Abstract
Background
Red blood cell (RBC) transfusions are given as “number of units” without considering the haemoglobin (Hb) content of these units. Donor factors influencing Hb level in whole blood donors and, ultimately, in RBC units have not been studied.
Materials and methods
Donor data for a period of 1.5 years were retrospectively analysed and the effects of age, gender and weight on the Hb level of the donors were determined. The correlation between donor’s Hb concentration with total Hb in the RBC unit was analysed. Additionally, actual Hb content of 125 RBC units was determined. The total Hb content of these RBC units was also mathematically calculated based on the blood donors’ Hb. The ability of this mathematically calculated Hb to predict actual Hb content per RBC unit was then analysed.
Results
The mean Hb level in female donors was 1.79 g/dL lower than in the male donors (p<0.001). Increasing age was associated with a lower mean Hb in the donors (p<0.01), while a higher body weight correlated weakly (r=0.06) but significantly with increased mean Hb (p<0.01). Logistic regression analysis showed that in blood donors, female gender had a stronger influence on lowering the mean Hb than either older age or lower weight. A variation of nearly 100% (42.3–80.8 g Hb per unit) was seen in the total Hb content of the RBC units tested. Mathematically calculated Hb content correlated well (r=0.6; p<0.01) with the actual Hb content of the RBC units.
Discussion
We demonstrated the effect of gender, age and weight on Hb levels in whole blood donors. Dissimilarities in the donor Hb caused nearly 100% variations in the Hb content of the RBC units. It would, therefore, be prudent to label RBC units with their total Hb content. This total Hb content can be predicted fairly accurately from the donor’s pre-donation Hb level.
Keywords: pre-donation Hb, blood transfusion, Hb increase, RBC overtransfusion, Hb content
Introduction
Red blood cell (RBC) transfusions are given working on the rule of thumb that one unit will increase the patient’s haemoglobin (Hb) concentration by approximately 1 g/dL1. This generalisation does not take into any account the total Hb content (THb) in a unit or the difference in THb content among the RBC units. The THb in a RBC unit is determined by the whole blood donor’s Hb, subtracting the Hb lost due to processing the collected blood unit, such as removing the buffy-coat or leucodepletion. Of these two determinants of the THb per unit, the Hb lost due to the processing remains fairly constant and has been quantified to some extent in the past2,3. In contrast, the main cause of variation in the THb among RBC units-the variation in the donor’s pre-donation Hb-has not yet been quantified adequately. Possibly for the same reason, the variation in the THb has also not yet been specifically studied.
Most blood centres across the world consider 12.5 g/dL as the lower limit for the pre-donation Hb in donors4. Even though most guidelines are silent on the upper limit for Hb in the donors, an Hb of up to 18 g/dL is considered normal for an adult5. Theoretically, therefore, the pre-donation Hb may vary up to 44% (12.5–18 g/dL) among blood donors donating at a blood centre. This variation in Hb among the donors could notionally lead to a similar variation in the final THb of the RBC units prepared from these donations.
Measuring the THb in each RBC unit is one way of quantifying this expected variation. However, to do so would add logistical and financial burdens to the blood collection or transfusion centre. We, therefore, planned to study the variation in pre-donation Hb of the accepted whole blood donors and the resultant variation in the THb content of the RBC units. We also intended determine whether the THb in a unit can be estimated without performing any extra tests on that particular unit.
Materials and methods
Donor selection
Data collected from whole blood donors accepted at our centre over 1.5 years were retrospectively analysed. The departmental standard operating procedure, incorporating criteria recommended by the national regulatory authorities, was followed for the donor selection. The donors’ pre-donation Hb was determined by a calibrated haemoglobin meter (Hemocontrol, EKF Diagnostics, Magdeburg, Germany) using the finger prick sample. The lower and upper cut-offs for pre-donation Hb in both men and women were 12.5 and 18.0 g/dL, respectively. The lower cut-off for the body weight of the donor was 45 kg in both the sexes. All female donors and those male donors weighing less than 55 kg donated 350 mL, while the rest of the male donors donated 450 mL of whole blood. The lower and the upper age limits for the donors were 18 and 65 years, respectively. The blood bank software was used to collect the donors’ demographic details, including age, gender, and repeat donation status. Repeat donors constituted a very small proportion of the total donors and were excluded from the study.
