Abstract
Background
Splenectomy is reported to increase the haemoglobin level in patients with haemoglobin H Constant Spring (HbH CS) disease; however, its impact on iron burden and the underlying mechanism remains unclear.
Materials and methods
From March through to May 2013, a total of 50 adults with HbH CS disease (25 cases splenectomised and 25 cases non-splenectomised) were enrolled. The patients’ general conditions, history of blood transfusion and iron chelator treatment were investigated. Levels of haemoglobin, nucleated red blood cell counts, and serum ferritin were measured. The percentage of apoptotic erythroid precursor cells in bone marrow, an index representing ineffective erythropoiesis, was determined in some cases.
Results
There were no significant differences in age, blood transfusion volume, and use of iron chelator drugs between the splenectomised group and the non-splenectomised group. Significantly higher haemoglobin levels, serum ferritin levels and nucleated red blood cell counts as well as a higher percentage of apoptotic erythroid progenitor cells were detected in the splenectomised group. Regression analysis revealed that age and nucleated red blood cell counts were independent risk factors affecting the serum ferritin level.
Discussion
Despite improving the haemoglobin level, splenectomy is associated with greater iron burden in HbH CS disease. A high nucleated red blood cell count is predictive of the risk of severe iron overload.
Keywords: haemoglobin H Constant Spring disease, splenectomy, serum ferritin, ineffective erythropoiesis
Introduction
Alpha-thalassaemia is a congenital haemolytic disease caused by disorders of α-globin synthesis due to deletions or mutations within the genes that encode the α-globin chain. This disease is prevalent in Southeast Asia1, Guangxi2 and the Guangdong province of Southern China3, and is also common in the Middle East and Mediterranean area. Normal individuals have an α-globin gene cluster on chromosome 16, which consists of four α-globin genes (αα/αα). If defects occur in three of the four α-globin genes, α-thalassaemia intermedia, also termed haemoglobin H disease (HbH disease), develops. HbH disease is classified into deletional HbH disease (--/−α) and non-deletional HbH disease (--/αTα) according to the genotype. One common form of α-thalassaemia in Southeast Asia results from a nucleotide change in the α2 gene termination codon α142 (Term→Gln, TAA>CAA), which is expressed as Hb Constant Spring (Hb CS). HbH CS was the most common non-deletional HbH in the Guangxi province of Southern China. Compared to deletional HbH disease, HbH CS has more severe clinical manifestations and an earlier onset4.
Beta-thalassaemia intermedia (TI) is another group of inherited blood disorders caused by problems in the production of β-globin induced by β-globin gene defects5. Due to the disorder of β-chain synthesis, erythroid progenitor cells undergo intramedullary apoptosis and do not develop into mature red cells, a phenomenon which is termed ineffective erythropoiesis. Patients with TI or HbH disease do not require regular blood transfusion, and so these conditions are also called non-transfusion-dependent thalassaemia (NTDT). Unlike in patients with TI, most of the red blood cells in patients with HbH CS disease are destroyed in the spleen6, and splenectomy is reported to be therapeutically effective7. Quite a few patients with HbH disease undergo splenectomy, and the therapeutic efficacy of this operation is greater in patients with HbH CS disease than in patients with deletional HbH disease8.
NTDT may be complicated by iron overload, which is mainly attributed to increased gastrointestinal iron absorption rather than to the iron burden induced by blood transfusion9. The fundamental cause of iron overload in patients with TI is the acceleration of the production of red blood cells caused by ineffective erythropoiesis10. Hepcidin is the key factor regulating gastrointestinal absorption of iron, mediated by growth differentiation factor 15 (GDF-15). The increase in the production of red blood cells leads to up-regulation of GDF-15, inhibition of hepcidin and an increase in the gastrointestinal absorption of iron, which provides more raw materials for the production of red blood cells11. The spleen also plays an important role in iron metabolism. Iron burden, represented by serum ferritin and liver iron concentration, has shown to be intensified in patients with TI who are splenectomised12,13. Splenectomy has been found to be an effective approach for the treatment of HbH CS disease, although the impact of splenectomy on iron burden and the underlying mechanisms remain unclear. This cross-sectional study was designed to evaluate the effect of splenectomy on iron burden and investigate the underlying mechanisms in patients with HbH CS disease. The information derived could be of great significance for choosing the best treatment option and for the prevention and control of iron overload in patients with HbH CS disease
Materials and methods
Study subjects and design
This study was a cross-sectional survey, and was approved by the ethics review committee of the 303rd Hospital of the People’s Liberation Army. Since March 2010, when the database of patients with thalassaemia was established in the Thalassaemia treatment centre of the 303rd Hospital of the People’s Liberation Army (Nanning, China), data from 81 adult patients (aged 18 years or older) with HbH CS disease have been entered. All patients were invited to participate in this study, and 61 cases accepted the invitation. Five cases were excluded due to complications (one case complicated by autoimmune haemolytic anaemia, and 4 cases complicated by β-thalassaemia), and 6 cases were excluded because of a history of fever or blood transfusion 3 months prior to the study. Finally, 50 eligible patients were enrolled in the study. The diagnosis of HbH CS disease was confirmed by genetic analyses.
