Abstract
Background
Iron homeostasis in humans is tightly regulated by mechanisms aimed to conserve iron for reutilisation, with a negligible role played by excretory mechanisms. In a previous study we found that mice have an astonishing ability to tolerate very high doses of parenterally administered iron dextran. Whether this ability is linked to the existence of an excretory pathway remains to be ascertained.
Materials and methods
Iron overload was generated by intraperitoneal injections of iron dextran (1 g/kg) administered once a week for 8 weeks in two different mouse strains (C57bl/6 and B6D2F1). Urinary and faecal iron excretion was assessed by inductively coupling plasma-mass spectrometry, whereas cardiac and liver architecture was evaluated by echocardiography and histological methods. For both strains, 24-hour faeces and urine samples were collected and iron concentration was determined on days 0, 1 and 2 after iron administration.
Results
In iron-overloaded C57bl/6 mice, the faecal iron concentration increased by 218% and 157% on days 1 and 2, respectively (p<0.01). The iron excreted represented a loss of 14% of total iron administered. Similar but smaller changes was also found in B6D2F1 mice. Conversely, we found no significant changes in the concentration of iron in the urine in either of the strains of mice. In both strains, histological examination showed accumulation of iron in the liver and heart which tended to decrease over time.
Conclusions
This study indicates that mice have a mechanism for removal of excess body iron and provides insights into the possible mechanisms of excretion.
Keywords: excretion, gut, iron overload, packed red blood cells, transfusional therapy
Introduction
Patients with chronic anaemia may require life-saving treatment consisting of prolonged transfusion therapy. A downside to this treatment is that it delivers about 250 mg of iron for every unit of transfused packed red blood cells. Humans have sophisticated molecular mechanisms which tightly regulate the absorption of dietary iron in the duodenum as well as the uptake of iron into the cells of other tissues1. When iron concentration increases in the cell, the metal binds iron regulatory proteins (IRP1/2) which in turn promote or inhibit the expression of genes involved in iron homeostasis such as TfR1/2, DMT-1, Dcytb, hephaestin and ferritin. However, the human body seems to have no significant physiological mechanisms for actively eliminating excess iron and, for this reason, following transfusions the iron accumulates in the body, especially in some organs such as the liver, heart, spleen, bone marrow and pituitary gland, leading to organ damage2. Adults with transfusional iron overload begin to exhibit signs and symptoms of organ damage after receiving 100 units of packed red blood cells or 25 g of iron (1 mL of packed red blood cells=1 mg iron; 1 unit of packed red blood cells=250 mL=250 mg iron), which represents a total dose of approximately 0.36 g/kg in a 70-kg man.
The leading cause of death in patients with secondary haemocromatosis is iron overload cardiomyopathy, a disease characterised by systolic and diastolic dysfunction, increased propensity to arrhythmias and end-stage dilated cardiomyopathy3. At present, the main strategy used to prevent the onset of transfusional iron overload is chelation therapy. Chelating agents allow active elimination of iron through urine and faeces, although it has been observed that therapy with chelating agents is often not sufficient to prevent the onset of the disease and, additionally, it is associated with significant toxic effects4. Preventing iron overload does, therefore, remain a major goal in the management of patients requiring chronic transfusion therapy.
Unlike human beings, other mammals such as rats and mice seem tolerate very high doses of iron, of the order of several grams per kilogram, i.e. 10–20 times higher than those that cause symptoms in humans. The existence of a excretory process rather than better distribution of iron within the body may be the cause of this astonishing resistance to iron overload-induced damage. Previous studies in rats support this hypothesis. Indeed, two independent groups reported that a significant loss of hepatic iron occurs when iron-overloaded rats are switched to a low iron diet5,6. Additionally, Oates et al.6 demonstrated the existence of an excretory process for iron in iron-overloaded rats. Whether a similar excretory process also occurs in mice remains to be ascertained. If this is the case, the murine models of iron overload provide a unique opportunity to study the physiological mechanisms that ensure the removal of excess iron.