All the 450 mL donations were collected into triple blood bags with an integral white blood cell filter (Fenwal Inc., Lake Zurich, IL, USA). All the 350 mL donations were collected into double blood bags (Terumo Penpol Ltd., Trivandrum, India) which was subsequently connected in a sterile manner to a white blood cell filter from the same company. All the RBC units were, therefore, leucofiltered and suspended in a RBC additive solution.
Total haemoglobin content in the red blood cell units
We intended to determine the following: (i)the actual THb of a representative number of RBC units, (ii)the mathematically calculated THb of the same RBC units (whose actual THb was tested), and (iii)the accuracy of the mathematically calculated THb in predicting the actual THb.
We determined the Hb, haematocrit (using a Sysmex cell counter, Sysmex Corp., Kobe, Japan) and volume of 125 complete RBC units (i.e. no low volume collections) at their expiry.
The actual THb in these units was determined as follows6:
The mathematical calculation of the THb was done as follows:
First, total Hb collected in these RBC units was calculated. The pre-donation Hb of the donor of these units was used for this purpose as follows:
Blood loss during leucofiltration and other procedures (e.g. transfer between satellite bags, tubing) was calculated prospectively on 50 RBC units by subtracting post-leucofiltration RBC volume from pre-leucofiltration RBC volume. The Hb lost was calculated as follows:
THb in these RBC units was then mathematically calculated as follows:
Statistical analysis
Statistical calculations were done using the Statistical Package for Social Sciences software (version 15). p values less than 0.05 are considered statistically significant. The independent sample t-test was used to assess the significance of differences between the two means. The correlation coefficient was calculated using both Pearson’s and Spearman’s rho tests. Logistic regression analysis was done to find the individual effects of gender, age and weight on the donors’ Hb level. The one sample t-test was used for the analysis of the data from the 125 RBC units tested at the end of their shelf-life.
Results
The donors’ characteristics and haemoglobin levels
In our study, out of a total of 7,000 accepted whole blood donors, 6,712 (95.9%) were male and 288 (4.1%) were female. Nearly one third of the female donors presenting for a blood donation were deferred, as their Hb was below the lower cut-off (Table I).
Table I.
Gender profile of the selected and deferred whole blood donors.
| Female (%)* | Male (%)* | Total (%)* | |
|---|---|---|---|
| Selected for whole blood donation | 288 (45.9) | 6,712 (90.6) | 7,000 (87.1) |
| Deferred due to low Hb alone | 213 (34.0) | 321 (4.4) | 534 (6.7) |
| Deferred due to other causes | 126 (20.1) | 373 (5.0) | 499 (6.2) |
|
| |||
| Total | 627 (100) | 7,406 (100) | 8,033 (100) |
Percentage of the total in the respective gender category.
Accepted blood donors had a mean Hb of 15.06±1.25 g/dL. Eighty percent of the male donors had a Hb level greater than 14 g/dL while the same proportion of the female donors had a Hb concentration below this level (Table II).
Table II.
Hb distribution in selected female and male whole blood donors (n=7,000).
| Hb range (g/dL) | Female N. (%)* | Male N. (%)* | Total N. (%)* |
|---|---|---|---|
| 12.5–13.0 | 127 (44.1) | 300 (4.5) | 427 (6.1) |
| 13.1–14.0 | 110 (38.2) | 1,066 (15.9) | 1,176 (16.8) |
| 14.1–15.0 | 42 (14.7) | 1,815 (27.0) | 1,857 (26.5) |
| 15.1–16.0 | 6 (2.0) | 1,962 (29.2) | 1,968 (28.1) |
| 16.1–17.0 | 3 (1.0) | 1,145 (17.1) | 1,148 (16.4) |
| 17.1–18.0 | 0 (0) | 424 (6.3) | 424 (6.1) |
|
| |||
| Total | 288 (100) | 6,712 (100) | 7,000 (100) |
Percentage of the total in the respective gender category.