Laboratory examinations
Overall, 50 patients were involved in this study during the period from March to May 2013. Informed consent was obtained from all subjects, following a detailed description of the potential benefits of the study. The medical history, history of blood transfusions and treatment with iron chelator drugs were recorded. Fasting blood was collected from all participants. Red cell parameters were analysed using an XE 5000 automatic blood cell analyser (Sysmex Corporation, Kobe, Japan) and included haemoglobin concentration, reticulocyte count and nucleated red blood cell (NRBC) count. The levels of HbH and haemoglobin Constant Spring (HbCS) were quantitatively determined using a BioRad Variant II high pressure liquid chromatograph (Bio-Rad, Hercules, CA, USA). For the detection of deletional defects, α-thalassaemia mutations (including – –Southern East Asian (SEA), α–3.7 and α–4.2) were determined using a multiplex polymerase chain reaction assay; for the detection of the non-deletional defects, the two common α-thalassaemia mutations αCS and Quong Size variant (αQS) were determined using a reverse dot blot hybridisation assay (YiShengTang Biological Products, Shenzhen, China)14. The serum ferritin level was measured with a chemiluminescence method (AxSYM Ferritin, Abbott Laboratories, Chicago, IL, USA), and a serum ferritin level of >500 ng/mL was defined as iron overload. The levels of hepcidin, GDF-15 and serum transferrin receptor (sTfR) were determined by enzyme-linked immunosorbent assays following the manufacturer’s instructions (Cusabio Biotech Co., Ltd., Wuhan, China).
Detection of apoptosis of erythroid precursor cells
The antibodies used to detect the apoptosis of erythroid precursor cells included CD45, glycophorin A (GPA) and annexin V (BD Pharmingen, San Diego, CA, USA). Under sterile conditions, 2 mL of heparin sodium-anticoagulated bone marrow was collected into a test-tube and the antibody was added to the tube at the volume required according to the panel. Blood samples, at a volume determined on the basis of the cell count, were transferred to the tube, mixed at a low speed for 3 seconds, and incubated at room temperature for 20 minutes. Following this, 2 mL of red blood cell lysis buffer were added to each tube, mixed evenly, and incubated at room temperature in the darkness for 10 minutes. The mixture was then centrifuged immediately at 1,500 rpm at room temperature for 5 minutes, washed twice with phosphate-buffered solution, re-suspended following addition of 0.5 mL of phosphate-buffered saline containing 1% paraformaldehyde, and examined on a BD FACS Calibur™ flow cytometer (BD Biosciences, San Jose, CA, USA). CD45-GPA is a marker of erythroid precursor cells, and annexin V is a marker of apoptosis. Therefore, the percentage of annexin V+ cells among the CD45-GPA+ cells indicated the proportion of apoptotic erythroid precursor cells.
Statistical analysis
All statistical analyses were performed using the statistical software Statistical Package for Social Sciences (SPSS) version 15.0 (SPSS Inc., Chicago, IL, USA). Differences in continuous variables that had a normal distribution were compared with analysis of variance (ANOVA), and comparisons between groups were made using the least significant difference (LSD). Differences in categorical data were tested for statistical significance using Pearson’s χ2 test. Pearson’s linear correlation analysis was used for the correlation analysis of the factors affecting the serum ferritin level, and variables for which statistically significant differences were revealed by the single-factor correlation analysis were entered into multivariate linear regression analysis. A p-value of <0.05 from a two-tailed test was considered statistically significant.
Results
General information
The 50 subjects studied comprised 25 patients who had been splenectomised and 25 patients who had not undergone splenectomy. There were 21 males and 29 females, with a mean age of 28.5 years (range, 18.3–47.7 years). The mean age of the 25 splenectomised patients at the time of surgery was 24.0 years (range, 8–44 years), and the mean time from splenectomy to enrolment in this study was 5.0 years (range, 1–16 years).
