Abstract
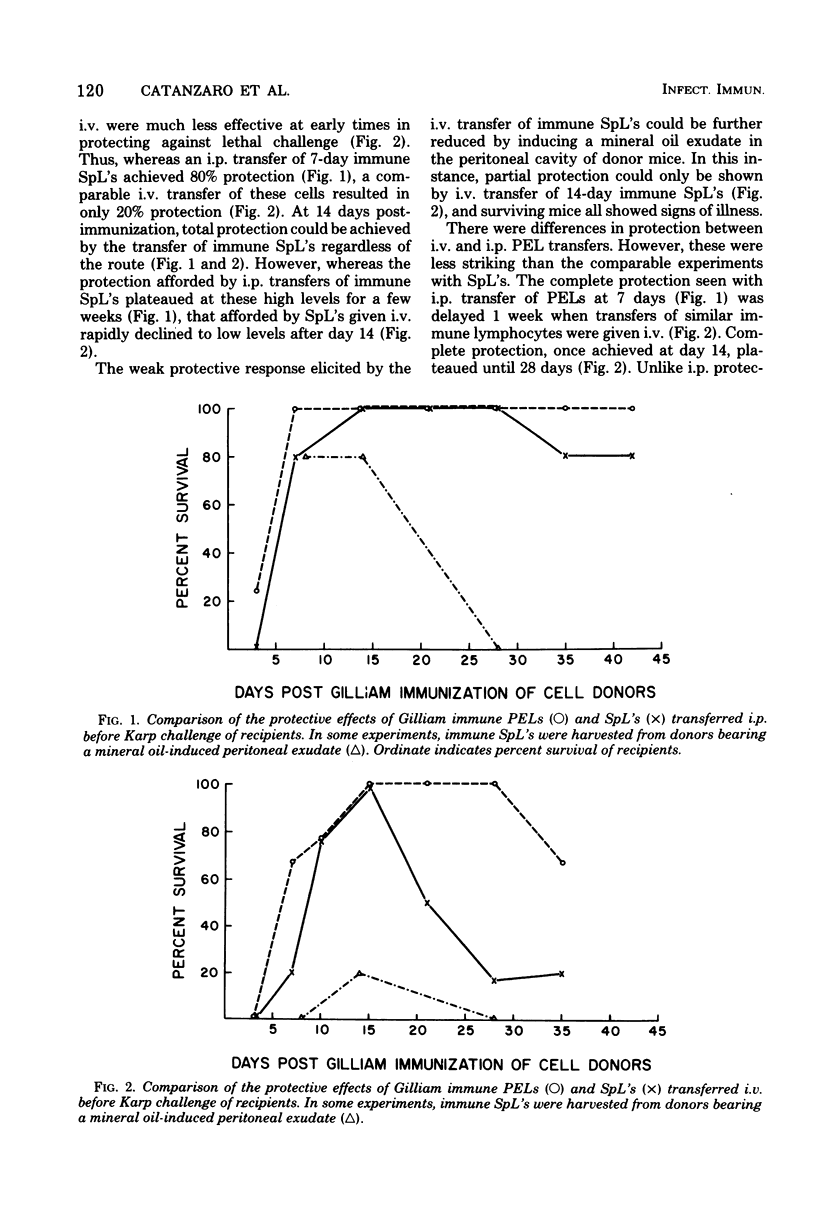
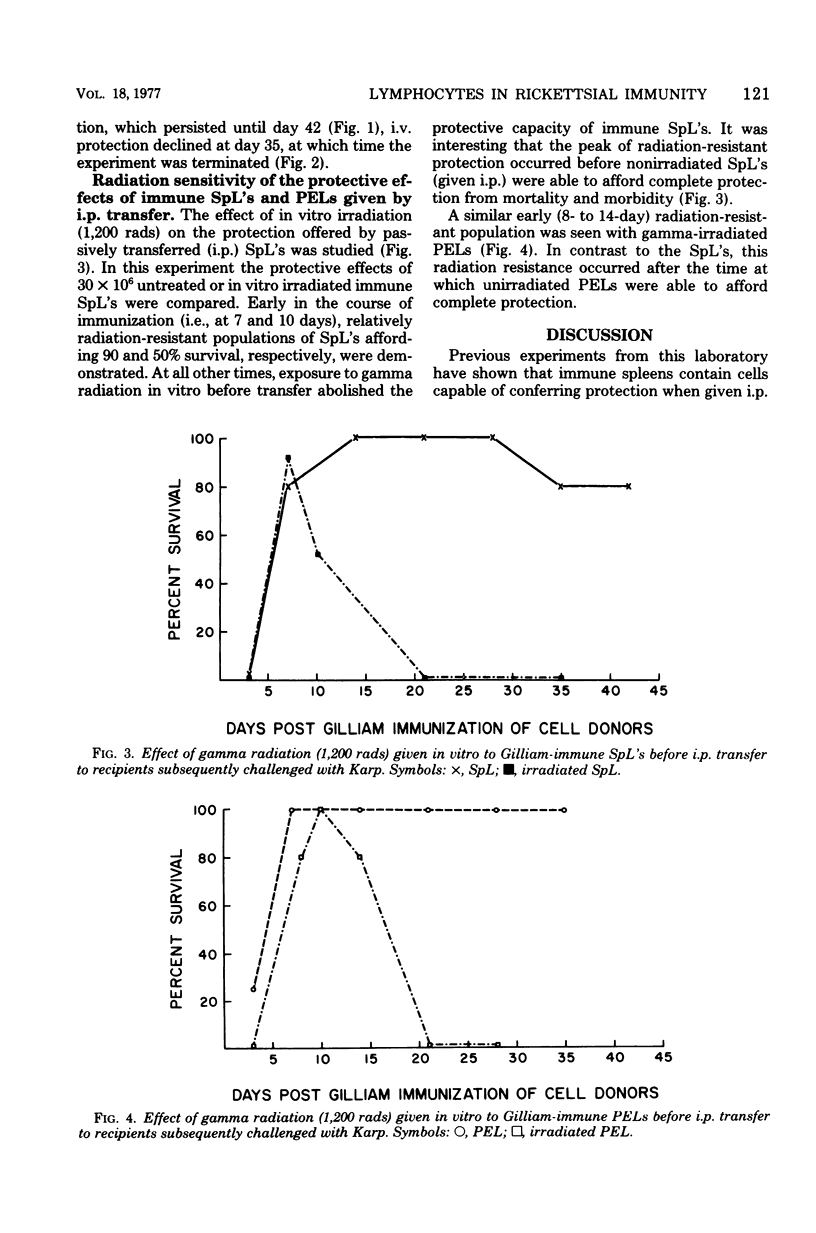
Lymphocytes obtained from spleens or peritoneal exudates of immune donor mice were evaluated for their ability to passively confer protection on recipients subsequently challenged with virulent scrub typhus rickettsiae. Peritoneal exudate lymphocytes (PELs) injected intraperitoneally were able to transfer complete protection against rickettsial challenge by 5 days after immunization, whereas splenic lymphocytes (SpL's) required 15 days to exhibit similar resistance. When immune lymphocytes were transferred intravenously, cells from both anatomical compartments required 15 days after immunization before they were able to completely protect recipients. PELs maintained this protective capacity for 2 weeks, but the passive immunity induced by intravenously transferred SpL's rapidly diminished to insignificant levels. It was particularly interesting that the protective effect of SpL's could be dramatically reduced by the concomitant presence of a mineral oil-induced peritoneal exudate. Almost total abrogation of resistance was observed when SpL's obtained from exudate-bearing mice were transferred intravenously. The protective capacity of both PELs and SpL's was resistant to 1,200 rads of gamma radiation at 7 to 10 days after immunization, but resistance was transient and by 3 weeks was undetectable. It was not possible to determine from this study whether the transferred lymphocytes were proximate mediators of protection in scrub typhus infection of mice or whether they served to recruit the host's own defenses, or both. However, it was possible to conclude that PEL's and SpLs exhibited functional heterogeneity and that PELs were more efficient mediators of protection.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Catanzaro P. J., Shirai A., Hilderbrandt P. K., Osterman J. V. Host defenses in experimental scrub typhus: histopathological correlates. Infect Immun. 