In a previous study, we found that parenteral administration of high doses of iron dextran, whose fate within the body is similar to that of haemoglobin iron, does not cause either cardiac dysfunction or pathological ventricular remodelling in C57bl/6 mice7. These results led us to speculate that the C57bl/6 genetic background confers cardioprotection against iron overload injury through a mechanism of elimination of excess iron. However, a comparison between different strains of mice is lacking and, in particularly, it is not known whether the C57bl/6 strain is able to eliminate excess iron through the digestive tract or the kidneys. To answer these questions, in the present study, iron overload was induced in two different strains, namely C57 bl/6 and B6D2F1, by injecting iron dextran for 2 months, as described by Tsay et al.8. During the study, urinary and faecal iron excretion were assessed by quadrupole inductively coupled plasma mass spectrometry (Q-ICP-MS) after wet-ashing, whereas cardiac function, ventricular remodelling and liver fibrosis were evaluated by echocardiography and histological methods.
Materials and methods
Animals
Eight-week old, male B6D2F1 and C57bl/6 mice (Charles River, Calco, Italy) were housed (4 per cage) in controlled conditions of humidity and temperature (22±1 °C) with a 12-hour/12-hour light-dark cycle (lights on at 7:00 am). The mice were left to adapt to their surroundings for a week before commencing treatments and were given access to food pellets and drinking water ad libitum; the rodent diet included 165 ppm of iron. All the procedures followed in this work were in compliance with the European Community Council Directive of 24 November 1986 (86/609/EEC). All efforts were made to minimise animal suffering, and to reduce the number of animals studied.
Mouse model of iron overload
To induce iron overload, B6D2F1 and C57bl/6 male mice were treated intraperitoneally once a week with iron dextran (1 g/kg), a complex of ferric hydroxide and dextran (Sigma Aldrich, Milan, Italy), or vehicle (phosphate-buffered saline, PBS) for 8 consecutive weeks, as described by Tsay et al.8. A total of 24 mice were studied, 12 of each strain. Both mouse strains were each divided into two further groups: one group (n=8 mice) was given iron dextran, the other, control group (n=4 mice) were administered the vehicle. Cardiac function and liver architecture were evaluated by echocardiography and histological methods 5 weeks after the last administration of iron (13-week group). In another series of experiments the animals were studied 1 week after the last administration of iron (9-week group) to check if there was proportionality between the duration of iron overload and tissue damage. Again, a total of 24 mice were studied, 12 of each strain.
Faecal and urinary iron measurements
In order to evaluate the faecal and urinary iron concentration, animals were located into acrylic glass metabolic cages designed for mice. Twenty-four hour faeces and urine samples were collected and iron concentration was determined only on days 0 (to determine the background level), 1 and 2 after iron administration. The amount of iron in the samples was determined by Q-ICP-MS using a DRCII spectrometer (Perkin-Elmer, Norwalk, CT, USA) equipped with a cyclonic quartz spray chamber. The Q-ICP-MS system was set up as follows: radiofrequency power, 1100 W; lens voltage ranging from 7 to 8 V; nebuliser gas flow ranging from 0.96 to 11 L/min; peristaltic pump speed, 1.0 mL/min. The analytical masses 56Fe and 57Fe were selected for quantification. Internal standardisation by 103Rh at a concentration of 2 μg/L in the analytical solutions was used to control for drifts of the instrument and matrix effects. The effect of the operating conditions of the Dynamic Reaction Cell system was studied to get the best signal-to-background ratio (NH3 flow 0.5 L/min, RPq=0.7). The method’s validation parameters such as detection limit, sensitivity, and precision have been described for all elements. Calibrators and internal standard solution (rhodium) were obtained daily from standard certified solutions containing 1 mg/mL of all elements (Carlo Erba Reagenti SpA, Rodano, Milan, Italy.), followed by dilution with acidified (HNO3) deionised water as necessary. Results are expressed as mg of iron excreted per kg sample weight.