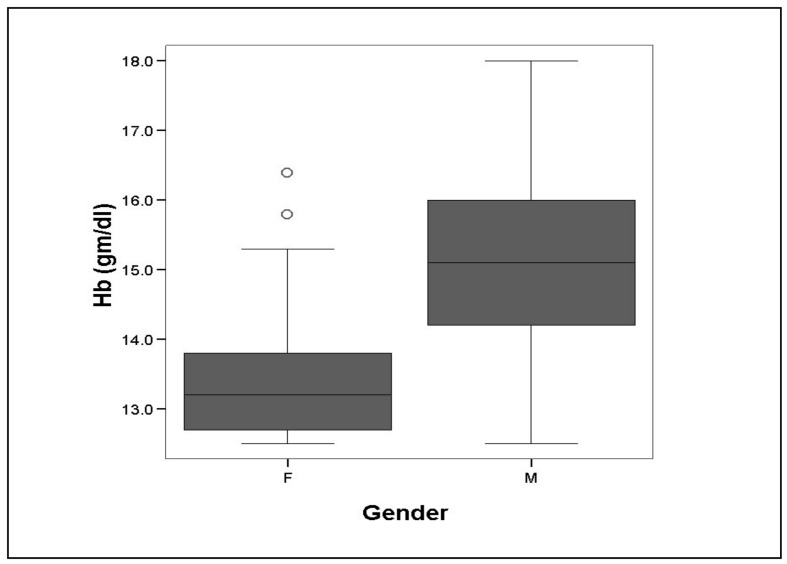
The female donors had a lower mean (±SD) Hb of 13.34±0.80 g/dL (95% CI-13.19 to 13.50 gm/dL) as compared to a mean Hb of 15.13±1.22 g/dL (95% CI of 15.08 to 15.18 gm/dL) in the male donors (Figure 1). This difference of 1.79 g/dL (95% CI of 1.62 to 1.95 g/dL) in the mean Hb of the two genders was statistically significant (p<0.001).
Figure 1.
Mean Hb in female (F) and male (M) whole blood donors.
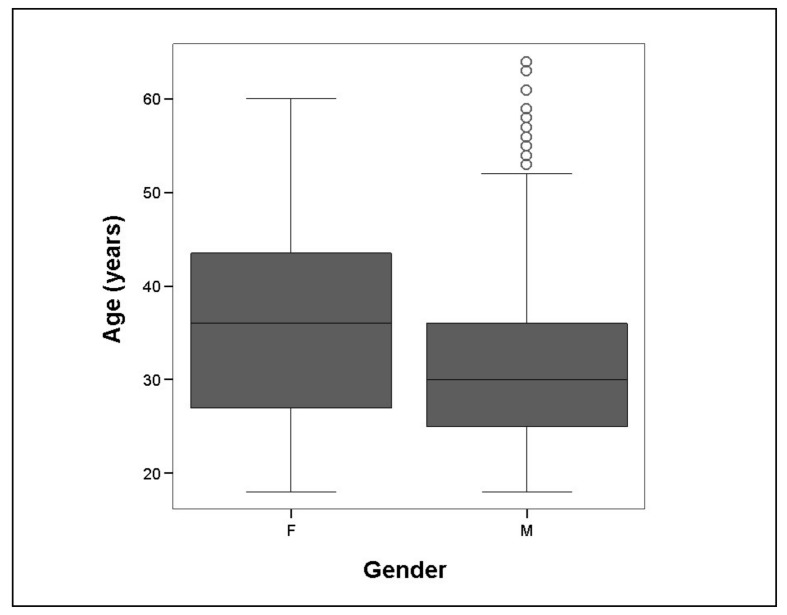
The median and mean age of the donors, irrespective of gender, was 30 and 31.4±8.7 years, respectively. Nearly 70% of the donors were below the age of 35 years. In our study, the male donors, whose mean age was 31.3±10.3 years, were 5 years younger (95% CI of 4.8 to 5.6 years; p<0.001) than the female donors, whose mean age was 36.3±8.6 years (Figure 2).
Figure 2.
Age (years) of female (F) and male (M) WBD.
The mean Hb level decreased with increasing age of the blood donor (Table III). The correlation coefficient for this reciprocal relationship was −0.12 (p<0.01). The mean Hb of the donors decreased from 15.1±1.1 g/dL at 18 years of age to 13.2±0.1 g/dL at 63 years of age (p<0.001).
Table III.