1976 Mar;13(3):861–875. doi: 10.1128/iai.13.3.861-875.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- JACKSON E. B., SMADEL J. E. Immunization against scrub typhus. II. Preparation of lyophilized living vaccine. Am J Hyg. 1951 May;53(3):326–331. doi: 10.1093/oxfordjournals.aje.a119457. [DOI] [PubMed] [Google Scholar]
- Koster F. T., McGregor D. D., Mackaness G. B. The mediator of cellular immunity. II. Migration of immunologically committed lymphocytes into inflammatory exudates. J Exp Med. 1971 Feb 1;133(2):400–409. doi: 10.1084/jem.133.2.400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koster F. T., McGregor D. D. The mediator of cellular immunity. 3. Lymphocyte traffic from the blood into the inflamed peritoneal cavity. J Exp Med. 1971 Apr 1;133(4):864–876. doi: 10.1084/jem.133.4.864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lefford M. J., McGregor D. D., Mackaness G. B. Properties of lymphocytes which confer adoptive immunity to tuberculosis in rats. Immunology. 1973 Oct;25(4):703–715. [PMC free article] [PubMed] [Google Scholar]
- McGregor D. D., Logie P. S. The mediator of cellular immunity. VI. Effect of the antimitotic drug vinblastine on the mediator of cellular resistance to infection. J Exp Med. 1973 Mar 1;137(3):660–674. doi: 10.1084/jem.137.3.660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shirai A., Catanzaro P. J., Phillips S. M., Osterman J. V. Host defenses in experimental scrub typhus: role of cellular immunity in heterologous protection. Infect Immun. 1976 Jul;14(1):39–46. doi: 10.1128/iai.14.1.39-46.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zatz M. M., Gershon R. K. Regulation of lymphocyte trapping: the negative trap. J Immunol. 1975 Aug;115(2):450–453. [PubMed] [Google Scholar]
- van Furth R. Origin and kinetics of monocytes and macrophages. Semin Hematol. 1970 Apr;7(2):125–141. [PubMed] [Google Scholar]


