Abstract
Background
The practice of transfusing red blood cells is still liberal in some centres suggesting a lack of compliance with guidelines recommending transfusion of red blood cells at haemoglobin levels of 6–8 g/dL in the non-bleeding patient. Few databases provide ongoing feedback of data on pre-transfusion haemoglobin levels at the departmental level. In a tertiary care hospital, no such data were produced before this study. Our aim was to establish a Patient Blood Management database based on electronic data capture in order to monitor compliance with transfusion guidelines at departmental and hospital levels.
Materials and methods
Hospital data on admissions, diagnoses and surgical procedures were used to define the populations of patients. Data on haemoglobin measurements and red blood cell transfusions were used to calculate pre-transfusion haemoglobin, percentage of transfused patients and transfusion volumes.
Results
The model dataset include 33,587 admissions, of which 10% had received at least one unit of red blood cells. Haemoglobin measurements preceded 96.7% of the units transfused. The median pre-transfusion haemoglobin was 8.9 g/dL (interquartile range 8.2–9.7) at the hospital level. In only 6.5% of the cases, transfusion was initiated at 7.3 g/dL or lower as recommended by the Danish national transfusion guideline. In 27% of the cases, transfusion was initiated when the haemoglobin level was 9.3 g/dL or higher, which is not recommended. A median of two units was transfused per transfusion episode and per hospital admission. Transfusion practice was more liberal in surgical and intensive care units than in medical departments.
Discussion
We described pre-transfusion haemoglobin levels, transfusion rates and volumes at hospital and departmental levels, and in surgical subpopulations. Initial data revealed an extensive liberal practice and low compliance with national transfusion guidelines, and identified wards in need of intervention.
Keywords: transfusion, guideline, database, blood management, haemoglobin
Introduction
Transfusion practice with red blood cells (RBC) varies and is still liberal in many centres1–4. This suggests a lack of compliance with existing transfusion guidelines. Most guidelines recommend restrictive use of RBC transfusion, suggesting that transfusion be considered if the haemoglobin (Hb) level goes below 6–8 g/dL in the non-bleeding patient5–9. Few hospital transfusion databases report an ongoing feedback on pre-transfusion haemoglobin levels at hospital and departmental levels10, whereas most studies and national databases report usage, transfusion rates and sometimes Hb at hospital discharge, mostly at hospital and national leve ls11–18. Starting in 2007, the Joint Commission elaborated a Patient Blood Management Performance Measures Project defining the patient populations to be investigated and the transfusion indicators to be measured19 (hereafter named the JC-PBM measures), among which is PBM-02: the number of units with pre-transfusion haemoglobin or haematocrit and clinical indication documented20. Preliminary data from The Danish Transfusion Database (DTDB)18 and from the Council of Europe21 suggested a general overuse of RBC in Denmark compared with other European countries. Preliminary data from Rigshospitalet, Copenhagen University Hospital22 likewise indicated an excessive use of RBC transfusion. A Patient Blood Management programme was initiated in 2009 at Rigshospitalet with the primary aim of reducing unnecessary RBC transfusions. Rigshospitalet is Denmark’s largest hospital with 1200 beds, a level I trauma centre and a case-mix of thoracic, vascular, urological and gastrointestinal surgery including solid organ transplantation, oncology, haematology including bone marrow transplantation, neurology, orthopaedic surgery/advanced trauma surgery, gynaecology, obstetrics, cardiology, infectious medicine, paediatric care and intensive care. Review of the existing quality data in the hospital in 2008 revealed a substantial lack of data on transfusion practices and compliance with the national transfusion guidelines, even though the hospital databases contained relevant source data. In order to establish a baseline before intervention and enable benchmarking and feedback of data to the hospital staff during the intervention, we needed data to describe transfusion practices and their compliance with national transfusion guidelines.
Danish national transfusion guideline
The Danish Health and Medicines Authority issued a transfusion guideline in 1998, with the latest revision in 200723. A restrictive approach to RBC transfusion is recommended, requiring “a reduction in the use of blood transfusion”, and recommending that transfusion should be used only “after consideration of other rational therapy for the clinical condition, in order to avoid blood transfusion”. It is stated that “the purpose of the treatment with blood transfusion is to improve the clinical symptoms of anaemia, not to correct the haemoglobin value”. In the non-bleeding patient, a general Hb value of 7.3 g/dL is suggested as a limit below which “transfusion of RBC is almost often beneficial”. In case of specific clinical conditions such as ischaemic heart disease or thalassaemia, transfusion may be used at Hb levels between 7.3 and 9.7 g/dL. Generally, transfusion is “not recommended at Hb levels above 9.7 g/dL”.
Aims
We aimed at monitoring RBC transfusion treatment, in particular the compliance with transfusion guidelines at both departmental and hospital level. We established a Patient Blood Management database based on an electronic data capture system in order to extract and combine data from the patient administrative system and laboratory and blood bank databases to produce relevant indicators with the smallest possible time delay between transfusion treatment and data feedback. The database should enable an initial internal evaluation of the hospital’s transfusion practice as well as an ongoing monitoring on at least a quarterly basis.
Materials and methods
Blood Bank structure and existing transfusion data
The Blood Bank is financed by the government and integrated in the hospital. It manufactures blood according to the standards and principles of the Council of Europe24 and delivers the blood components directly to the hospital’s departments. When a department picks up a unit of RBC, it is checked out by scanning a bar code on the unit, which simultaneously records the date, hour and minute, the recipient identity (see below) and the ISBT-128 identity code25 of the blood component in the Blood Bank database. By national law, the fate of every blood component is reported back to the Blood Bank and recorded in the database. Traceability of blood components is 99%. Monitoring the documentation on the patient’s consent and clinical indication in the patient’s record (part of JC-PBM-02) is included in the existing hospital quality model together with correct identification of the patient and the laboratory samples for type and screen procedures, and correct blood administration procedures including control of vital parameters. This was not electronically monitored (but subject to regular paper audits), and thus these data are not available.