Splenectomy was performed in patients with a haemoglobin level stably below 8.0 g/dL, patients with splenomegaly, or patients requiring regular transfusions. There were no significant differences in age, gender composition, age at onset and diagnosis, and age at starting blood transfusions between the splenectomised group and the non-splenectomised group. All subjects had been transfused with fewer than 20 units of blood and most patients had received fewer than 10 units. There was no significant difference in the blood transfusion volume between the two groups. Three patients were receiving iron chelator therapy, of whom two were non-splenectomised and had received deferiprone treatment for 3 and 6 months and one had been splenectomised and had been receiving combined treatment with deferoxamine and deferiprone for 5 months. A higher haemoglobin level, white blood cell count, and platelet count were detected in the splenectomised group as compared to those in the non-splenectomised group, while the total and direct bilirubin levels were lower in the splenectomised group than in the non-splenectomised group. The reticulocyte count was not, however, significantly different between the splenectomised and non-splenectomised groups (Table I).
Table I.
Comparison of clinical characteristics in patients with haemoglobin H disease according to whether they had been splenectomised or not. The results are expressed as mean±standard deviation.
| Characteristics | Splenectomised group | Non-splenectomised group | p |
|---|---|---|---|
| Number | 25 | 25 | |
| Age (years) | 29.0±8.2 | 27.9±6.0 | 0.628 |
| Sex (male/female) | 9/16 | 12/13 | 0.567 |
| Age of onset (years) | 7.4±6.4 | 8.5±7.5 | 0.559 |
| Age at diagnosis (years) | 11.4±8.6 | 12.3±8.9 | 0.724 |
| Height (cm) | 157.3±6.2 | 159.3±5.7 | 0.250 |
| Weight (kg) | 49.6±6.3 | 48.5±5.8 | 0.510 |
| Age at blood transfusion (years) | 13.1±9.2 | 9.0±6.7 | 0.079 |
| Accumulated volume of blood transfused* | - | - | 0.714 |
| Never transfused | 4 (16%) | 3 (12.0%) | - |
| 0.5–10 units | 16 (64%) | 14 (56.0%) | - |
| 10–20 units | 5 (19.3%) | 8 (32.0%) | - |
| Use of iron chelator agents (N, %) | 1 (4.0%) | 2 (8.0%) | 1.00 |
|
| |||
| Blood cell parameters | |||
| Platelet count (×109/L) | 490.6±119.2 | 175.8±70.0 | <0.001 |
| White blood cell count (×109/L) | 10.4±3.2 | 7.2±2.1 | <0.001 |
| Haemoglobin (g/dL) | 9.1±1.1 | 8.0±1.3 | 0.002 |
| Reticulocyte count (×109/L) | 342.2±124.8 | 317.4±105.1 | 0.452 |
|
| |||
| Liver function | |||
| Total bilirubin (μmol/L) | 34.8±17.9 | 68.4±37.4 | <0.001 |
| Indirect bilirubin (μmol/L) | 25.2±14.8 | 55.7±36.8 | 0.001 |
| Alanine transaminase (IU/L) | 28.6±26.8 | 22.7±17.0 | 0.409 |
|
| |||
| Haemoglobin analysis | |||
| Haemoglobin H (%) | 12.8±3.2 | 14.3±3.2 | 0.086 |
| Haemoglobin H (g/dL) | 1.2±0.4 | 1.2±0.4 | 0.830 |
| Haemoglobin CS (%) | 1.3±0.5 | 1.2±0.5 | 0.882 |
| Haemoglobin CS (g/dL) | 0.12±0.05 | 0.10±0.05 | 0.234 |
1 unit means red blood cells from 200 mL whole blood.
Levels of serum ferritin and iron-metabolism regulators
Of all 50 patients, 33 (66%) met the criteria for iron overload, with the incidence being 80% and 52% in the splenectomised group and the non-splenectomised group, respectively. Furthermore, 24% patients had a ferritin level higher than 1,000 ng/mL. The serum ferritin level was higher in the splenectomised group than in the non-splenectomised group (Table II).
Table II.
Comparison of iron metabolism-associated parameters in patients with haemoglobin H disease who had been splenectomised or not. The results are expressed as mean ± standard deviation.
| Carachteristics | Splenectomised group | Non-splenectomised group | Normal group | p |
|---|---|---|---|---|
| Number | 25 | 25 | 24 | - |
| Ferritin level (ng/mL) | 1,219.0±1,032.5a, b | 643.8±482.1 a | 236.5±163.6 | <0.001 |
| Iron overload (N, %) | 20(80.0) | 13(52.0) | - | 0.037 |
| sTfR (ng/mL) | 21,811.4±13,338.6a | 19,016.5±13,905.4a | 12,521.9±7,287.8 | 0.025 |
| Hepcidin (ng/mL) | 884.2±697.8 | 870.8±669.5 | 627.2±344.5 | 0.244 |
| GDF-15 (pg/mL) | 322.9±79.6 | 303.4±82.7 | 335.5±90.9 | 0.409 |
| Hepcidin/ferritin | 1.27±1.04a | 1.70±1.00a | 2.76±0.56 | <0.001 |
sTfR: serum transferrin receptor; GDF-15: growth differentiation factor 15;
p<0.05 vs the normal group;
p<0.05 vs the non-splenectomised group.