Echocardiography
Thirteen weeks after starting the iron overload procedure, the mice of both strains were intubated and anaesthetised with isoflurane (1% in 100% of oxygen) and an echocardiographic examination was performed with a SONOLINE G50 (Siemens AG, Erlangen, Germany) equipped with a 13-MHz imaging transducer. After good-quality, two-dimensional, short-axis images of the left ventricle had been obtained, M-mode freeze frames were printed on common echocardiographic paper and digitalised. Left ventricular end-systolic (LVESD) and end-diastolic (LVEDD) internal diameters, and posterior wall end-diastolic thickness were measured by an image-analysis system (Metamorph, Universal Image Corporation, West Chester, Pennsylvania, USA). Percent fractional shortening was calculated as (LVEDD–LVESD/LVEDD)×100. An identical experimental protocol was also performed in the 9-week group.
Histological analysis
Histological analysis was performed as reported previously7. Briefly, the left ventricle and a part of the liver were cut, fixed and stained with haematoxylin and eosin, with the sirius red/picric acid method to determine fibrosis, or Prussian blue to assess iron stores.
Statistical analysis
Group means (±standard error, SE) were calculated for all relevant variables. The statistical analysis was performed using Student’s t test or ANOVA with Bonferroni’s multiple comparison test for post-hoc analyses. P values less than 0.05 were considered to be statistically significant.
Results
Iron administration affects body growth, heart and liver mass
At 13 weeks, neither mortality nor apparent signs of illness or heart failure (e.g. lethargy, ascites, peripheral oedema) were observed in any of the groups of animals. However, iron-treated mice of both strains showed coarse fur with gradually darkening skin, a significant delay in body weight increase and a decrease in heart weight along with a marked increase in liver weight (hepatomegaly) (Table I). Similar results were also found in the 9-week groups after administration of iron (data not shown).
Table I.
Weights of the body, heart and liver in 13-week old mice treated with iron dextran or vehicle.
| Groups | Body weight (g) | Heart-to-body weight ratio (mg/g) | Liver-to-body weight ratio (mg/g) |
|---|---|---|---|
| C57bl/6 + PBS | 28.4±1.3 | 3.7±0.2 | 41.8±3.2 |
| C57bl/6 + Fe | 25.8±1.6 | 3.3±0.1 | 124.7±6.3 |
| B6D2F1 +PBS | 31.9±2.0 | 5.1±0.2 | 47.8±2.4 |
| B6D2F1 + Fe | 27.9±2.2 | 3.7±0.2 | 120.4±11.7 |
PBS: phosphate-buffered saline (vehicle); Fe: iron.
Iron accumulates in the liver
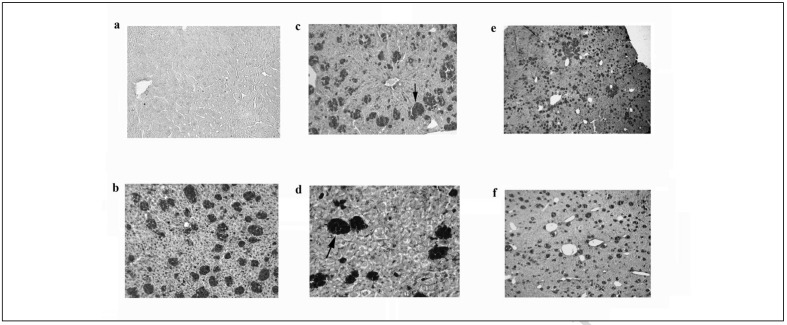
At 13 weeks, the liver sections of vehicle-treated mice of both strains showed normal cell morphology with well-preserved cytoplasm and a normal lobular architecture with no apparent iron accumulation (Figure 1a). As expected, parenteral iron dextran administration increased reticuloendothelial and also parenchymal iron in the liver of both strains. The liver sections of iron-overloaded C57bl/6 mice showed large periportal and centrilobular siderophage accumulations that did not alter liver lobular architecture and moderate amounts of iron in the parenchyma (Figure 1b–d). No signs of either necrosis or fatty changes appeared. Furthermore, no signs of fibrosis were observed (Figure 1c). Similar results were found in the B6D2F1 mice after iron administration (data not shown). Although we did not measure the amount of liver iron, the iron stores in the liver appeared reduced in C57bl/6 mice evaluated at 13 weeks compared to those at 9 weeks suggesting that a process of elimination was in progress (Figure 1e, f). Again, similar findings were also observed in the B6D2F1 mice after iron administration.