Definition of patient populations
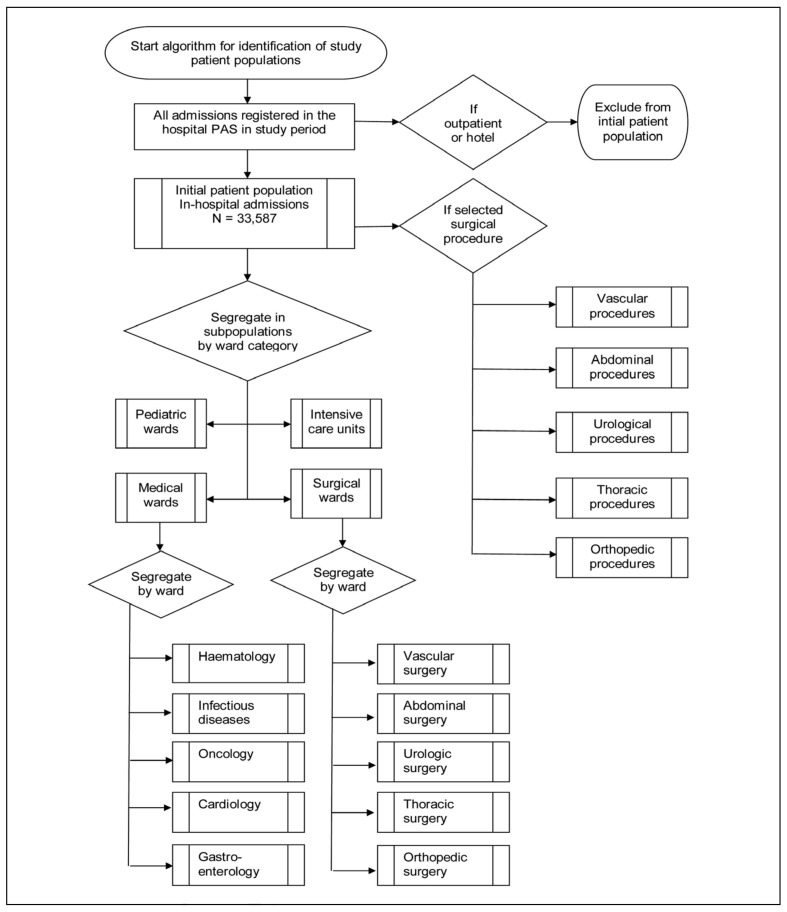
Before data extraction, we defined the patient populations and surgical procedures to be investigated according to the method described by the Joint Commission19, using the algorithm shown in Figure 1. In Denmark, every citizen is assigned a unique personal identification number (personal ID) from birth or at the time of obtaining a residence permit, based on the date of birth in a unique combination with a four digit code registered by the Danish Civil Registry. The personal ID was used as a key to identify and combine data (Figure 2). According to the principles in the JC-PBM measures, we defined two main types of patient populations: (i) the initial patient population of this study, i.e. admitted patients, and theirs subpopulations; and (ii) the surgical procedure population and its subpopulations (Figure 1). For each indicator we first defined the relevant patient population (denominator), before identifying the transfused part of this population (numerator). The initial patient population included all patients admitted to and discharged from the hospital within the study inclusion period. For an overview, we grouped the initial patient population, admitted into 41 wards, into the following subpopulations: surgical admissions (admissions to surgical wards, regardless of surgical procedures), medical admissions (admissions to medical wards, regardless of any procedures), intensive care admissions, and paediatric admissions. We chose to present detailed data at the departmental level from 10 selected wards (5 surgical and 5 medical). One patient could contribute to more than one subpopulation, but not at the same time (e.g. by being admitted to a surgical department and later in the study period to a medical department). During an admission, one patient could contribute with more than one surgical procedure. Selected surgical procedure populations within all transfusing surgical specialties were defined by searching the initial patient population for the patients with a specific surgical procedure code or combination of codes, using the NOMESCO Classification of Surgical Procedures, Version 1.12:2008 Copenhagen 2007 (http://nomesco-eng.nom-nos.dk) and the Danish translation of ICD10 codes for diagnoses (http://www.who.int/classifications/icd/en/) according to the search criteria listed below. One patient could contribute to both an admission subpopulation (e.g. thoracic ward) and a selected surgical procedure, but could only contribute with one selected surgical procedure per admission (e.g. coronary artery bypass grafting), except for combined heart surgery (see below). The surgical codes chosen to define a surgical procedure were determined and validated in cooperation with each surgical speciality.
Figure 1.
Patient population algoritm. The algorithm for identifying the initial patient population and subpopulations as suggested by the Joint Commission.
Figure 2.
Data linkage algorithm. The unique personal ID-number was used to combine hospital data on the patient level and create a patient-specific chronology.
Permission
The Danish data protection agency granted permission to extract and combine patients’ data from the different data sources (http://www.datatilsynet.dk/english/).
Design of the Patient Blood Management Database and transfusion indicators
The study inclusion period was from the 4th July to 31st December 2008, both days inclusive. Data were extracted from three existing data sources within the hospital: the Patient Administrative System (PAS), the Blood Bank Database and the laboratory system (LAB-data). After data extraction and validation, we linked the data as illustrated in Figure 2 and calculated the following transfusion indicators in the transfused patient populations both at hospital level and within the pre-defined patient populations (described above): pre-transfusion Hb level, including the proportion of RBC transfused with a pre-transfusion Hb; the mean time (hours) between the pre-transfusion Hb and the initial RBC transfusion, the median pre-transfusion Hb and distribution of pre-transfusion Hb levels according to the national guideline. Transfusion rate was calculated as the percentage of the initial patient population or surgical patient population who had at least one RBC transfusion, and transfusion volume was calculated as the number of RBC units per patient admission or until the third post-operative day. RBC usage was described as activity-indexed usage, e.g. number of RBC units as the numerator divided by the number of patient admissions or selected surgical procedures as the denominator. After validation of the data extracted from each source, we randomly selected the records for ten patients, whose admissions, procedures and transfusions were looked up by an independent person in the user interface of the PAS, Lab-data and Blood Bank database. These patients’ records showed full correspondence to the data obtained by data capture.