Of the 25 patients who had undergone splenectomy, 10 had records of serum ferritin levels immediately before splenectomy and a history of blood transfusion before and after splenectomy. As shown in Table III, after splenectomy, although the haemoglobin level increased and blood transfusion requirement decreased in patients with HbH CS, the serum ferritin level continued to increase.
Table III.
Clinical characteristics of patients with HbH CS before and after splenectomy.
| Case N. | Gender | Before splenectomy | At investigation | Transfusion (unit)* | Years post-operation (y) | Difference of ferritin (ng/mL) | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
| ||||||||||||
| Age (y) | Hb (g/dL) | Ferritin (ng/mL) | Age (y) | Hb (g/dL) | Ferritin (ng/mL) | NRBC (109/L) | Before splenectomy | After splenectomy | ||||
| 1 | Female | 15.6 | 65 | 285.6 | 18.6 | 83 | 737.31 | 0.32 | 2–10 | 0 | 3.0 | 451.71 |
| 2 | Male | 11.0 | 77 | 638.8 | 17.3 | 106 | 643.18 | 0.32 | 1 | 0 | 6.3 | 4.38 |
| 3 | Male | 16.8 | 60 | 841.2 | 23.1 | 101 | 710.51 | 0.29 | 2–10 | 0 | 6.3 | −130.69 |
| 4 | Female | 23.3 | 71 | 144.9 | 26.6 | 90 | 367.02 | 0.15 | 0 | 0 | 3.3 | 222.12 |
| 5 | Female | 20.6 | 69 | 235.12 | 25.6 | 95 | 1,054.04 | 0.3 | 10–20 | 0 | 5.0 | 818.92 |
| 6 | Female | 37.6 | 61 | 346.8 | 42.6 | 82 | 581.98 | 0.32 | 2–10 | 1 | 4.9 | 235.18 |
| 7 | Female | 25.1 | 68 | 103.5 | 26.8 | 87 | 504.24 | 0.12 | 0 | 0 | 1.7 | 400.74 |
| 8 | Male | 24.8 | 79 | 685 | 26.6 | 116 | 3,381.66 | 0.72 | 10–20 | 0 | 1.7 | 2,696.66 |
| 9 | Female | 20.2 | 75 | 352.6 | 20.9 | 77 | 585.54 | 0.29 | 0 | 0 | 0.8 | 232.94 |
| 10 | Female | 22.4 | 69 | 228.4 | 28.9 | 86 | 1,346.32 | 0.85 | 10–20 | 5 | 6.6 | 1,117.92 |
1 unit means red blood cells from 200 mL of whole blood.
The sTfR levels in both the splenectomised and non-splenectomised group were higher than those in normal populations, while no significant difference in the sTfR level was found between the splenectomised group and the non-splenectomised group. GDF-15 and hepcidin levels in patients with HbH CS disease, whether splenectomised or not, were not significantly different from those in normal populations (Table II).
Nucleated red blood cell count in peripheral blood and percentage of apoptotic erythroid precursor cells in bone marrow
NRBC were detected in 24% (6/25) of non-splenectomised patients and 100% (25/25) splenectomised patients (p<0.001). The NRBC count was higher in the splenectomised group than in the non-splenectomised group (0.412±0.435×109/L vs 0.013±0.026×109/L, p<0.001).
The percentages of apoptotic erythroid precursor cells were 1.4±0.90%, 2.8±1.5% and 1.2±0.36% in six non-splenectomised patients, six splenectomised patients and five normal subjects, respectively, with differences being statistically significant (p=0.047). Multiple comparisons showed that the percentage of apoptotic erythroid precursor cells in patients who had been splenectomised was significantly higher than that in patients who had not undergone splenectomy (p=0.036) or in the normal population (p=0.029), while no significant difference in the percentage of apoptotic erythroid precursor cells was detected between non-splenectomised patients and normal subjects (p=0.820).
