and cell cycle regulators p21 and p27. ERβ antitumor effects are inhibited by oxidative stress and paracrine signals including TGF-β signaling and hypoxia.
Despite its tumor-suppressing role, ERβ has also been proposed as a cancer-promoting factor. Interestingly, higher levels of ERβ correspond to lower survival in HNPC cells through the activation of pAR(s210), although the exact mechanism remains unknown. Additionally, ERβ1 has been proposed to form a complex with AR, resulting in the transcription of AR-dependent genes in PCa (76). Moreover, its spliced variants, ERβ2 and ERβ5, have tumor-promoting actions and are stimulated after dimerization with ERβ1. Although regulation of ERβ1 expression occurs mainly at the transcriptional level, ERβ2 and ERβ5 expression is controlled at both the transcriptional and posttranscriptional levels, via complex interactions that involves promoter methylation on CpG islands and uORFs. Further findings are needed to elucidate the exact molecular functions of these isoforms.
Future studies should focus on understanding the molecular mechanism governing the controversial findings on ERβ function and its spliced variants in cancerous prostate to develop ERβ-based therapeutic agents for prostate cancer treatment and other estrogen-dependent processes (83).
Footnotes
Online address: http://www.molmed.org
DISCLOSURES
The authors declare they have no competing interests as defined by Molecular Medicine, or other interests that might be perceived to influence the results and discussion reported in this paper.
REFERENCES
- 1.Huggins C, Stevens RE, Jr, Hodges CV. Studies on prostatic cancer: Ii. the effects of castration on advanced carcinoma of the prostate gland. Arch Surg. 1941;43:209–23. [Google Scholar]
- 2.Koutsilieris M, Tolis G. Long-term follow-up of patients with advanced prostatic carcinoma treated with either buserelin (HOE 766) or orchiectomy: classification of variables associated with disease outcome. Prostate. 1985;7:31–9. doi: 10.1002/pros.2990070105. [DOI] [PubMed] [Google Scholar]
- 3.Klotz L, McNeill I, Fleshner N. A phase 1–2 trial of diethylstilbestrol plus low dose warfarin in advanced prostate carcinoma. J Urol. 1999;161:169–72. [PubMed] [Google Scholar]
- 4.Jensen EV. Fate of steroid estrogens in target tissues. In: Vollmer PE, editor. Biological Activities of Steroids in Relation to Cancer. Academic Press; New York: 1960. pp. 61–174. [Google Scholar]
- 5.Kuiper GG, Enmark E, Pelto-Huikko M, Nilsson S, Gustafsson JA. Cloning of a novel receptor expressed in rat prostate and ovary. Proc. Natl. Acad. Sci. U. S. A. 1996;93:5925–30. doi: 10.1073/pnas.93.12.5925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hartman J, Ström A, Gustafsson JA. Current concepts and significance of estrogen receptor β in prostate cancer. Steroids. 2012;77:1262–6. doi: 10.1016/j.steroids.2012.07.002. [DOI] [PubMed] [Google Scholar]
- 7.Enmark E, et al. Human estrogen receptor beta-gene structure, chromosomal localization, and expression pattern. J. Clin. Endocrinol. Metab. 1997;82:4258–65. doi: 10.1210/jcem.82.12.4470. [DOI] [PubMed] [Google Scholar]
- 8.Fixemer T, Remberger K, Bonkhoff H. Differential expression of the estrogen receptor beta (ERbeta) in human prostate tissue, premalignant changes, and in primary, metastatic, and recurrent prostatic adenocarcinoma. Prostate. 2003;54:79–87. doi: 10.1002/pros.10171. [DOI] [PubMed] [Google Scholar]
- 9.Royuela M, et al. Estrogen receptors alpha and beta in the normal, hyperplastic and carcinomatous human prostate. J Endocrinol. 2001;168:447–54. doi: 10.1677/joe.0.1680447. [DOI] [PubMed] [Google Scholar]