Figure 1.
Histological effects of iron accumulation on mice liver after iron dextran administration.
(a,b) Representative haematoxylin and eosin staining of liver sections. Panel (a) shows the histology of C57bl/6 control mouse liver (×100), with normal lobular architecture and without signs of iron accumulation. Panel (b) shows marked iron deposition in Küppfer cells (arrow) and, to a lesser extent, in hepatocytes of C57bl/6 mice treated with iron dextran (×100). (c, d) Picrosirius red (×100) and Prussian blue (×200) staining of liver sections in C57bl/6 iron-overloaded mice, respectively. Iron accumulation is clearly evident (arrow). However no signs of fibrosis are present. (e, f) Representative haematoxylin and eosin staining (×25) of iron-treated mouse liver at 9 and 13 weeks, respectively (see Material and methods section for more details). The iron stores in the liver appeared reduced in mice evaluated at 13 weeks compared to those at 9 weeks. Vehicle, phosphate buffer saline-treated mice; Iron, iron-treated mice.
Cardiac effects of iron overload
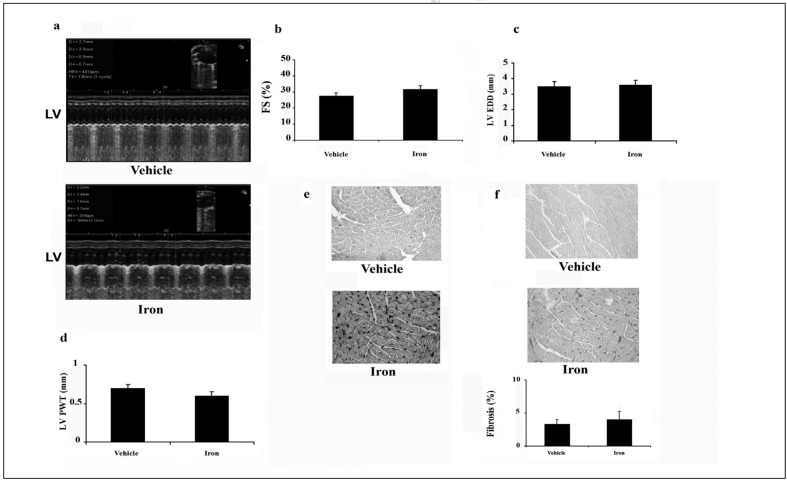
At 13 weeks, no significant differences in systolic function, left ventricular wall thickness or diastolic dimension were found between iron- and vehicle-treated C57bl/6 mice as revealed by transthoracic echocardiography (Figure 2a–d), which confirms our previous results6. Iron staining of myocytes was observed in all hearts from iron-overloaded C57bl/6 mice (Figure 2e). Iron staining was also noted in the interstitium of the myocardium, endocardium and epicardium. However, iron overload was not accompanied by cardiac interstitial fibrosis as evidenced by histological and histomorphometric analyses (Figure 2f). Control hearts showed no evidence of iron accumulation or fibrosis (Figure 2e,f). Similar results were also observed in the B6D2F1 mice after iron administration (data not shown).
Figure 2.
Preserved cardiac structure and function in iron dextran-loaded mice after 13 weeks.
(a) Representative transthoracic echocardiographic tracings from the vehicle-treated and iron-treated mice; LV, left ventricle. (b) Fractional shortening (FS), (c) left ventricular end-diastolic diameter (LV EDD), and (d) left ventricular posterior wall thickness (LV PWT). Echocardiographic analysis revealed no significant changes between vehicle and iron-treated groups. (e and f) Representative Prussian blue and picrosirius red staining. (e) Prussian blue staining shows visible accumulation of iron both inside and outside of cardiomyocytes in iron-treated mice (bottom panel). (f) Conversely, there is no collagen accumulation in iron-treated mice as evidenced by picrosirius red staining (middle and bottom panels). Vehicle, phosphate buffer saline-treated mice; iron, iron-treated mice.