Correlation analysis and multivariate linear regression analysis of factors affecting serum ferritin level
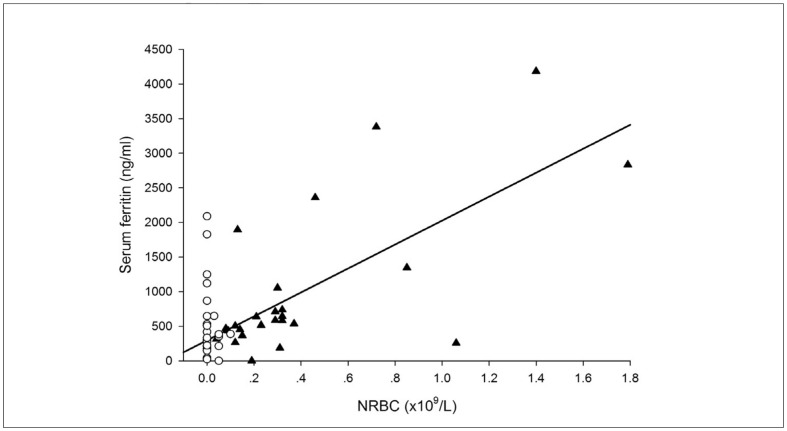
Correlation analysis revealed that serum ferritin level correlated with age, white blood cell count, NRBC count, reticulocyte count and haemoglobin level, but did not correlate with blood transfusion volume. When the patients were divided into splenectomised and non-splenectomised groups, the NRBC count was found to be associated with the serum ferritin level only in patients who had been splenectomised, while the haemoglobin level correlated with the serum ferritin level only in patients who had not undergone splenectomy (Table IV, Figure 1).
Table IV.
Correlation analysis of serum ferritin levels in patients with HbH CS disease divided according to whether they had or had not been splenectomised. The results are expressed as mean ± standard deviation.
| Affecting factor | Splenectomised group (N=25) | Non-splenectomised group (N=25) | Total (N=50) | |||
|---|---|---|---|---|---|---|
|
| ||||||
| r | p | R | p | r | p | |
| HbH | 0.163 | 0.441 | 0.585 | 0.002 | 0.170 | 0.237 |
| NRBC | 0.530 | 0.006 | 0.057 | 0.244 | 0.564 | <0.001 |
| Reticulocytes | 0.347 | 0.089 | 0.196 | 0.348 | 0.312 | 0.027 |
| Haemoglobin | 0.066 | 0.754 | 0.452 | 0.023 | 0.305 | 0.031 |
| Age | 0.589 | 0.002 | 0.625 | 0.001 | 0.574 | <0.001 |
| Transfusion | 0.170 | 0.416 | 0.055 | 0.793 | 0.039 | 0.789 |
| GDF-15 | 0.594 | 0.002 | 0.467 | 0.019 | 0.523 | <0.001 |
| sTfR | 0.170 | 0.170 | 0.683 | <0.001 | 0.131 | 0.365 |
| Hepcidin | 0.325 | 0.113 | 0.382 | 0.059 | −0.091 | 0.530 |
HbH: haemoglobin H; NRBC: nucleated red blood cells; sTfR: serum transferrin receptor
Figure 1.
Correlation analysis of NRBC and serum ferritin level in patients with HbH CS who had or had not been splenectomised.
NRBC: nucleated red blood cells; S: splenectomised; N: non-splenectomised. SF: serum ferritin. NRBC is significantly correlated with serum ferritin in the S group (▲) but not in the N group (○).
The variables with statistical significance revealed by correlation analysis were entered into the multivariate linear regression analysis, and age was found to be an independent risk factor affecting the serum ferritin level in patients who had been splenectomised (b=63.1±18.8, p=0.003) and in those who had not undergone splenectomy (b=43.9±10.8, p=0.001). In addition, multivariate linear regression analyses revealed that NRBC count was an independent risk factor affecting the serum ferritin level in patients with splenectomy (b=999.1±223.8, p=0.009).
Discussion
It is commonly accepted that the incidence of iron overload is low in patients with HbH disease. In one Chinese study, serum ferritin levels were found to be higher than 1,000 ng/mL in only four out of 104 patients (3.8%) with HbH disease14. In another study by Origa and colleagues, only five of 261 patients (1.9%) with HbH disease had a serum ferritin around 1,000 ng/mL15. In the current study, if iron overload is defined as a serum ferritin >500 ng/mL, the incidence of iron overload was 66% in adult patients with HbH CS disease. Furthermore, 24% of patients had a ferritin level higher than 1,000 ng/mL. This may be associated with the following factors: (i) a higher iron burden in patients with HbH CS disease than in patients with deletional HbH disease16, and (ii) a higher iron burden in adult patients than in children with HbH disease17, because serum ferritin levels are age-related4. Hsu et al.18 reported a 50% incidence of iron overload in adult patients with HbH disease, which was similar to the findings in this study. This demonstrates that iron overload is not rare in patients with HbH CS disease and indicates that vigilance is required.