- 10.Heldring N, et al. Estrogen receptors: how do they signal and what are their targets. Physiol Rev. 2007;87:905–31. doi: 10.1152/physrev.00026.2006. [DOI] [PubMed] [Google Scholar]
- 11.Powell E, et al. Identification of estrogen receptor dimer selective ligands reveals growth-inhibitory effects on cells that co-express ERα and ERβ. PLoS ONE. 2012;7:e30993. doi: 10.1371/journal.pone.0030993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chakraborty S, Willett H, Biswas PK. Insight into estrogen receptor beta–beta and alpha–beta homo- and heterodimerization: A combined molecular dynamics and sequence analysis study. Biophys Chem. 2012;170:42–50. doi: 10.1016/j.bpc.2012.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Walton TJ, et al. Quantitative RT-PCR analysis of estrogen receptor gene expression in laser microdissected prostate cancer tissue. Prostate. 2009;69:810–9. doi: 10.1002/pros.20929. [DOI] [PubMed] [Google Scholar]
- 14.Hurtado A, et al. Estrogen receptor beta displays cell cycle-dependent expression and regulates the G1 phase through a non-genomic mechanism in prostate carcinoma cells. Cell Oncol. 2008;30:349–65. doi: 10.3233/CLO-2008-0430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Leav I, et al. Comparative studies of the estrogen receptors beta and alpha and the androgen receptor in normal human prostate glands, dysplasia, and in primary and metastatic carcinoma. Am. J. Pathol. 2001;159:79–92. doi: 10.1016/s0002-9440(10)61676-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Leung YK, Mak P, Hassan S, Ho SM. Estrogen receptor (ER)-beta isoforms: a key to understanding ER-beta signaling. Proc. Natl. Acad. Sci. U. S. A. 2006;103:13162–7. doi: 10.1073/pnas.0605676103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Leung YK, et al. Estrogen receptor beta2 and beta5 are associated with poor prognosis in prostate cancer, and promote cancer cell migration and invasion. Endocr. Relat. Cancer. 2010;17:675–89. doi: 10.1677/ERC-09-0294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Moore JT, et al. Cloning and characterization of human estrogen receptor beta isoforms. Biochem. Biophys. Res. Commun. 1998;247:75–8. doi: 10.1006/bbrc.1998.8738. [DOI] [PubMed] [Google Scholar]
- 19.Younes M, Honma N. Estrogen receptor β. Arch. Pathol. Lab. Med. 2011;135:63–6. doi: 10.5858/2010-0448-RAR.1. [DOI] [PubMed] [Google Scholar]
- 20.Peng B, Lu B, Leygue E, Murphy LC. Putative functional characteristics of human estrogen receptor-beta isoforms. J. Mol. Endocrinol. 2003;30:13–29. doi: 10.1677/jme.0.0300013. [DOI] [PubMed] [Google Scholar]
- 21.Celhay O, et al. Expression of estrogen related proteins in hormone refractory prostate cancer: association with tumor progression. J Urol. 2010;184:2172–8. doi: 10.1016/j.juro.2010.06.089. [DOI] [PubMed] [Google Scholar]
- 22.Ghosh D, Griswold J, Erman M, Pangborn W. Structural basis for androgen specificity and oestrogen synthesis in human aromatase. Nature. 2009;457:219–23. doi: 10.1038/nature07614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ellem SJ, Risbridger GP. Aromatase and regulating the estrogen:androgen ratio in the prostate gland. J. Steroid Biochem. Mol. Biol. 2010;118:246–51. doi: 10.1016/j.jsbmb.2009.10.015. [DOI] [PubMed] [Google Scholar]