Hb level with the increasing age of the blood donor.
| Hb (g/dL) | Age (years) | ||||
|---|---|---|---|---|---|
|
| |||||
| 18–25 | 26–35 | 36–45 | 46–55 | 56–65 | |
|
| |||||
| N. (%) | N. (%) | N. (%) | N. (%) | N. (%) | |
| Mean (±SD) Hb | 15.3 (1.3) | 15.3 (1.2) | 14.9 (1.3) | 14.8 (1.2) | 14.8 (1.3) |
| 12.5–13.0 | 130 (6.4) | 124 (4.3) | 130 (8.8) | 37 (7.4) | 6 (7.3) |
| 13.1–14.0 | 308 (15.1) | 424 (14.6) | 288 (19.4) | 124 (24.8) | 31 (37.8) |
| 14.1–15.0 | 495 (24.3) | 772 (26.7) | 418 (28.2) | 161 (32.2) | 11 (13.4) |
| 15.1–16.0 | 577 (28.3) | 862 (29.8) | 396 (26.7) | 113 (22.6) | 20 (24.4) |
| 16.1–17.0 | 393 (19.3) | 515 (17.8) | 184 (12.4) | 48 (9.6) | 8 (9.8) |
| 17.1–18.0 | 136 (6.7) | 198 (6.8) | 68 (4.6) | 17 (3.4) | 6 (7.3) |
|
| |||||
| Total | 2,039 (100) | 2,895 (100) | 1,484 (100) | 500 (100) | 82 (100) |
The median and mean body weight of the blood donor, irrespective of gender, was 76.6 and 77.4±13.6 kg (range, 46.2–159 kg), respectively. The mean weight of the female donors was 69.2±13.6 kg (range, 46–107 kg) while that of the male donors was 77.7±13.5 kg (range, 47–159 kg). On average, the female donors weighed 8.5 kg less than the male donors (p<0.001).
There was a weak (correlation coefficient of 0.06) but statistically significant (p<0.01) correlation between the weight and the mean Hb of the donors. The mean Hb increased from 14.7±1.3 g/dL at 46 kg of body weight to 15.3±1.3 at 105 kg (p<0.02) before declining again to similar lower levels of 14.9±1.3 g/dL at 159 kg (p=0.12 for the difference between the mean Hb at the lowest and the highest weight category; Table IV).
Table IV.
Body weight and mean Hb levels in the blood donors.
| Body weight (in kg) | Mean Hb (g/dL) | Standard Deviation | N. of donors (%) |
|---|---|---|---|
| 46–55 | 14.74 | 1.32 | 260 (3.7) |
| 56–65 | 14.97 | 1.30 | 1,133 (16.2) |
| 66–75 | 15.18 | 1.24 | 1,883 (26.9) |
| 76–85 | 15.28 | 1.27 | 1,911 (27.3) |
| 86–95 | 15.22 | 1.17 | 1,151 (16.4) |
| 96–105 | 15.31 | 1.25 | 450 (6.4) |
| 106–130 | 14.94 | 1.09 | 201 (2.9) |
| 131–160 | 14.90 | 1.31 | 11 (0.2) |
The female donors at our centre were, on an average, 5 years older and weighed 8.5 kg less than the male donors. Since, female gender, an advanced age and a lower weight were all found to be associated with a lower mean Hb in the blood donors (p<0.01), logistic regression analysis was done to evaluate the independent effect of each variable. This analysis showed that all three factors, female gender, advanced age and a lower body weight, were independent and significant (p<0.001) predictors of a lower mean Hb in the donors. Female gender was, however, a stronger predictor (standardized coefficient, b=−0.259) of a lower mean Hb in the donors as compared to higher age (b=−0.101) or lower weight (b=0.043).
Total haemoglobin content in the red blood cell units
Prospective testing of 50 RBC units found that, at our centre, an average of 35±2.3 mL blood was lost during the preparing of a unit of leucofiltered RBC from the whole blood.