Iron excretion in iron-overloaded mice
To assess whether mice have a mechanism to excrete excess iron that protects them from organ damage, iron concentration was determined before, which represents the amount of iron that is derived from the diet and is not absorbed from the digestive tract, and after the parenteral administration of iron dextran at the dose of 1g/kg. Iron excretion in urine and faeces was followed for 2 days after iron administration. In both mouse strains, the urinary iron clearance was negligible (<0.5% of the iron administered). Conversely, in iron-overloaded C57bl/6 mice, the faecal iron concentration increased by 118% and 58% on days 1 and 2, respectively (p<0.01). The iron excreted represented a loss of 14% of total iron administered. Similar but smaller changes was also found in iron-overloaded B6D2F1 mice in which the iron excreted in the faeces was 7% of the total iron administered.
Discussion
In a previous study, we found that the well-characterized, inbred C57bl/6 mouse strain does not display either cardiac dysfunction or pathological ventricular remodelling in response to parenteral iron overload7. These results led us to speculate that the C57bl/6 genetic background confers cardioprotection against iron overload injury through a mechanism of elimination of excess iron. In view of this possibility, we measured iron in the urine and faeces of mice under conditions of iron overload. Here we found that the amount of iron in the faeces is much larger in iron-overloaded animals than in controls indicating that C57bl/6 mice are able to remove a significant amount of the excess iron and that the gastrointestinal tract is a major route of iron excretion in this mouse strain. To our knowledge this is the first study to report that mice have an efficient mechanism of excretion of excess iron and to show an association between iron elimination and the lack of cardiac remodelling or dysfunction in an animal model of iron overload.
Although the amount of iron eliminated within 48 hours was approximately 14% of the total iron administered and the rate of elimination of iron did not differ significantly during the observation period, at the moment, we do not know whether the ability to remove iron observed in the present study would be enough to restore iron-overloaded animals to a normal iron status or the level of body iron stores at which this excretory process becomes active. For example, Bonkovsky et al.4 reported that, in iron-overloaded rats, the change to a diet free of iron caused a fall of 50% in total liver iron which stabilised at a level 10 times higher than that in controls. Whether this also occurs in mice remains to be determined.
We also wondered if the ability to eliminate excess iron is a specific feature of C57bl/6 mice or is shared by other strains. Here, we show that even the hybrid B6D2F1 strain has the ability to eliminate excess iron and, like the C57bl/6 strain, does not show signs of myocardial dysfunction or cardiac fibrosis despite the administration of large amounts of iron dextran by injection. These results seem to be in contradiction with those of another study in which the effects of chronic administration of iron dextran on cardiac function and geometry were determined9–11. Indeed, it was reported that the parenteral administration of iron dextran in hybrid B6D2F1 mice at a total cumulative dose of about 200 mg per 25g of body weight caused cardiac iron accumulation, myocardial dysfunction and cardiac fibrosis after 13 weeks of dosing9–11. Although the reasons for these differences in response to the same cumulative dose of iron dextran remain to be elucidated, they may be due to the different treatment schedules (one injection per day for 5 days/week for 13 weeks versus one injection per week for 8 weeks) or to the development of infections due to repeated intraperitoneal injections, which can greatly complicate the interpretation of results.
It is thought that iron stores in the body are exclusively regulated by mechanisms related to the rate of absorption of iron from the diet, with no significant role played by iron excretion processes. This is definitely true for humans, but in other species the situation seems to be rather different. For example, in the rat, it has been reported that there is a major excretory process for iron that results in the loss of significant amounts of liver iron when animals are switched to a diet free of iron6. The results of our study indicate that the mouse also possesses an efficient mechanism for iron removal. However, whether the elimination of the iron takes place via the bile, the enterocytes or other cells of the digestive tract remains to be investigated in the future studies.