Patient administrative data
Standardised data extraction was performed from the PAS (The Green System Open, CSC Scandihealth, Aarhus, Denmark), including registration details of all admissions (date/hour/minute), diagnoses, surgical procedures, different departments with care responsibility visited during the hospital admission, and discharge (date/hour/minute) for each personal ID. In-hospital death was also registered (date/hour/minute; if death occurred after admission and before discharge). Only hospital admissions were included; psychiatric wards, outpatient clinic and patient hotel visits were excluded. We validated the data with respect to registration completeness, number and distribution of admissions and procedures, and validity of the extracted data on the patient level. Registration completeness was checked by comparing the percentage of registrations on the hospital and departmental levels as a function of time until the final deadline for registration in the PAS. This revealed a varying delay among the wards in registration between the time of admission and the time of registration. We chose to use a 3-month delay when extracting PAS data, since 97% of the registrations were complete at this time throughout the study period. We checked that the numbers and distribution of admissions in the wards and the number and type of surgical procedures corresponded to the numbers and the distribution registered by the wards themselves. Duplicate and erroneous registrations were very few, and were excluded.
Haemoglobin data
From the in-house biochemistry database, Lab-data (Klinisk Biokemisk System, Rigshospitalet, Copenhagen, Denmark), we extracted data on Hb analyses from phlebotomies (daily routine rounds as well as acute analyses) using the time when the Hb result was visible to the staff in the electronic system. Even though Hb was sometimes measured using point of care (POC) devices, such as Hemocue or arterial blood gas machines in some surgical wards and intensive care units, data were not automatically transmitted to Lab-data in 2008. We, therefore, chose not to include POC Hb results.
Blood Bank data
From the in-hospital Blood Bank database (Blodflödet, CSC Scandihealth, Aarhus, Denmark) we extracted data (BB-data) on component class25, the electronic issuing (the pickup time from the Blood Bank) and transfusion status of all units of RBC in the hospital in the entire study period, excluding any returned or wasted components.
Calculation of transfusion indicators
All extracted source data were imported and further processed using SAS 9.3.1 software (SAS Institute, Cary, NC, USA). From PAS a table with all admissions was created identifying admission and surgical periods, and from the Blood Bank database we created a table with all RBC transfusions by personal ID and by chronology. By merging these two tables, we created a table listing all RBC transfusions given to each transfused patient during one admission to a department thereby identifying admissions with RBC transfusion and their RBC transfusion chronology.
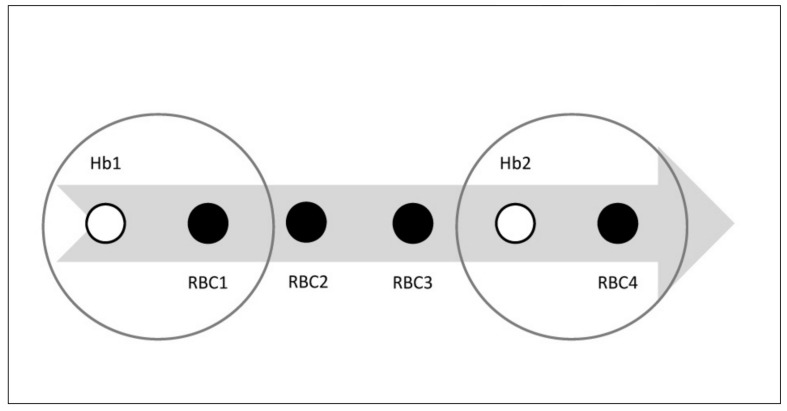
For pre-transfusion Hb, we included all Hb values for which the results were visible to the staff between 30 minutes and 72 hours before the electronic issuing time of each RBC unit from the Blood Bank. We examined the shortest possible time between seeing the Hb result and starting the transfusion at the patient in three departments with different distances from the Blood Bank. These intervals were 15–30 minutes, most often 30 minutes. We then tested the effect of different time intervals on the data output, and found that changing time intervals to between 10–50 minutes affected the median pre-transfusion Hb result by <5%, and settled for a 30-minute interval for the calculation of pre-transfusion Hb. If more than one Hb result was available the result closest to the issue time minus 30 minutes was chosen. If more than one RBC unit was issued after the Hb result, only the initial RBC was used to calculate the pre-transfusion Hb, since the source data from the Blood Bank database had revealed a high number of two-unit RBC transfusions, which would potentially have confounded the results. After coupling the initial RBC to the closest Hb result, we named this Hb result “the pre-transfusion Hb” (Figure 3).
Figure 3.
Pre-transfusion Hb and Hb-linked transfusion volume. Pre-transfusion Hb and Hb-linked transfusion volume.
The arrow illustrates the time flow of one admission to hospital (example). The patient receives 4 units of RBCs in total (smaller filled circles marked RBC1–4) throughout the admission period. Hb is measured twice (smaller open circles, Hb1 and Hb2). There are two pre-transfusion Hb results (bigger open circles): Pre-transfusion Hb1=Hb1+RBC1 and pre-transfusion Hb2=Hb2+RBC4. Hb-linked transfusion volume for Hb1=3 units corresponding to RBC1+RBC2+RBC3. Hb2-linked transfusion volume=1 unit corresponding to RBC4.
The Hb-linked transfusion volume, i.e. the number of RBC units transfused in the same transfusion sequence, was calculated by merging the pre-transfusion Hb with the table of all transfusions, including the initial RBC unit and the following RBC units up till 24 hours after the initial RBC unit, until the next Hb result, discharge from the hospital or death. Five medical wards (haematology, infectious diseases, oncology, cardiology and gastroenterology) and five surgical wards (vascular, abdominal, urological, thoracic and orthopaedic) were selected for detailed data presentation on a departmental level.