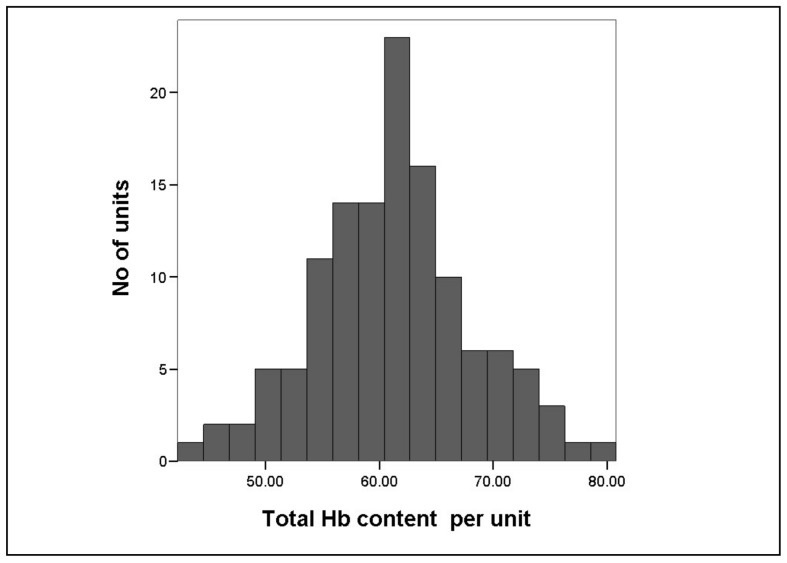
The actual Hb content in the tested RBC units (n=125) varied significantly and ranged from 42.3 to 80.8 g with a mean of 61.3±6.9 g/unit (p<0.001; Figure 3 and Table V). As expected, a higher pre-donation Hb of the blood donor resulted in a higher THb of the RBC unit (p<0.01).
Figure 3.
Total Hb content of RBC units tested at end of shelf life.
Table V.
Descriptive statistics of the tested RBC units (n=125).
| Parameter | Mean | Standard Deviation | 95% CI |
|---|---|---|---|
| Hb of RBC units (g/dL) | 18.94 | 2.04 | 18.58–19.30 |
| Volume (mL) | 323.92 | 23.47 | 319.76–328.08 |
| Haematocrit | 59.57 | 5.70 | 58.56–60.58 |
| Actual Hb content per unit (g) | 61.25 | 6.91 | 60.04–62.46 |
| Calculated Hb content per unit (g) | 62.12 | 5.57 | 61.15–63.10 |
| Blood Donor’s Hb of the tested RBC units (g/dL) | 15.07 | 1.24 | 14.85–15.29 |
The mathematically calculated THb per RBC unit (calculated using pre-donation Hb) correlated well with the actual Hb content of those units (correlation coefficient of 0.6 and p<0.01). The actual and the mathematically calculated THb in the RBC units differed by 0.87 g/unit. This difference between the two values was not statistically significant (p=0.72; Table V)
Discussion
In the current era of evidence-based medicine and individualised care of patients, RBC transfusion continues to be administered on the basis of conventional wisdom and the notion of an average benefit per unit. Gorlin and Cable proposed in the past that a RBC unit should be defined by its therapeutic equivalent of the RBC mass7. The RBC mass is, in turn, better expressed in terms of THb per unit. The existing unscientific blood transfusion practice based on the “number of units transfused” ignores the fact that the THb varies markedly among the individual RBC units. This dissimilarity in the Hb content of the RBC units is, in fact, a natural extension of the dissimilar Hb levels in the blood donors.
To the best of our knowledge this variation in Hb level in the whole blood donors has not been investigated previously. We studied this variation in Hb in our whole blood donor population to identify its extent and modifiers, if any. We also studied the variation in the THb content of the RBC units occurring due to this variation in the blood donors’ Hb. Finally, we propose a formula to calculate the THb content in an RBC unit, based on the donor’s Hb.
With female donors contributing a meagre 4.1% of the total donors, most of our whole blood donors were first-time, male donors. A high deferral rate due to anaemia in female donors is an established fact in our region8, as in other developing countries. Even among the accepted female donors, the mean Hb was significantly lower (1.8 g/dL) than that in the male donors.
Although not specifically studied earlier in the accepted blood donors, it is common knowledge that the Hb is lower in the general female population than in the male population. The third National Health And Nutritional Examination Survey (NHANES III), as well as a study by Beutler and Waalen9, found that Caucasian females have a mean Hb level 1.5 g/dL lower than that of Caucasian males. Hence, RBC units donated by female donors will have a lower THb than those donated by male donors.