- 24.Muthusamy S, et al. Estrogen receptor β and 17?β-hydroxysteroid dehydrogenase type 6, a growth regulatory pathway that is lost in prostate cancer. Proc. Natl. Acad. Sci. U. S. A. 2011;108:20090–4. doi: 10.1073/pnas.1117772108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sugiyama N, Barros RP, Warner M, Gustafsson JA. ERbeta: recent understanding of estrogen signaling. Trends Endocrinol Metab. 2010;21:545–52. doi: 10.1016/j.tem.2010.05.001. [DOI] [PubMed] [Google Scholar]
- 26.Beranič N, Stefane B, Brus B, Gobec S, Rižner TL. New enzymatic assay for the AKR1C enzymes. Chem. Biol. Interact. 2013;202:204–9. doi: 10.1016/j.cbi.2012.12.003. [DOI] [PubMed] [Google Scholar]
- 27.Baker ME. What are the physiological estrogens? Steroids. 2013;78:337–40. doi: 10.1016/j.steroids.2012.12.011. [DOI] [PubMed] [Google Scholar]
- 28.Weihua Z, et al. A role for estrogen receptor beta in the regulation of growth of the ventral prostate. Proc. Natl. Acad. Sci. U. S. A. 2001;98:6330–5. doi: 10.1073/pnas.111150898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Grubisha MJ. Local endocrine, paracrine and redox signaling networks impact estrogen and androgen crosstalk in the prostate cancer microenvironment. Steroids. 2013;78:538–41. doi: 10.1016/j.steroids.2013.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Dondi D, et al. Estrogen receptor beta and the progression of prostate cancer: role of 5alpha-androstane-3beta,17beta-diol. Endocr. Relat. Cancer. 2010;17:731–42. doi: 10.1677/ERC-10-0032. [DOI] [PubMed] [Google Scholar]
- 31.Dey P, Ström A, Gustafsson JA. Estrogen receptor β upregulates FOXO3a and causes induction of apoptosis through PUMA in prostate cancer. Oncogene. 2013 2013 Sept. doi: 10.1038/onc.2013.384. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 32.Marker PC, Donjacour AA, Dahiya R, Cunha GR. Hormonal, cellular, and molecular control of prostatic development. Dev Biol. 2003;253:165–74. doi: 10.1016/s0012-1606(02)00031-3. [DOI] [PubMed] [Google Scholar]
- 33.Omoto Y, Imamov O, Warner M, Gustafsson JA. Estrogen receptor alpha and imprinting of the neonatal mouse ventral prostate by estrogen. Proc. Natl. Acad. Sci. U. S. A. 2005;102:1484–9. doi: 10.1073/pnas.0409168102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Prins GS, Huang L, Birch L, Pu Y. The role of estrogens in normal and abnormal development of the prostate gland. Ann. N. Y. Acad. Sci. 2006;1089:1–13. doi: 10.1196/annals.1386.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Shapiro E, et al. Immunolocalization of estrogen receptor alpha and beta in human fetal prostate. J Urol. 2005;174:2051–3. doi: 10.1097/01.ju.0000176472.90432.5b. [DOI] [PubMed] [Google Scholar]
- 36.Adams JY, Leav I, Lau KM, Ho SM, Pflueger SM. Expression of estrogen receptor beta in the fetal, neonatal, and prepubertal human prostate. Prostate. 2002;52:69–81. doi: 10.1002/pros.10103. [DOI] [PubMed] [Google Scholar]
- 37.Imamov O, et al. Estrogen receptor beta regulates epithelial cellular differentiation in the mouse ventral prostate. Proc. Natl. Acad. Sci. U. S. A. 2004;101:9375–80. doi: 10.1073/pnas.0403041101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Mak P, et al. ERbeta impedes prostate cancer EMT by destabilizing HIF-1alpha and inhibiting VEGF-mediated snail nuclear localization: implications for Gleason grading. Cancer Cell. 2010;17:319–32. doi: 10.1016/j.ccr.2010.02.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Mak P, Chang C, Pursell B, Mercurio AM. Estrogen receptor β sustains epithelial differentiation by regulating prolyl hydroxylase 2 transcription. Proc. Natl. Acad. Sci. U. S. A. 2013;110:4708–13. doi: 10.1073/pnas.1221654110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.McPherson SJ, et al. Essential role for estrogen receptor beta in stromal-epithelial regulation of prostatic hyperplasia. Endocrinology. 2007;148:566–74. doi: 10.1210/en.2006-0906. [DOI] [PubMed] [Google Scholar]