For hospital transfusion rates the number of transfused admissions (nominator) was divided by the total number of admissions (denominator). For surgical transfusion rates, the number of a selected surgical procedure served as the denominator and the number of patients with this selected procedure with at least one transfusion of RBC within 24 hours prior to and 72 hours after starting surgery, served as the nominator. The following surgical procedures were selected: (i) cardiac surgery: coronary artery bypass graft (CABG), aortic valve replacement, mitral valve replacement and combinations; (ii) gastric surgery: liver resections (excluding liver transplantation which always required transfusion), oesophageal/gastric resections, and colonic resections; (iii) urological surgery: prostatic cancer with prostatectomy, renal cancer with nephrectomy, excision of retroperitoneal and testicular cancer, and bladder cancer with cystectomy; (iv) orthopaedic surgery: primary and secondary hip replacement, primary and secondary knee replacement, and hip fracture; and (v) vascular surgery: acute abdominal aortic aneurysm rupture+surgery, elective abdominal aortic aneurysm surgery, peripheral ileo-femoral bypass, and endoscopic vascular artery resection (EVAR).
The surgical transfusion volume was calculated as the number of RBC units transfused within 24 hours prior to and 72 hours after the start of surgery. Median values and interquartile ranges are given for length of stay in hospital, age at admission, pre-transfusion Hb results, Hb-linked transfusion volume and transfusion volume during the hospital admission.
Results
The identification of the study populations is shown in the flowchart in Figure 1. The initial patient population included 33,587 admissions by 22,073 patients. The characteristics of the patient populations are shown in Table I for the initial patient population and the four major subpopulations (number of specialty wards in parenthesis): medical (n=14), surgical (n=18), intensive care (n=2), and paediatric wards (n=7). The most frequent type of admission was surgical admissions, followed by medical admissions, which together constituted 85% of all admissions. There were more male admission days and males were older. The median length of hospital stay per admission was between 1.21–2.17 days for all populations except the intensive care units, where patients stayed for 6.29 days.
Table I.
Patient population characteristics.
| Characteristic | Number | % of total | |
|---|---|---|---|
| Patients | Total number of patients | 22,073 | 100 |
| Female patients | 11,679 | 53 | |
| Male patients | 10,394 | 47 | |
| Admissions | Total number of admissions | 33,587 | 100 |
| Female admissions | 17,017 | 51 | |
| Male admissions | 16,570 | 49 | |
| Admissions to surgical departments | 16,290 | 49 | |
| Admissions to medical departments | 11,932 | 36 | |
| Admissions to intensive care units | 1,025 | 3 | |
| Admissions to paediatric departments | 4,340 | 13 | |
| Procedures | Total number of main surgical procedures* | 30,190 | 100 |
| Total number of surgical interventions* | 15,994 | 100 | |
| Admission days | Total number of admission days | 139,827 | 100 |
| Females admission days | 66,879 | 48 | |
| Male admission days | 72,948 | 52 | |
| Surgical admission days | 60,036 | 43 | |
| Medical admission days | 54,960 | 39 | |
| Intensive care unit admission days | 8,183 | 6 | |
| Paediatric admission days | 16,648 | 12 | |
|
| |||
| Median | IQR | ||
|
| |||
| Length of stay | Days per hospital admission | 2.11 | 0.91–4.43 |
| Days per female admission | 2.10 | 0.86–4.21 | |
| Days per male admission | 2.13 | 0.94–4.92 | |
| Days per surgical admission | 2.17 | 1.02–4.29 | |
| Days per medical admission | 2.10 | 0.93–4.41 | |
| Days per intensive care unit admission | 6.29 | 1.94–10.18 | |
| Days per paediatric admission | 1.21 | 0.42–3.43 | |
| Age in years at admission | All admissions | 45 | 20–63 |
| Female admissions | 39 | 24–62 | |
| Male admissions | 51 | 15–64 | |
| Surgical admissions (excl. Paediatric adm) | 38 | 23–62 | |
| Medical admissions (excl. Paediatric adm) | 58 | 45–67 | |
| Intensive care unit admissions | 60 | 40–69 | |
| Paediatric admissions | 5 | 1–11 | |
The characteristics of the initial patient population and subpopulations admitted to the hospital during the observation period of June–December 2008.
One surgical intervention may include one or more main surgical procedures.
Red blood cell transfusion
In total 13,674 units of RBC were transfused to 3,346 (10%) of the initial patient population (Table II). Medical, surgical and intensive care units consumed 92% of the RBC units, with an almost even share of 30–31% of all transfused RBC. The number of RBC units/1,000 admissions was 10 to 20-fold higher in the intensive care units than in the initial patient population and in the other subpopulations, since intensive care consumed one-third of the blood for only 3% of the admissions. Correspondingly, 37% of the intensive care admissions were transfused, which is more than three times higher than in the initial patient population.
Table II.
Pre-transfusion haemoglobin and activity-indexed usage of red blood cells in the different patient populations.