Our study also showed that the THb will be less in a RBC unit donated by an ‘older’ donor. Although this inverse relationship between age of the donor and THb was not very strong (−0.12), it was still significant. Poor dietary intake of iron and the diminished overall bone marrow reserves might explain the lower Hb levels in older donors. As for the lower Hb in female donors, this age-related decrease in Hb could also be a global phenomenon. Parikh A. et al.10 suggested that a lower mean Hb associated with iron-deficiency anaemia is seen uniformly across the globe and is not restricted to developing countries only.
In our study body weight had a weak relationship with Hb level of the blood donor. Both lower weight as well as grossly overweight donors had lower mean Hb levels, probably reflecting the donors’ poor nutritional status. However, this should be interpreted with caution because body mass index is a better marker of an individual’s overall nutritional status than weight alone. We could not study this relationship between body mass index and Hb level of donors because height was not routinely recorded for the whole blood donors.
Female gender and increasing age were thus two independent and significant predictors of a lower mean Hb in blood donors and thus in RBC units. In developed countries, female and older donors constitute a higher proportion (nearly 50%) of total blood donors as against 10% or less in underdeveloped and developing countries11–13. Hence, we can presume that the mean THb per RBC unit would be lower in developed countries than in underdeveloped and developing countries.
Our assumption is supported by the fact that the mean THb of RBC units in our study was 61.3±6.9 g/unit. This is nearly 20% higher than the mean THb of 50.9±5.4 g/unit found by Chabanel and colleagues in their 5-year quality control of RBC units in France3. Another study by Susanne and colleagues also showed a lower mean Hb of 46.8±5.1 g/unit in RBC units collected from German blood donors14. Thus, in terms of Hb content, RBC units are different not only within a particular blood centre but also across different blood centres.
Nonetheless, the variation in Hb of whole blood donors and, therefore, of RBC units should be further studied across different geographies. The effect of other donor variables, for example repeat donation status, on a donor’s Hb level, also needs to be evaluated in future studies.
Now, let us consider the theoretical effect of this variable Hb content in RBC units on patients and the implications for the treating clinician. In our study, the THb of the RBC units ranged from 42–81 g/unit. When given to a 70 kg, non-bleeding, euvolemic, adult, male patient, a RBC unit containing 42 g of Hb would be expected to increase his Hb by 0.85 g/dL. Another RBC unit with a THb of 81 g, given to a clinically similar 60 kg female patient, would raise her Hb by 2.07 g/dL. Thus depending on the characteristics of the transfused patient, an assumption of a 1g/dL increase in the recipient’s Hb per RBC unit transfused could be an under-estimate or an over-estimate.
For the same reason, transfusion appropriateness studies based on number of RBC units transfused (while ignoring the THb transfused) may not present the true picture. The THb of a RBC unit transfused should be considered as a confounding factor in these studies, especially those taking discharge Hb as the marker of overtransfusion15–17. Additionally, great differences seen in blood transfusion practices of clinicians18,19 may actually be due to unpredictable increases in Hb in patients, caused by this variable Hb in different RBC units.
The presence of this variation in THb of RBC units and its probable clinical effect were postulated in the past. Hogman and Knutson, hypothesising this variability and its consequences, even proposed the concept of a “standardised unit of RBC” based on a fixed THb per unit20. They suggested the use of automated apheresis devices to achieve this goal of a standardised unit with a fixed THb content per unit. However, such automated collections, besides being too expensive21, are also very “demanding” in terms of the donor selection criteria. Thus, while trying to achieve any level of cost-effectiveness and standardisation with such automated collections, we might have to defer nearly 50% of male donors and almost all female donors20. Considering the already shrinking donor base and ever increasing blood costs, this standardised RBC unit cannot be currently viewed as an option.