- 41.Ricke WA, et al. Prostatic hormonal carcinogenesis is mediated by in situ estrogen production and estrogen receptor alpha signaling. FASEB J. 2008;22:1512–20. doi: 10.1096/fj.07-9526com. [DOI] [PubMed] [Google Scholar]
- 42.Gabal SM, Habib FM, Helmy DO, Ibrahim MF. Expression of estrogen receptor-B (ER-B ) in benign and malignant prostatic epithelial cells and its correlation with the clinico-pathological features. J. Egypt Natl. Canc. Inst. 2007;19:239–48. [PubMed] [Google Scholar]
- 43.Gallardo F, et al. Expression of androgen, oestrogen alpha and beta, and progesterone receptors in the canine prostate: differences between normal, inflamed, hyperplastic and neoplastic glands. J. Comp. Pathol. 2007;136:1–8. doi: 10.1016/j.jcpa.2006.08.007. [DOI] [PubMed] [Google Scholar]
- 44.Bardin A, Boulle N, Lazennec G, Vignon F, Pujol P. Loss of ERbeta expression as a common step in estrogen-dependent tumor progression. Endocr. Relat. Cancer. 2004;11:537–51. doi: 10.1677/erc.1.00800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Li LC, Yeh CC, Nojima D, Dahiya R. Cloning and characterization of human estrogen receptor beta promoter. Biochem. Biophys. Res. Commun. 2000;275:682–9. doi: 10.1006/bbrc.2000.3363. [DOI] [PubMed] [Google Scholar]
- 46.Zhang X, Leung YK, Ho SM. AP-2 regulates the transcription of estrogen receptor (ER)-beta by acting through a methylation hotspot of the 0N promoter in prostate cancer cells. Oncogene. 2007;26:7346–54. doi: 10.1038/sj.onc.1210537. [DOI] [PubMed] [Google Scholar]
- 47.Cotrim CZ, Fabris V, Doria ML, Lindberg K, Gustafsson J. Estrogen receptor beta growth-inhibitory effects are repressed through activation of MAPK and PI3K signalling in mammary epithelial and breast cancer cells. Oncogene. 2013;32:2390–402. doi: 10.1038/onc.2012.261. [DOI] [PubMed] [Google Scholar]
- 48.Lee MT, Ouyang B, Ho SM, Leung YK. Differential expression of estrogen receptor beta isoforms in prostate cancer through interplay between transcriptional and translational regulation. Mol. Cell. Endocrinol. 2013;376:125–35. doi: 10.1016/j.mce.2013.06.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Gupta-Elera G, Garrett AR, Robison RA, O’Neill KL. The role of oxidative stress in prostate cancer. Eur. J. Cancer Prev. 2012;21:155–62. doi: 10.1097/CEJ.0b013e32834a8002. [DOI] [PubMed] [Google Scholar]
- 50.Kumar B, Koul S, Khandrika L, Meacham RB, Koul HK. Oxidative stress is inherent in prostate cancer cells and is required for aggressive phenotype. Cancer Res. 2008;68:1777–85. doi: 10.1158/0008-5472.CAN-07-5259. [DOI] [PubMed] [Google Scholar]
- 51.Thapa D, Ghosh R. Antioxidants for prostate cancer chemoprevention: challenges and opportunities. Biochem Pharmacol. 2012;83:1319–30. doi: 10.1016/j.bcp.2011.12.027. [DOI] [PubMed] [Google Scholar]
- 52.Miró AM, et al. 17β-estradiol regulates oxidative stress in prostate cancer cell lines according to ERalpha/ERbeta ratio. J. Steroid Biochem. Mol. Biol. 2011b;123:133–9. doi: 10.1016/j.jsbmb.2010.12.004. [DOI] [PubMed] [Google Scholar]
- 53.Rebillard A, Lefeuvre-Orfila L, Gueritat J, Cillard J. Prostate cancer and physical activity: adaptive response to oxidative stress. Free Radic. Biol. Med. 2013;60:115–24. doi: 10.1016/j.freeradbiomed.2013.02.009. [DOI] [PubMed] [Google Scholar]