| Initial patient population (all admissions ) | Surgical wards | Medical wards | Intensive care units | Paediatric wards | |
|---|---|---|---|---|---|
|
|
|||||
| Total n. of RBC units transfused (% of total) | 13,674 (100.0) | 4,113 (30.1) | 4,265 (31.2) | 4,239 (31.0) | 1,057 (7.7) |
| N. of RBC units preceded by a haemoglobin result (% of total n. of RBC units) | 13,227 (96.7) | 3,829 (93.1) | 4,244 (99.5) | 4,196 (99.0) | 958 (90.6) |
| Median pre-transfusion haemoglobin (IQR) | 8.9 (8.2–9.7) | 9.4 (8.4–11.3) | 8.9 (8.2–9.4) | 9.0 (8.2–10.2) | 8.4 (7.7–8.9) |
| N. of RBC units/1000 admissions | 407 | 252 | 357 | 4136 | 244 |
| N. of RBC units/1000 surgical procedures | 453 | 174 | -- | -- | -- |
| N. of admissions with RBC transfusion (% of all admissions) | 3,346 (10) | 1,282 (8) | 1,243 (10) | 378 (37) | 443 (10) |
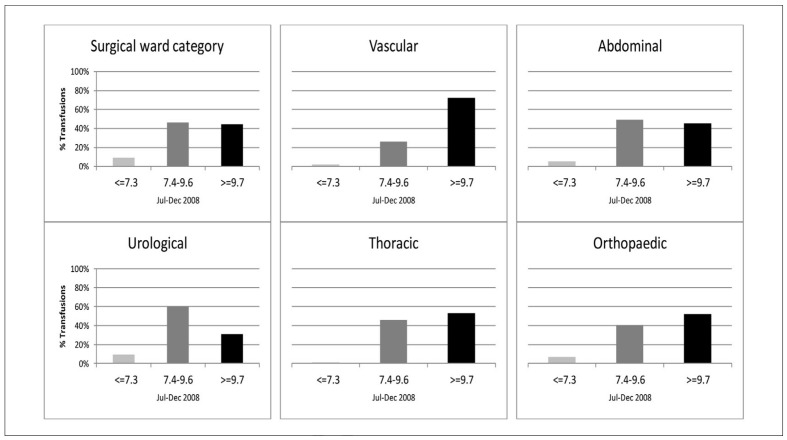
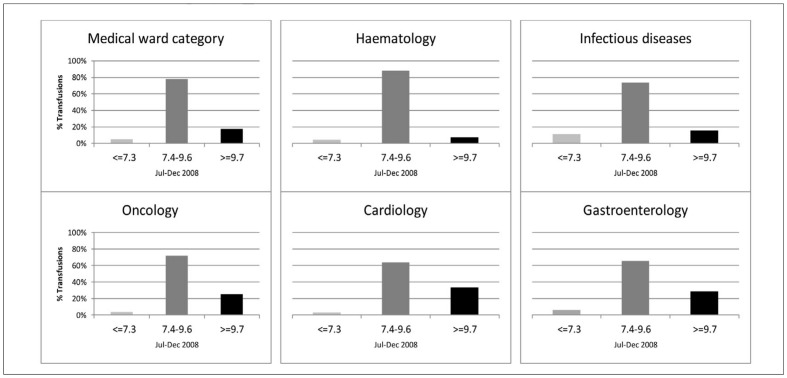
A Hb result could be identified within 72 hours prior to transfusion for 96.7% of the total of 13,674 RBC units transfused. Pre-transfusion Hb results were available for 99% of the RBC units transfused in medical wards and intensive care units, 90% of those transfused in surgical units and 93% of those transfused in paediatric wards. The median pre-transfusion Hb for transfusion was 8.9 g/dL in the initial patient population and in the medical subpopulation, whereas the pre-transfusion Hb was higher in the surgical wards and intensive care units. In total 6,112 pre-transfusion Hb were identified, with a median time between the Hb result and the initial RBC transfusion of 5 hours (interquartile range [IQR], 3–11). Extending the time frame to 7 days prior to transfusion had no impact on data. Only 6.5% of the pre-transfusion Hb followed the general recommendation of transfusion at 7.3 g/dL or lower, and 27.2% of the pre-transfusion Hb were 9.7 g/dL or higher. The distribution of pre-transfusion Hb compared with the recommendations in the Danish national transfusion guideline is shown in Figure 4a for the surgical and in Figure 4b medical subpopulations together with the chosen medical and surgical wards. In surgical wards, 44% of the pre-transfusion Hb were above the national guidelines, as opposed to 34% in intensive care units and 17% in the medical wards. Among surgical wards, vascular surgery had the highest median pre-transfusion Hb and percentage above the upper limit of the guideline. Among medical wards cardiology and oncology had the highest median pre-transfusion Hb, whereas haematology only had 7.5% pre-transfusion Hb above the upper guideline limit. The median Hb-linked transfusion volume was two units of RBC (IQR 1–2) in the initial patient population, one unit (IQR 1–1) in the medical and paediatric subpopulations, two (IQR 1–2) in the surgical subpopulation, and two (IQR 2–3) in the intensive care subpopulation.
Figure 4a.
Distribution of pre-transfusion Hb in surgical wards.
The distribution of pre-transfusion Hb according to the national transfusion guideline in all surgical wards (upper left) and five selected surgical wards.
Light grey columns: Pre-transfusion Hb ≤7.3 g/dL. Medium dark grey columns: 7.4–9.6 g/dL.
Black columns: ≥9.7 g/dL.
Figure 4b.
Distribution of pre-transfusion Hb in medical wards.
The distribution of pre-transfusion Hb according to the national transfusion guideline in all medical wards (upper left) and in five selected medical wards.
Light grey columns: Pre-transfusion Hb ≤7.3 g/dL.
Medium dark grey columns: 7.4–9.6 g/dL. Black columns: ≥9.7 g/dL.
Transfusion rates and surgical transfusion volumes for the selected surgical procedures are shown in Figure 5. Vascular surgery had the highest surgical transfusion rates, especially for aneurysms, followed by combined open heart surgery. The lowest transfusion rates were seen in EVAR, prostatic cancer and orthopaedic surgery. A median of two RBC units were transfused per hospital admission.
Figure 5.
RBC transfusion rates and volumes in selected surgical procedures. Transfusion rates (percentage of patients transfused) in selected surgical procedures (upper lane, filled bars).
Transfusion volume i.e. median number of transfused units of RBCs per selected surgical procedure (lower lane, open bars showing interquartile range and median. Median is shown as a dot, if equal to upper or lower quartile). Procedures from the left: AAA: acute abdominal aortic aneurysm, EAA: elective abdominal aortic aneurysm, P bypass: peripheral bypass. EVAR: endovascular aortic repair. Liver: liver resection, Oes/cardia: oesophageal/cardia surgery, Colon: colonic resections, C. Prost: prostatic cancer+resection, C. Renis: kidney cancer+nephrectomy, Tx: renal transplantation, Cyst: bladder cancer+cystectomy, Retro: resection of retroperitoneal tumours, CABG: coronary artery bypass graft, A-valve: aortic valve replacement, M-valve: mitral valve replacement, Comb: combined heart surgery, P-THR: primay total hip replacement, S-THR: secondary total hip replacement, P-TKR: primary total knee replacement, S-TKR: secondary total knee replacement, H-frac: hip fracture.
Discussion
A Patient Blood Management database was established in Rigshospitalet Copenhagen University Hospital in 2008 to evaluate the hospital’s transfusion practice, both as a baseline before intervention and as an ongoing data monitoring system. This was necessary because the hospital wanted to commence a Patient Blood Management programme in 2009 to optimise transfusion treatment, as it was suspected that there was an ongoing overuse of RBC transfusions. The two major questions asked before commencing the programme were; (i) whether recent and precise data could be produced to show where and how blood was used within the hospital wards, and (ii) how the RBC transfusion practice complied with the national guideline.