We, therefore, recommend labelling RBC units with their THb content and letting the transfusing clinician know the actual Hb content per unit. The need to label RBC units with their THb content was also advocated by Davenport21. He suggested that we should test each RBC unit for its Hb value and measure the total Hb content per unit before this labelling21. However, implementing Davenport’s suggestion of labelling the RBC units would further increase the cost of the final product. We, therefore, propose a simple formula to calculate the THb per RBC unit, without requiring any additional testing on the unit itself. This formula is based on the donor’s pre-donation Hb level and the haemoglobin lost during the processing of a unit. The donor’s pre-donation Hb level is measured in almost all blood centres and thus no additional testing is required to obtain this value. Only those blood centres using a cut-off method (e.g. copper sulphate) may need to switch to point-of-care testing to determine this value. Similarly, as shown in our study and in other studies20, the Hb lost during processing of a blood unit remains fairly constant and does not need be tested for every RBC unit. The calculated THb, derived using the simple formula, predicted the actual THb of the RBC units fairly accurately in our study. Hence, it seems feasible in most blood centres to label RBC units with their THb content without incurring any additional cost.
If units are labelled with their THb content, there are better chances of rationalising RBC transfusions in an institution. Arslan and colleagues6 showed the utility of a THb content-based transfusion policy in successfully decreasing the number of RBC units required in an institution. They also showed that such a Hb content-based RBC transfusion strategy can successfully (96.6% of times) achieve the target Hb in the patients. A Hb content-based transfusion policy may facilitate rational ordering and use of RBC units, which currently varies across as well as within institutions18,19. Using the donor’s pre-donation Hb to calculate and label the THb in a RBC unit appears to be an easy and inexpensive way of achieving rational use of RBC donations.
Conclusion
In the context of current-day evidence-based medical practice, the salt content of any drugs administered is known to the last milligram. However, it is an irony that RBC units are still routinely prepared and transfused with no more than a guess as to their Hb content.
Our study proved the hypothesis of a variable Hb content in RBC units and highlighted the factors causing this variability. We showed that characteristics such as gender, age and weight of the donors influence and vary the donors’ pre-donation Hb levels. This variation in pre-donation Hb levels among donors is, in turn, the main cause of the variable Hb content of RBC units. Donor demographics do, therefore, have a direct impact on the THb content in RBC units prepared at a blood centre. We showed that RBC units given by females and older donors would contain less Hb content than those given by males and younger donors. More studies, preferably in different donor populations and with higher percentages of females and repeat donors, are, however, required to further establish the concept.
Since donor demographics differ, we can be certain that THb in RBC units also differs both within and across geographic settings. We demonstrated this effect by showing that the mean Hb in the RBC units at our centre was much higher than that reported by western centres. This probably reflects the fact that the majority of the donations at our centre were made by younger, male donors.
We also proposed a formula which could be used to label RBC units with their THb content without doing any additional testing. This formula would spare the blood centres from incurring any extra cost for the THb labelling. The logical and inexpensive Hb-content labelling of RBC units is, therefore, advisable. It is to be hoped that such labelling of RBC units will facilitate precise ordering of this precious resource and make transfusion practice scientific, comparable and more rational.
Footnotes
The Authors declare no conflicts of interest.
References
- 1.Brecher ME. AABB Technical Manual. 15th ed. Bethesda, Maryland: AABB publications; 2005. [Google Scholar]
- 2.Hogman CF, Meryman HT. Red blood cells intended for transfusion: quality criteria revisited. Transfusion. 2006;46:137–42. doi: 10.1111/j.1537-2995.2006.00681.x. [DOI] [PubMed] [Google Scholar]