Tso and colleagues19 did not observe an effect of splenectomy on the serum ferritin level in patients with HbH disease. However, only three patients with HbH disease underwent splenectomy in their study, so the conclusions cannot be considered very persuasive. In our previous study we found a high serum ferritin level in patients with HbH CS disease undergoing splenectomy8, a finding which was validated in the present study excluding the impact of blood transfusion and iron chelator therapy, suggesting that splenectomy does not alleviate the iron burden in patients with HbH CS. In addition, we observed a rapid rise in the serum ferritin level in patients with HbH CS disease following splenectomy. Of the ten subjects with records of serum ferritin levels before and after splenectomy, most did not receive blood transfusions after splenectomy; however, the 4-year mean rise in the serum ferritin level was about 700 ng/mL, indicating a mean annual rise in the serum ferritin level of 175 ng/mL, whereas the mean annual rise in the serum ferritin level in the patients who had not undergone splenectomy was only about 40 ng/mL.
Ineffective erythropoiesis has been found to be the basic mechanism of iron overload in patients with TI. It appears that NRBC can partly reflect the degree of ineffective erythropoiesis20, and NRBC count has been found to be increased in splenectomised patients with TI21. We found that the rate of NRBC and NRBC count in the peripheral blood of the patients with HbH CS disease who had been splenectomised were higher than those in the patients who had not been splenectomised. The increase in the NRBC count could be attributed to a reduction in NRBC destruction following splenectomy. However, we noted that the count of reticulocytes, another type of prophase red blood cells, was not significantly different between the splenectomised group and the non-splenectomised group, demonstrating that reduced destruction is not sufficient to explain the increased NRBC count after splenectomy in patients with HbH CS disease. It has been demonstrated that splenectomy does not cause the increase in the NRBC count in another form of haemoglobin diseases that lacks ineffective erythropoiesis - sickle cell anaemia12. The increase in the NRBC count, therefore, infers the existence of ineffective erythropoiesis in patients with HbH CS disease after splenectomy. The percentage of apoptotic bone marrow erythroid precursor cells is the most direct indicator of ineffective erythropoiesis22. Like the results from the study by Pootrakul et al.23, there was no significant difference in the percentage of apoptotic bone marrow erythroid precursor cells between non-splenectomised patients with HbH CS disease and the normal population. However, the percentage of apoptotic bone marrow erythroid precursor cells in patients with HbH CS disease who had been splenectomised was higher than that in the normal population and in patients who had not been splenectomised. A prospective study was required to clarify the relationship between ineffective erythropoiesis and splenectomy in patients with HbH CS.
It has been reported that the iron overload induced by ineffective erythropoiesis is associated with the GDF-15 and hepcidin in patients with TI24,25. GDF-15 and sTfR levels have been shown to reflect the status of haematopoiesis26,27. As indicated by Papassotiriou et al.28, an increase in the sTfR level was detected in the non-splenectomised patients with HbH CS disease, indicating exuberant compensatory haematopoiesis induced by anaemia. Our findings showed that splenectomy alleviated anaemia but did not reduce the sTfR level. A remarkable rise in the GDF-15 level and significant decline in the hepcidin level have been detected in patients with TI, but were not observed in patients with HbH CS disease, whether splenectomised or not. The hepcidin/ferritin ratio reflects the influence of iron burden on hepcidin29,30. Patients with HbH CS disease had a lower hepcidin/ferritin ratio than that of normal subjects and there was a tendency for splenectomised patients to have a lower hepcidin/ferritin ratio than that of non-splenectomised patients (p=0.077), demonstrating the existence of excessive haematopoiesis and disordered regulation of iron metabolism in patients with HbH CS disease.
Multivariate linear regression analysis revealed that age was an independent risk factor affecting the serum ferritin level in patients with HbH CS disease whether splenectomised or not. There was no significant correlation between blood transfusions and serum ferritin level, demonstrating that the iron burden mainly originated from gastrointestinal absorption of iron in patients with HbH CS disease. When the patients with HbH CS disease were divided into those who had or had not been splenectomised, the NRBC count was found to be an independent risk factor affecting the serum ferritin level in the splenectomised group, indicating that the impact of splenectomy on serum ferritin level may be associated with intensified erythroid haematopoiesis. It has also been suggested that the spleen is functional in eradicating non-transferrin bound iron13, thus an increasing ferritin level in splenectomised patients with HbH CS may be a reflection of abnormal redistribution of body iron.