In spite of a relatively restrictive national transfusion guideline issued 10 years prior to this study, Denmark has a history of an extensive use of RBC transfusions, measured by the European Council21 and by DTDB18. When reviewing DTDB data, we found that they were useful for determining the overall usage of RBC units at national and hospital levels. However, when trying to answer our major questions we found some limitations in the data provided by the DTDB: (i) data were reported once a year with a 1–2 year delay; (ii) no data were provided at the departmental level; (iii) the patient populations were of limited relevance, since they were a list of the national top 10 most blood-using diagnoses (e.g. pneumonia or myeloid leukaemia) and procedures (e.g. vascular surgery), regardless of the procedures performed in the different hospitals. This set-up provided transfusion indicators for only 25–33% of the RBC units used in the hospital, even though it is the largest in Denmark. Furthermore, the patient populations were not defined by admission to wards and the diagnoses, pneumonia for example, could represent a clinically variable mix of patients in many departments, and one could speculate that pneumonia might be associated with a variety of different major diseases, really causing the transfusion; (iv) both diagnoses and procedures on the top 10 list would tend to represent cases with heavy bleeding/transfusion, however, none of the transfusion indicators was useful for bleeding; (v) the transfusion indicators were not consistent with the recommendations in the national guideline for non-bleeding patients, as there was no pre-transfusion Hb to monitor the compliance with the pre-transfusion levels recommended in the guideline.
Instead Hb on discharge was used as a surrogate marker. Similar limitations have appeared in other databases and epidemiological studies16,26, some of which have reported discharge Hb, with data coverage percentages being only 27–48% of all RBC transfusions15,27. At Rigshospitalet the data coverage of Hb after RBC transfusion was only 25%, and since measuring Hb after transfusion is not recommended in the guideline, we suspect that measuring Hb after transfusion may be done because of ongoing bleeding and/or because further transfusions are considered. We, therefore, chose not to include post-transfusion Hb in our database. In other studies the daily nadir Hb levels have been used as a surrogate marker28. The lack of pre-Hb results in previous databases/studies may have been caused by technical factors such as limited electronic data access29, however, pre-Hb monitoring has recently been established in Western Australia10, and our data system and data coverage of 97% are similar to those in this model. Furthermore, the establishment of a pre-transfusion Hb is a central issue in the PBM-02 indicator recommended in the JC-PBM measures19. Finally, the pre-transfusion Hb enables important feedback to the staff on their behaviour at the time of the decision to transfuse or not, which may be a difficult decision30, as knowledge of the pre-Hb may help the evaluation of whether the staff are using the recommendations regarding pre-Hb appropriately. The patient population definitions in our model are comparable to those used in Western Australia10 and in the JC-PBM measures19, even though some minor discrepancies in data registration (e.g. in diagnosis and procedure codes) must be expected due to differences in the code definitions and data platforms.
Our Patient Blood Management database demonstrated an overuse of RBC transfusions in the hospital, with 27% of the transfusion series initiated when the patient’s Hb was 9.7 g/dL or higher, and less than 7% initiated at the restrictive Hb value of 7.3 g/dL recommended by the guideline. This result showed a lack of compliance with the national guideline, in contrast to the findings in Western Australia in 2008, where 9% of the transfusions were given to patients with Hb above 10 g/dL and more than 18% to those with a Hb below 7 g/dL. We found a high degree of transfusions of two units of RBC, both within 24 hours after transfusion initiation, and also during the hospital admission, which is similar to findings of others10,15. Theoretically, this could mean that in a stable patient, the frequent “second” unit of RBC could have been administered at even higher Hb levels. However, we were not able to demonstrate this, as the data coverage of post-transfusion Hbs was insufficient. Transfusion rates were highly variable between specialties and procedures. For example almost 45% of the transfusions in the thoracic surgery department were initiated at the upper guideline limit and 40% were double-unit transfusions, resulting in a 40–60% transfusion rate in open heart surgery. This made thoracic surgery a target for further investigation and intervention in contrast to the haematology department, in which 11% of all RBC units were used, but only 7.5% were transfused at the upper guideline limit, with 74% being single-unit transfusions. Combining ward-specific pre-transfusion Hb and Hb-linked transfusion volume with transfusion rates and RBC usage data provided a valuable tool for evaluating transfusion practice within hospital wards, linked to specific procedures. Transfusion rates could also be used for external benchmarking. Compared with rates in other centres, the transfusion rates for some procedures, e.g. open heart surgery and primary hip replacement, were relatively high31–33, and together with the data on pre-transfusion Hb, suggested that intervention in these wards might be relevant.