- 3.Chabanel A, Masse M, Begue S EFS group of blood component QC laboratory managers. National French observatory of the quality of blood components for transfusion. Transfus Clin Biol. 2008;15:85–90. doi: 10.1016/j.tracli.2008.04.006. [DOI] [PubMed] [Google Scholar]
- 4.Karp JK, King KE. International variation in volunteer whole blood donor eligibility criteria. Transfusion. 2010;50:507–13. doi: 10.1111/j.1537-2995.2009.02392.x. [DOI] [PubMed] [Google Scholar]
- 5.Fauci AS, Braunwald E, Isselbacher KJ, et al. Harrison’s Principles of Internal Medicine. 14th ed. Singapore: McGraw Hill; 1998. [Google Scholar]
- 6.Aslan O, Toprak S, Arat M, Kayalak Y. Hb content-based transfusion policy successfully reduces the number of RBC units transfused. Transfusion. 2004;44:485–8. doi: 10.1111/j.1537-2995.2004.03225.x. [DOI] [PubMed] [Google Scholar]
- 7.Gorlin JB, Cable R. What is a unit? Transfusion. 2000;40:263–5. doi: 10.1046/j.1537-2995.2000.40030263.x. [DOI] [PubMed] [Google Scholar]
- 8.Agnihotri N. Whole blood donor deferral analysis at a center in Western India. Asian J Transfus Sci. 2010;4:116–22. doi: 10.4103/0973-6247.67035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Beutler E, Waalen J. The definition of anemia: what is the lower limit of normal of the blood hemoglobin concentration? Blood. 2006;107:1747–50. doi: 10.1182/blood-2005-07-3046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Parikh A, Natarajan S, Lipsitz SR, Katz SD. Iron deficiency in community-dwelling US adults with self-reported heart failure in the National Health and Nutrition Examination Survey III: prevalence and associations with anemia and inflammation. Circ Heart Fail. 2011;4:599–606. doi: 10.1161/CIRCHEARTFAILURE.111.960906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Atsma F, Veldhuizen I, de Vegt F, et al. Cardiovascular and demographic characteristics in whole blood and plasma donors: results from the Donor InSight study. Transfusion. 2011;51:412–20. doi: 10.1111/j.1537-2995.2010.02867.x. [DOI] [PubMed] [Google Scholar]
- 12.Glynn SA, Schreiber GB, Busch MP, et al. Demographic characteristics, unreported risk behaviors, and the prevalence and incidence of viral infections: a comparison of apheresis and whole-blood donors. The Retrovirus and Epidemiology Donor Study. Transfusion. 1998;38:350–8. doi: 10.1046/j.1537-2995.1998.38498257373.x. [DOI] [PubMed] [Google Scholar]
- 13.Agnihotri N, Marwaha N, Sharma RR. Analysis of adverse events and predisposing factors in replacement and voluntary whole blood donors: a study from North India. Asian J Transfus Sci. 2012;6:158–63. doi: 10.4103/0973-6247.98922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Picker SM, Radojska SM, Gathof BS. In vitro quality of red blood cells (RBCs) collected by multicomponent apheresis compared to manually collected RBCs during 49 days of storage. Transfusion. 2007;47:687–96. doi: 10.1111/j.1537-2995.2007.01172.x. [DOI] [PubMed] [Google Scholar]
- 15.Edwards J, Morrison C, Mohiuddin M, et al. Patient blood transfusion management: discharge hemoglobin level as surrogate marker for red blood cell utilization appropriateness. Transfusion. 2012;52:2445–51. doi: 10.1111/j.1537-2995.2012.03591.x. [DOI] [PubMed] [Google Scholar]
- 16.Andreasen JJ, Sindby JE, Brocki BC, et al. Efforts to change transfusion practice and reduce transfusion rates are effective in coronary artery bypass surgery. J Cardiothorac Vasc Anesth. 2012;26:545–9. doi: 10.1053/j.jvca.2012.02.006. [DOI] [PubMed] [Google Scholar]
- 17.Barr PJ, Donnelly M, Cardwell CR, et al. The appropriateness of red blood cell use and the extent of overtransfusion: right decision? Right amount? Transfusion. 2011;51:1684–94. doi: 10.1111/j.1537-2995.2011.03130.x. [DOI] [PubMed] [Google Scholar]
- 18.Maddux FW, Dickinson TA, Rilla D, et al. Institutional variability of intraoperative red blood cell utilization in coronary artery bypass graft surgery. Am J Med Qual. 2009;24:403–11. doi: 10.1177/1062860609339384. [DOI] [PubMed] [Google Scholar]
- 19.Frank SM, Savage WJ, Rothschild JA, et al. Variability in blood and blood component utilization as assessed by an anesthesia information management system. Anesthesiology. 2012;117:99–106. doi: 10.1097/ALN.0b013e318255e550. [DOI] [PubMed] [Google Scholar]
- 20.Hogman CF, Knutson F. Standardized units of RBCs: Is it time for implementation? Transfusion. 2000;40:330–4. doi: 10.1046/j.1537-2995.2000.40030330.x. [DOI] [PubMed] [Google Scholar]
- 21.Davenport R. Blood components should be labeled for content. Transfusion. 2005;45:3–4. doi: 10.1111/j.1537-2995.2005.04323.x. [DOI] [PubMed] [Google Scholar]