This study had some shortcomings. It was a cross-sectional survey and only patients with severe HbH CS disease choose to undergo splenectomy. It cannot, therefore, be excluded that the high serum ferritin level in the splenectomised group was caused by severe disease before the splenectomy. Furthermore, it has been found that the serum ferritin level does not accurately reflect the iron burden in the liver and heart of patients with TI9. Serum ferritin level alone cannot be used to comprehensively compare iron burden between the two groups. Further prospective studies with more advanced techniques of detection are required to evaluate the effect of splenectomy on ineffective erythropoiesis and iron burden in patients with HbH CS disease.
Acknowledgements
This study was sponsored by Guangxi Science-Tech Tackle Key Project (1355005-2-1) and the Natural Science Foundation of Guangxi, China (2011GXNSFA018196 and 2012GXNSFDA053018).
Footnotes
The Authors declare no conflicts of interest.
References
- 1.Xiong F, Sun M, Zhang X, et al. Molecular epidemiological survey of haemoglobinopathies in the Guangxi Zhuang Autonomous Region of southern China. Clin Genet. 2010;78:139–48. doi: 10.1111/j.1399-0004.2010.01430.x. [DOI] [PubMed] [Google Scholar]
- 2.Xu XM, Zhou YQ, Luo GX, et al. The prevalence and spectrum of alpha and beta thalassaemia in Guangdong Province: implications for the future health burden and population screening. J Clin Pathol. 2004;57:517–22. doi: 10.1136/jcp.2003.014456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Tan JA, Lee PC, Wee YC, et al. High prevalence of alpha- and beta-thalassemia in the Kadazandusuns in East Malaysia: challenges in providing effective health care for an indigenous group. J Biomed Biotechnol. 2010;2010:706872. doi: 10.1155/2010/706872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chen FE, Ooi C, Ha SY, et al. Genetic and clinical features of hemoglobin H disease in Chinese patients. N Engl J Med. 2000;343:544–50. doi: 10.1056/NEJM200008243430804. [DOI] [PubMed] [Google Scholar]
- 5.Rachmilewitz EA, Giardina PJ. How I treat thalassemia. Blood. 2011;118:3479–88. doi: 10.1182/blood-2010-08-300335. [DOI] [PubMed] [Google Scholar]
- 6.Rigas DA, Koler RD. Decreased erythrocyte survival in hemoglobin H disease as a result of the abnormal properties of hemoglobin H: the benefit of splenectomy. Blood. 1961;18:1–17. [PubMed] [Google Scholar]
- 7.Al-Salem AH, Nasserulla Z. Splenectomy for children with thalassemia. Int Surg. 2002;87:269–73. [PubMed] [Google Scholar]
- 8.Yin XL, Zhang XH, Wu ZK, et al. Pulmonary hypertension risk in patients with hemoglobin H disease: low incidence and absence of correlation with splenectomy. Acta Haematol. 2013;130:153–9. doi: 10.1159/000347177. [DOI] [PubMed] [Google Scholar]
- 9.Musallam KM, Cappellini MD, Wood JC, Taher AT. Iron overload in non-transfusion-dependent thalassemia: a clinical perspective. Blood Rev. 2012;26:S16–9. doi: 10.1016/S0268-960X(12)70006-1. [DOI] [PubMed] [Google Scholar]
- 10.Taher AT, Musallam KM, Cappellini MD, Weatherall DJ. Optimal management of beta thalassaemia intermedia. Br J Haematol. 2011;152:512–23. doi: 10.1111/j.1365-2141.2010.08486.x. [DOI] [PubMed] [Google Scholar]
- 11.Piperno A, Mariani R, Trombini P, Girelli D. Hepcidin modulation in human diseases: from research to clinic. World J Gastroenterol. 2009;15:538–51. doi: 10.3748/wjg.15.538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kalpravidh RW, Tangjaidee T, Hatairaktham S, et al. Glutathione redox system in beta -thalassemia/Hb E patients. Scientific World Journal. 2013;2013:543973. doi: 10.1155/2013/543973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Taher A, Musallam KM, El Rassi F, et al. Levels of non-transferrin-bound iron as an index of iron overload in patients with thalassaemia intermedia. Br J Haematol. 2009;146:569–72. doi: 10.1111/j.1365-2141.2009.07810.x. [DOI] [PubMed] [Google Scholar]