Our database has some limitations: using existing hospital data in a retrospective data model, there are some built-in limitations to the design and to the nature of the data. Defining the patient population is dependent on the possibilities and limitations of the PAS, such as the accuracy of the registration and the validity of the coding of diagnoses and surgical procedures. The accuracy with regards to patient identification and timing is high because of the link between the PAS and the national registry, regular audits of the system and our validation steps. However we cannot exclude that some variation may exist in the coding practices between departments. A major limitation is the lack of clinical indications for transfusion in our data system. Even though quality surveillance of the documentation of informed consent and clinical indications is in place in the hospital, this is not electronically accessible, and the results should be interpreted with caution. Linking a RBC transfusion to a prior Hb level may not depict the whole picture of the decision to transfuse. The current lack of POC Hb results is also a limitation, since the pre-transfusion Hb in the electronic system may have been taken pre-operatively, whereas the actual pre-transfusion Hb may have been done on a POC device in the operating room following a bleeding episode. This could result in a false high pre-transfusion Hb in the database. However POC data were gradually registered electronically from 2009 and onwards, and will be included in the database, as they become available. Preliminary comparisons of recent median pre-transfusion Hbs before and after inclusion of POC Hb results have shown that median Hb was unaltered at the hospital level, and that inclusion of POC Hb only affected the median pre-transfusion Hb in intensive care units and in a few surgical wards, the greatest difference seen in a single ward being 0.5 g/dL. We therefore believe that the lack of POC results will not change the fact that our hospital had a liberal transfusion practice in 2008. Another limitation is the lack of indicators for transfusion of the bleeding patient. Bleeding episodes are not systematically registered e.g. as diagnoses or procedures in the PAS, and we were not able to identify a valid surrogate electronic marker for bleeding and bleeding treatment. Our results should, therefore, be interpreted with caution with regard to bleeding episodes, since some of the transfusions may have been given to patients with haemodynamic instability or acute severe anaemia with clinical signs of limited physiological compensation. However this most probably concerns a small proportion of the transfusions, since only 10% of the transfusion episodes resulted in a transfusion volume exceeding three units within 24 hours after the initial RBC transfusion. This is slightly higher than found in the Western Australia data and may reflect a different case mix in our hospital, but our data were not sufficient to investigate this further within the frames of this study. Work is ongoing to establish appropriate markers for bleeding and bleeding treatment in the future. Other future indicators could be pre-operative anaemia, use of cell-salvage and tranexamic acid. Using the hospital data systems common to all hospitals in the area of greater Copenhagen, this system is feasible for linking data from other hospitals or even other countries, provided that a single unique patient identification code can be provided to link the data on the individual level.
Conclusion
Existing hospital data were extracted and linked together in a Patient Blood Management database form useful RBC indicators at both departmental and hospital levels. Data on admissions, diagnoses and surgery were linked to pre-transfusion Hb levels and, combined with transfusion rates and usage data, provided a valuable tool for comparing the hospital’s transfusion practice to the existing transfusion guideline and identifying wards in need of intervention. The use of the unique personal ID as a key for data linkage is a great advantage together with the high traceability of the Blood Bank data. Limitations were the lack of clinical context of the transfusion decision and the lack of POC data. We demonstrated an excessive use of RBC transfusion in Rigshospitalet, reflecting a lack of compliance with national transfusion guidelines. The Patient Blood Management database served as a baseline measurement, as well as a tool for ongoing monitoring, and was very useful for reporting quarterly to the staff in the hospital departments as well as to hospital managers. The data model is similar to that in the Western Australia system10 and used the patient population definitions and the pre-transfusion Hb measurement criterion recommended by the Joint Commission20 and does, therefore, have the potential to be used for national or international benchmarking. This model dataset in our pilot hospital may also serve as a basis for expanding the data model to other hospitals in the region, and further inclusion of patient blood management indicators will be pursued.
Acknowledgements
We are very grateful to Danielle Poulsen and Birthe Hansen for helping with the data extraction from the Blood Bank, and to Svend Høime Hansen for helping with the biochemistry data. We also wish to thank Michael Saabye for sharing his insight into the patient administrative system, helping with data extraction and validation, and for invaluable help with the establishment of the database. We are also very grateful to Henrik Ravn for helping to design and start programming the database. Finally we want to thank Maibritt De Cordier for inspiration and contribution to the development of the database according to the Joint Commission principles, for facilitating the implementation, and for evaluating its usefulness in the hospital quality management setting, through presentations and engaging discussions.
Footnotes
The Authors declare no conflicts of interest.
References
- 1.Westbrook A, Pettila V, Nichol A, et al. Transfusion practice and guidelines in Australian and New Zealand intensive care units. Intensive Care Med. 2010;36:1138–46. doi: 10.1007/s00134-010-1867-8. [DOI] [PubMed] [Google Scholar]
- 2.Walsh TS, Garrioch M, Maciver C, et al. Red cell requirements for intensive care units adhering to evidence-based transfusion guidelines. Transfusion. 2004;44:1405–11. doi: 10.1111/j.1537-2995.2004.04085.x. [DOI] [PubMed] [Google Scholar]
- 3.Niemi TT, Kuitunen AH, Haukka J, Lepantalo M. Red blood cell transfusions in patients undergoing lower extremity artery bypass surgery. Scand J Surg. 2006;95:39–43. doi: 10.1177/145749690609500108. [DOI] [PubMed] [Google Scholar]
- 4.Cohen J, Kagan I, Hershcovici R, et al. Red blood cell transfusions--are we narrowing the evidence-practice gap? An observational study in 5 Israeli intensive care units. J Crit Care. 2011;26:106. doi: 10.1016/j.jcrc.2010.03.010. [DOI] [PubMed] [Google Scholar]
- 5.Carson JL, Grossman BJ, Kleinman S, et al. Red blood cell transfusion: a clinical practice guideline from the AABB. Ann Intern Med. 2012;157:49–58. doi: 10.7326/0003-4819-157-1-201206190-00429. [DOI] [PubMed] [Google Scholar]
- 6.Danish National Board of Health [Sundhedsstyrelsen] Guideline for the transfusion of blood components. [Vejledning om blodtransfusion]. 1998. [Google Scholar]
- 7.Ferraris VA, Brown JR, Despotis GJ, et al. 2011 update to the Society of Thoracic Surgeons and the Society of Cardiovascular Anesthesiologists blood conservation clinical practice guidelines. Ann Thorac Surg. 2011;91:944–82. doi: 10.1016/j.athoracsur.2010.11.078. [DOI] [PubMed] [Google Scholar]
- 8.Murphy MF, Wallington TB, Kelsey P, et al. Guidelines for the clinical use of red cell transfusions. Br J Haematol. 2001;113:24–31. doi: 10.1046/j.1365-2141.2001.02701.x. [DOI] [PubMed] [Google Scholar]
- 9.Patient Blood Management Guidelines National Blood Authority of Australia. [Accessed on 10/10/2013]. Available at: www.blood.gov.au/pbm-guidelines.