- 54.Grubisha MJ, Cifuentes ME, Hammes SR, Defranco DB. A local paracrine and endocrine network involving TGFβ, Cox-2, ROS, and estrogen receptor β influences reactive stromal cell regulation of prostate cancer cell motility. Mol Endocrinol. 2012;26:940–54. doi: 10.1210/me.2011-1371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Whittal RM, et al. Preferential oxidation of zinc finger 2 in estrogen receptor DNA-binding domain prevents dimerization and, hence, DNA binding. Biochemistry. 2000;39:8406–17. doi: 10.1021/bi000282f. [DOI] [PubMed] [Google Scholar]
- 56.Zellweger T, et al. Estrogen receptor β expression and androgen receptor phosphorylation correlate with a poor clinical outcome in hormone-naive prostate cancer and are elevated in castration-resistant disease. Endocr. Relat. Cancer. 2013;20:403–13. doi: 10.1530/ERC-12-0402. [DOI] [PubMed] [Google Scholar]
- 57.Savoy RM, Ghosh PM. The changing roles of steroid nuclear receptors with prostate cancer progression. Endocr. Relat. Cancer. 2013;20:C9–11. doi: 10.1530/ERC-13-0193. [DOI] [PubMed] [Google Scholar]
- 58.Dey P, et al. Estrogen receptors β1 and β2 have opposing roles in regulating proliferation and bone metastasis genes in the prostate cancer cell line PC3. Mol Endocrinol. 2012;26:1991–2003. doi: 10.1210/me.2012.1227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Alonso-Magdalena P, et al. A role for epithelial-mesenchymal transition in the etiology of benign prostatic hyperplasia. PNAS. 2009;106:2859–63. doi: 10.1073/pnas.0812666106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Nagasaki S, et al. Immunohistochemical analysis of gastrin-releasing peptide receptor (GRPR) and possible regulation by estrogen receptor βcx in human prostate carcinoma. Neoplasma. 2012;59:224–32. doi: 10.4149/neo_2012_029. [DOI] [PubMed] [Google Scholar]
- 61.Huber MA, Kraut N, Beug H. Molecular requirements for epithelial-mesenchymal transition during tumor progression. Curr. Opin. Cell Biol. 2005;17:548–58. doi: 10.1016/j.ceb.2005.08.001. [DOI] [PubMed] [Google Scholar]
- 62.Cavallaro U, Christofori G. Multitasking in tumor progression: signaling functions of cell adhesion molecules. Ann. N. Y. Acad. Sci. 2004;1014:58–66. doi: 10.1196/annals.1294.006. [DOI] [PubMed] [Google Scholar]
- 63.Pez F, et al. The HIF-1–inducible lysyl oxidase activates HIF-1 via the Akt pathway in a positive regulation loop and synergizes with HIF-1 in promoting tumor cell growth. Cancer Res. 2011;71:1647–57. doi: 10.1158/0008-5472.CAN-10-1516. [DOI] [PubMed] [Google Scholar]
- 64.Yang MH, et al. Direct regulation of TWIST by HIF-1alpha promotes metastasis. Nat Cell Biol. 2008;10:295–305. doi: 10.1038/ncb1691. [DOI] [PubMed] [Google Scholar]
- 65.Walton TJ, et al. DNA demethylation and histone deacetylation inhibition co-operate to re-express estrogen receptor beta and induce apoptosis in prostate cancer cell-lines. Prostate. 2008;68:210–22. doi: 10.1002/pros.20673. [DOI] [PubMed] [Google Scholar]
- 66.Cheng J, Lee EJ, Madison LD, Lazennec G. Expression of estrogen receptor beta in prostate carcinoma cells inhibits invasion and proliferation and triggers apoptosis. FEBS Lett. 2004;566:169–72. doi: 10.1016/j.febslet.2004.04.025. [DOI] [PubMed] [Google Scholar]
- 67.Nakamura Y, et al. Cyclin D1 (CCND1) expression is involved in estrogen receptor beta (ERβ) in human prostate cancer. Prostate. 2013;73:590–5. doi: 10.1002/pros.22599. [DOI] [PubMed] [Google Scholar]
- 68.Lai JS, et al. Metastases of prostate cancer express estrogen receptor-beta. Urology. 2004;64:814–20. doi: 10.1016/j.urology.2004.05.036. [DOI] [PubMed] [Google Scholar]