- 14.Yin XL, Zhang XH, Zhou TH, et al. Hemoglobin H disease in Guangxi province, Southern China: clinical review of 357 patients. Acta Haematol. 2010;124:86–91. doi: 10.1159/000314058. [DOI] [PubMed] [Google Scholar]
- 15.Origa R, Sollaino MC, Giagu N, et al. Clinical and molecular analysis of haemoglobin H disease in Sardinia: haematological, obstetric and cardiac aspects in patients with different genotypes. Br J Haematol. 2007;136:326–32. doi: 10.1111/j.1365-2141.2006.06423.x. [DOI] [PubMed] [Google Scholar]
- 16.Lal A, Goldrich ML, Haines DA, et al. Heterogeneity of hemoglobin H disease in childhood. N Engl J Med. 2011;364:710–8. doi: 10.1056/NEJMoa1010174. [DOI] [PubMed] [Google Scholar]
- 17.Chui DH, Fucharoen S, Chan V. Hemoglobin H disease: not necessarily a benign disorder. Blood. 2003;101:791–800. doi: 10.1182/blood-2002-07-1975. [DOI] [PubMed] [Google Scholar]
- 18.Hsu HC, Lin CK, Tsay SH, et al. Iron overload in Chinese patients with hemoglobin H disease. Am J Hematol. 1990;34:287–90. doi: 10.1002/ajh.2830340410. [DOI] [PubMed] [Google Scholar]
- 19.Tso SC, Loh TT, Todd D. Iron overload in patients with haemoglobin H disease. Scand J Haematol. 1984;32:391–4. doi: 10.1111/j.1600-0609.1984.tb00694.x. [DOI] [PubMed] [Google Scholar]
- 20.Mathias LA, Fisher TC, Zeng L, et al. Ineffective erythropoiesis in beta-thalassemia major is due to apoptosis at the polychromatophilic normoblast stage. Exp Hematol. 2000;28:1343–53. doi: 10.1016/s0301-472x(00)00555-5. [DOI] [PubMed] [Google Scholar]
- 21.Taher AT, Musallam KM, Karimi M, et al. Splenectomy and thrombosis: the case of thalassemia intermedia. J Thromb Haemost. 2010;8:2152–8. doi: 10.1111/j.1538-7836.2010.03940.x. [DOI] [PubMed] [Google Scholar]
- 22.Yuan J, Angelucci E, Lucarelli G, et al. Accelerated programmed cell death (apoptosis) in erythroid precursors of patients with severe beta-thalassemia (Cooley’s anemia) Blood. 1993;82:374–7. [PubMed] [Google Scholar]
- 23.Pootrakul P, Sirankapracha P, Hemsorach, et al. A correlation of erythrokinetics, ineffective erythropoiesis, and erythroid precursor apoptosis in Thai patients with thalassemia. Blood. 2000;96:2606–12. [PubMed] [Google Scholar]
- 24.Gardenghi S, Marongiu MF, Ramos P, et al. Ineffective erythropoiesis in beta-thalassemia is characterized by increased iron absorption mediated by down-regulation of hepcidin and up-regulation of ferroportin. Blood. 2007;109:5027–35. doi: 10.1182/blood-2006-09-048868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rivella S. The role of ineffective erythropoiesis in non-transfusion-dependent thalassemia. Blood Rev. 2012;26(Suppl 1):S12–5. doi: 10.1016/S0268-960X(12)70005-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Musallam KM, Taher AT, Duca L, et al. Levels of growth differentiation factor-15 are high and correlate with clinical severity in transfusion-independent patients with beta thalassemia intermedia. Blood Cells Mol Dis. 2011;47:232–4. doi: 10.1016/j.bcmd.2011.07.005. [DOI] [PubMed] [Google Scholar]
- 27.Camaschella C, Gonella S, Calabrese R, et al. Serum erythropoietin and circulating transferrin receptor in thalassemia intermedia patients with heterogeneous genotypes. Haematologica. 1996;81:397–403. [PubMed] [Google Scholar]
- 28.Papassotiriou I, Traeger-Synodinos J, Kanavakis E, et al. Erythroid marrow activity and hemoglobin H levels in hemoglobin H disease. J Pediatr Hematol Oncol. 1998;20:539–44. [PubMed] [Google Scholar]
- 29.El BA, Alaraby I, Abdel KMS, et al. Study of serum hepcidin in hereditary hemolytic anemias. Hemoglobin. 2012;36:555–70. doi: 10.3109/03630269.2012.721151. [DOI] [PubMed] [Google Scholar]
- 30.Origa R, Galanello R, Ganz T, et al. Liver iron concentrations and urinary hepcidin in beta-thalassemia. Haematologica. 2007;92:583–8. doi: 10.3324/haematol.10842. [DOI] [PubMed] [Google Scholar]