- 10.Mukhtar SA, Leahy MF, Koay K, et al. Effectiveness of a patient blood management data system in monitoring blood use in Western Australia. Anaesth Intensive Care. 2013;41:207–15. doi: 10.1177/0310057X1304100210. [DOI] [PubMed] [Google Scholar]
- 11.Cobain TJ, Vamvakas EC, Wells A, Titlestad K. A survey of the demographics of blood use. Transfus Med. 2007;17:1–15. doi: 10.1111/j.1365-3148.2006.00709.x. [DOI] [PubMed] [Google Scholar]
- 12.Kamper-Jorgensen M, Edgren G, Rostgaard K, et al. Blood transfusion exposure in Denmark and Sweden. Transfusion. 2009;49:888–94. doi: 10.1111/j.1537-2995.2008.02081.x. [DOI] [PubMed] [Google Scholar]
- 13.Madsen JT, Kimper-Karl ML, Sprogoe U, et al. One-year period prevalence of blood transfusion. Transfus Med. 2010;20:191–5. doi: 10.1111/j.1365-3148.2009.00977.x. [DOI] [PubMed] [Google Scholar]
- 14.Titlestad K, Georgsen J, Jorgensen J, Kristensen T. Monitoring transfusion practices at two university hospitals. Vox Sang. 2001;80:40–7. doi: 10.1046/j.1423-0410.2001.00001.x. [DOI] [PubMed] [Google Scholar]
- 15.Titlestad K, Kristensen T, Jorgensen J, Georgsen J. Monitoring transfusion practice--a computerized procedure. Transfus Med. 2002;12:25–34. doi: 10.1046/j.1365-3148.2002.00352.x. [DOI] [PubMed] [Google Scholar]
- 16.Palo R, li-Melkkila T, Hanhela R, et al. Development of permanent national register of blood component use utilizing electronic hospital information systems. Vox Sang. 2006;91:140–7. doi: 10.1111/j.1423-0410.2006.00814.x. [DOI] [PubMed] [Google Scholar]
- 17.Borkent-Raven BA, Janssen MP, van der Poel CL, et al. The PROTON study: profiles of blood product transfusion recipients in the Netherlands. Vox Sang. 2010;99:54–64. doi: 10.1111/j.1423-0410.2010.01312.x. [DOI] [PubMed] [Google Scholar]
- 18.A report on the usage of Blood Components in Denmark: Dansk Transfusionsdatabase 2009 (data year 2007 and 2008) DTDB. [Accessed on 10/10/2013]. Available at: www.dtdb.dk/download/DTDB_Rapport_2009_43.pdf.
- 19.Joint Commission. Blood Management Performance Measurements Project. 2011. [Accessed on 16/10/2013]. Available at: http://www.jointcommission.org/patient_blood_management_performance_measures_project.
- 20.Gammon HM, Waters JH, Watt A, et al. Developing performance measures for patient blood management. Transfusion. 2011;51:2500–9. doi: 10.1111/j.1537-2995.2011.03406.x. [DOI] [PubMed] [Google Scholar]
- 21.Report on the Collection, Testing and Use of Blood and Blood Components in Europe van der Poel CLj, n M.P., Behr-Gross M-E. 2008. [Accessed on 16/10/2013]. Available at: http://www.edqm.eu/medias/fichiers.
- 22.Johansson PI, Sorensen H. Transfusions in the Copenhagen Hospital Corporation, 2000–2002. (in Danish) Ugeskr Laeger. 2005;167:2785–8. [PubMed] [Google Scholar]
- 23.The Danish National Transfusion guideline: Vejledning om blodtransfusion 2007. Danish Health and Medicines Authority. 2007. Dec 20, [Accessed on 16/10/2013]. Available at: http://sundhedsstyrelsen.dk/publ/publ2007/EFT/blodtransfusion/vejl_blodtransfusion.pdf.
- 24.Guide to the Preparation, Use and Quality Assurance of Blood Components. Council of Europe. 2013. [Accessed on 16/10/2013]. Available at: http://www.edqm.eu/en/blood-organ-guides-1131.html.
- 25.Distler P. ISBT 128: a global information standard. Cell Tissue Bank. 2010;11:365–73. doi: 10.1007/s10561-010-9196-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Verlicchi F, Desalvo F, Zanotti G, et al. Red cell transfusion in orthopaedic surgery: a benchmark study performed combining data from different data sources. Blood Transfus. 2011;9:383–7. doi: 10.2450/2011.0095-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Edwards J, Morrison C, Mohiuddin M, et al. Patient blood transfusion management: discharge hemoglobin level as a surrogate marker for red blood cell utilization appropriateness. Transfusion. 2012;52:2445–51. doi: 10.1111/j.1537-2995.2012.03591.x. [DOI] [PubMed] [Google Scholar]
- 28.Hebert PC, Fergusson DA, Stather D, et al. Revisiting transfusion practices in critically ill patients. Crit Care Med. 2005;33:7–12. doi: 10.1097/01.ccm.0000151047.33912.a3. [DOI] [PubMed] [Google Scholar]
- 29.De Leon EM, Szallasi A. Transfusion indication RBC (PBM-02): gap analysis of a Joint Commission Patient Blood Management Performance Measure at a community hospital. Blood Transfus. 2012;29:1–5. doi: 10.2450/2012.0088-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Francis JJ, Tinmouth A, Stanworth SJ, et al. Using theories of behaviour to understand transfusion prescribing in three clinical contexts in two countries: development work for an implementation trial. Implement Sci. 2009;4:70. doi: 10.1186/1748-5908-4-70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Snyder-Ramos SA, Mohnle P, Weng YS, et al. The ongoing variability in blood transfusion practices in cardiac surgery. Transfusion. 2008;48:1284–99. doi: 10.1111/j.1537-2995.2008.01666.x. [DOI] [PubMed] [Google Scholar]
- 32.Kotze A, Carter LA, Scally AJ. Effect of a patient blood management programme on preoperative anaemia, transfusion rate, and outcome after primary hip or knee arthroplasty: a quality improvement cycle. Br J Anaesth. 2012;108:943–52. doi: 10.1093/bja/aes135. [DOI] [PubMed] [Google Scholar]
- 33.Jans O, Kehlet H, Hussain Z, Johansson PI. Transfusion practice in hip arthroplasty--a nationwide study. Vox Sang. 2011;100:374–80. doi: 10.1111/j.1423-0410.2010.01428.x. [DOI] [PubMed] [Google Scholar]