The presence of a mechanism for the elimination of excess iron in mammals such as mice raises a number of very important issues. Is there a similar mechanism in humans? Is it dormant? Can it be regulated pharmacologically? At the moment it is very difficult to predict whether the results of this study have clinical implications. Further studies are needed to understand the molecular mechanism underlying the process of iron removal in mice.
In conclusion, in the present study we demonstrated that, under conditions of iron overload, the mouse, in particular the strains C57bl/6 and B6D2F1, is capable of eliminating significant amounts of iron through the digestive tract via an unknown mechanism. Although this mouse model of secondary haemochromatosis does not mimic the human condition, it provides an unique opportunity to study the mechanisms of iron removal under iron-overloaded conditions.
Acknowledgements
This work was supported in part by grants from the National Blood Centre (CNS8/2012) to Giuseppe Marano.
Footnotes
The Authors declare no conflict of interest.
References
- 1.Ganz T, Nemeth E. Iron imports. IV. Hepcidin and regulation of body iron metabolism. Am J Physiol. 2006;290:G199–G203. doi: 10.1152/ajpgi.00412.2005. [DOI] [PubMed] [Google Scholar]
- 2.Porter JB. Practical management of iron overload. Br J Haematol. 2001;115:239–52. doi: 10.1046/j.1365-2141.2001.03195.x. [DOI] [PubMed] [Google Scholar]
- 3.Borgna-Pignatti C, Rugolotto S, De Stefano P, et al. Survival and complications in patients with thalassemia major treated with transfusion and deferoxamine. Haematologica. 2004;89:1187–93. [PubMed] [Google Scholar]
- 4.Silvestri L, Pagani A, Camaschella C, et al. The serine protease matriptase-2 (TMPRSS6) inhibits hepcidin activation by cleaving membrane hemojuvelin. Cell Metab. 2008;8:502–11. doi: 10.1016/j.cmet.2008.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bonkovsky HL, Healey JF, Lincoln B, et al. Hepatic heme synthesis in a new model of experimental hemochromatosis: studies in rats fed finely divided elemental iron. Hepatology. 1987;7:1195–203. doi: 10.1002/hep.1840070605. [DOI] [PubMed] [Google Scholar]
- 6.Oates PS, Jeffrey GP, Basclain KA, et al. Iron excretion in iron-overloaded rats following the change from an iron-loaded to an iron-deficient diet. J Gastroenterol Hepatol. 2000;15:665–74. doi: 10.1046/j.1440-1746.2000.02210.x. [DOI] [PubMed] [Google Scholar]
- 7.Musumeci M, Maccari S, Catalano L, et al. The C57BL/6 genetic background confers cardioprotection in iron-overloaded mice. Blood Transfus. 2013;11:88–93. doi: 10.2450/2012.0176-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tsay J, Yang Z, Ross FP, et al. Bone loss caused by iron overload in a murine model: importance of oxidative stress. Blood. 2010;116:2582–9. doi: 10.1182/blood-2009-12-260083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Oudit GY, Trivieri MG, Khaper N, et al. Taurine supplementation reduces oxidative stress and improves cardiovascular function in an iron-overload murine model. Circulation. 2004;109:1877–85. doi: 10.1161/01.CIR.0000124229.40424.80. [DOI] [PubMed] [Google Scholar]
- 10.Oudit GY, Sun H, Trivieri MG, et al. L-type Ca2+ channels provide a major pathway for iron entry into cardiomyocytes in iron-overload cardiomyopathy. Nat Med. 2003;9:1187–94. doi: 10.1038/nm920. [DOI] [PubMed] [Google Scholar]
- 11.Bartfay WJ, Bartfay E. Iron-overload cardiomyopathy: evidence for a free radical–mediated mechanism of injury and dysfunction in a murine model. Biol Res Nursing. 2000;2:49–59. doi: 10.1177/109980040000200106. [DOI] [PubMed] [Google Scholar]