- 69.Hofland J, et al. Evidence of limited contributions for intratumoral steroidogenesis in prostate cancer. Cancer Res. 2010;70:1256–64. doi: 10.1158/0008-5472.CAN-09-2092. [DOI] [PubMed] [Google Scholar]
- 70.Gross M, et al. Expression of androgen and estrogen related proteins in normal weight and obese prostate cancer patients. Prostate. 2009;69:520–7. doi: 10.1002/pros.20901. [DOI] [PubMed] [Google Scholar]
- 71.Meyers MJ, et al. Estrogen receptor-beta potency-selective ligands: structure-activity relationship studies of diarylpropionitriles and their acetylene and polar analogues. J. Med. Chem. 2001;44:4230–51. doi: 10.1021/jm010254a. [DOI] [PubMed] [Google Scholar]
- 72.McPherson SJ, et al. Estrogen receptor-beta activated apoptosis in benign hyperplasia and cancer of the prostate is androgen independent and TNFalpha mediated. Proc. Natl. Acad. Sci. U. S. A. 2010;107:3123–8. doi: 10.1073/pnas.0905524107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Lau KM, LaSpina M, Long J, Ho SM. Expression of estrogen receptor (ER)-alpha and ER-beta in normal and malignant prostatic epithelial cells: regulation by methylation and involvement in growth regulation. Cancer Res. 2000;60:3175–82. [PubMed] [Google Scholar]
- 74.Rossi V, et al. Raloxifene induces cell death and inhibits proliferation through multiple signaling pathways in prostate cancer cells expressing different levels of estrogen receptor α and β. J. Cell. Physiol. 2011;226:1334–9. doi: 10.1002/jcp.22461. [DOI] [PubMed] [Google Scholar]
- 75.Kim IY, et al. Raloxifene, a selective estrogen receptor modulator, induces apoptosis in androgen-responsive human prostate cancer cell line LNCaP through an androgen-independent pathway. Cancer Res. 2002;62:3649–53. [PubMed] [Google Scholar]
- 76.Nelson AW, Tilley WD, Neal DE, Carroll J. Estrogen receptor beta in prostate cancer: friend or foe? Endocr. Relat. Cancer. 20142014Jan. doi: 10.1530/ERC-13-0508. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 77.Patisaul HB, Jefferson W. The pros and cons of phytoestrogens. Front Neuroendocrinol. 2010;31:400–19. doi: 10.1016/j.yfrne.2010.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Slusarz A, et al. Aggressive prostate cancer is prevented in ERαKO mice and stimulated in ERβKO TRAMP mice. Endocrinology. 2012;153:4160–70. doi: 10.1210/en.2012-1030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Thelen P, Wuttke W, Seidlová-Wuttke D. Phytoestrogens selective for the estrogen receptor beta exert anti-androgenic effects in castration resistant prostate cancer. J. Steroid Biochem. Mol. Biol. 2014;139:290–3. doi: 10.1016/j.jsbmb.2013.06.009. [DOI] [PubMed] [Google Scholar]
- 80.Matsumura K, Tanaka T, Kawashima H, Nakatani T. Involvement of the estrogen receptor beta in genistein-induced expression of p21(waf1/cip1) in PC-3 prostate cancer cells. Anticancer Res. 2008;28:709–14. [PubMed] [Google Scholar]
- 81.Atawia RT, Tadros MG, Khalifa AE, Mosli HA, Abdel-Naim AB. Role of the phytoestrogenic, pro-apoptotic and anti-oxidative properties of silymarin in inhibiting experimental benign prostatic hyperplasia in rats. Toxicol Lett. 2013;219:160–9. doi: 10.1016/j.toxlet.2013.03.002. [DOI] [PubMed] [Google Scholar]
- 82.Yan L, Spitznagel EL. Soy consumption and prostate cancer risk in men: a revisit of a meta-analysis. Am. J. Clin. Nutr. 2009;89:1155–63. doi: 10.3945/ajcn.2008.27029. [DOI] [PubMed] [Google Scholar]
- 83.Koutsilieris M. Pathophysiology of uterine leiomyomas. Biochem. Cell. Biol. 1992;70:273–8. doi: 10.1139/o92-043. [DOI] [PubMed] [Google Scholar]