Abstract
Background
Recent studies have shown large increases in non-transferrin-bound iron (NTBI) and biomarkers of oxidative stress in the extracellular medium of packed red blood cell units during storage. It has been further suggested that transfusion-mediated iron and oxidative load may contribute to transfusion-related morbidity in premature babies. The origin and nature of the NTBI is currently unclear, but the release of iron from oxidatively modified haemoglobin and haem has been suggested. The purpose of this study was to investigate whether this may be the case.
Materials and methods
The concentration of haem in the extracellular fluid of paediatric packed cell units stored from 3 to 35 days was measured using a commercial haem assay. In vitro studies were conducted using haem (haemin; ferriprotoporphyrin IX chloride) to determine whether the NTBI assay was able to react with and measure iron associated with haem in the presence and absence of oxidising agents.
Results
The level of haem in the extracellular fluid of paediatric packed cell units rose gradually from day 3 to day 21, then more rapidly to day 35. Very little NTBI was released from haem in the absence of oxidising agents, but the amount rose in a dose- and time-dependent manner in proportion to the oxidation of haem by incubation with H2O2.
Discussion
The results of the study imply that the NTBI measured in previous studies may derive from the oxidatively modified haem that builds up in the extracellular fluid of packed red blood cell units during storage. The potential influence of this on transfusion mediated morbidity is discussed.
Keywords: iron, haem, oxidative stress, packed cells, storage
Introduction
A number of studies have shown large increases in non-transferrin-bound iron (NTBI) during storage in packed red blood cell units1,2, and this is associated with transient increases in circulating NTBI in premature babies following the receipt of blood3. The degree of the increase in post-transfusion NTBI is positively correlated with the duration of storage of the transfused blood. This may contribute to transfusion-related morbidity in babies and adults4–7 which is also influenced by the storage age of the blood8,9.
The origin and nature of the NTBI in packed cell units is currently unclear. Although the build-up of extracellular haemoglobin in the stored packs in most studies is well below that which would be expected if the level of haemolysis approached the maximal recommended level1,10,11, the level of haem rises considerably during storage12. Studies have also shown that the level of oxidative damage in stored units increases during storage1,13,14. Haem is able to release its iron when damaged by reactive oxygen species15,16, although it is not known whether this iron is detectable as NTBI.
In this study, extracellular fluid from stored paediatric packed cell units was used to examine to what extent haem levels vary as a function of storage age, as they do in adult units12. In addition, in vitro studies were conducted using haem (haemin) (ferriprotoporphyrin IX chloride) to determine whether the NTBI assay used in previous studies1,17 is able to react with and measure iron associated with haem in the presence and absence of oxidising agents. Data derived from this study, used in conjunction with data from previous studies1, provide a greater understanding of the origin of the iron detected in paediatric packed cell units, and will help to predict possible consequences to the recipients.
Materials and methods
Paediatric packed cell units
Paediatric packed cell units were prepared at the NHS Blood and Transplant Centre (Bristol, UK) as described previously1. The adult blood is anticoagulated with citrate, phosphate, dextrose (sodium citrate 89 mmol/L; citric acid 16 mmol/L; glucose 128 mmol/L; sodium phosphate 16 mmol/L) at a ratio of 63 mL anticoagulant to 450 mL blood. The blood is filtered to remove leucocytes and centrifuged to yield the red blood cells. Most of the plasma is removed (leaving about 20 mL) and replaced with 100 mL of SAGM additive (NaCl 150 mmol/L; adenine 1.25 mmol/L; glucose 45.4 mmol/L; mannitol 28.8 mmol/L). This provides a unit containing approximately 200 mL of red blood cells and 100 mL of additive. The adult pack is then split into six paediatric units of 45–50 mL each. In this study a total of ten adult packs were used providing ten sets of paediatric packs for study. Each set of six paediatric units was transported using the regular NHS blood transport carriers to the blood centre at Derriford Hospital (Plymouth, UK) and stored in the hospital blood bank in a quarantined section in the same refrigerator that houses units for clinical use. One pack was removed from each set on arrival at the blood bank (3 days after donation), one at 7 days after donation, and then every 7 days up to 35 days of storage.
The packed cell units were gently inverted a few times to mix the contents. The blood was removed and centrifuged in plain vacutainers. The tubes were centrifuged at 1,500×g for 10 minutes to separate the packed cells from the extracellular phase24. The extracellular phase was removed and stored at −80 °C for later analysis. This study was conducted on the same stored samples used in the previous study1, which allowed direct comparison with data from that study.
Measurement of haem
In order to help compare findings, haem was measured in the stored extracellular fluid in this study by the same method used by Ozment et al12, which was a commercially available assay kit, the QuantiChrom Heme Assay Kit (BioAssay Systems, Gentaur Ltd., London, UK). The haem was analysed according to the manufacturer’s instructions. Samples (50 μL) were placed in individual wells of a 96-well plate followed by 200 μL of the reagent. Samples were incubated for 5 minutes at room temperature before absorbance was measured at 400 nm in a Molecular Devices VersaMax ELISA Microplate reader (Molecular Devices, Wokingham, UK). The amount of haem was quantified using the supplied calibrator and SAGM blanks.
Measurement of non-transferrin-bound iron
NTBI was measured using a slight modification of the high performance liquid chromatography (HPLC) methods of Kime et al.18 and Pafetti et al.19 as described in detail previously1. Briefly, 300 μL of haem solution in phosphate-buffered saline was incubated with 30 μL of 0.8 M nitrilotriacetic acid for 20 minutes at room temperature to chelate loosely bound iron. The samples were then placed in 30 kDa Amicon Ultra 0.5 ml filters (Millipore, Watford, UK) and centrifuged at 13,000×g at 4 °C for 30 minutes. Next, 250 μL of the ultrafiltrate were removed and incubated with 25 μL of 35 mM 3-hydroxy-1-propyl-2-methyl-pyridon-4-one for 5 minutes before injecting into the HPLC system (sample loop 20 μL). The mobile phase consisted of 5 mM PIPES buffer pH 7.0 containing 3.5 mM 3-hydroxy-1-propyl-2-methyl-pyridon-4-one and 5% acetonitrile. The column was a polyethyl ethyl ketone (PEEK)-lined 100mmx5 mm C18 column (Hichrom, Reading, UK). All tubing was PEEK. The mobile phase was pumped at a flow rate of 1 mL/minute using a Dionex pump. The absorbance of the iron-chromophore complex was determined using a Dionex UV/VIS detector at a wavelength of 450 nm, and chromatography conducted using Chromeleon software (Thermo Fisher Scientific, Loughborough, UK). The concentration of NTBI was computed from blanks and standards taken through the whole procedure with each batch of samples.
Preparation of haem solutions
Haem (haemin, ferriprotoporphyrin IX chloride; Frontier Scientific, Inochem Ltd., Carnforth, UK) was initially made up in dimethyl sulphoxide at a concentration of 5 mM. This was diluted down in phosphate-buffered saline to working concentrations of 2 μM to 50 μM. These concentrations were chosen to reflect the levels found in the paediatric packs.
Haem solutions were oxidatively modified by incubation at room temperature with various concentrations of H2O2 for periods of time from 30 to 120 minutes.
Statistical analysis
Descriptive and correlational statistics were conducted using Stat 200 (Biosoft, Cambridge, UK).
Results
Haem concentration in stored packs
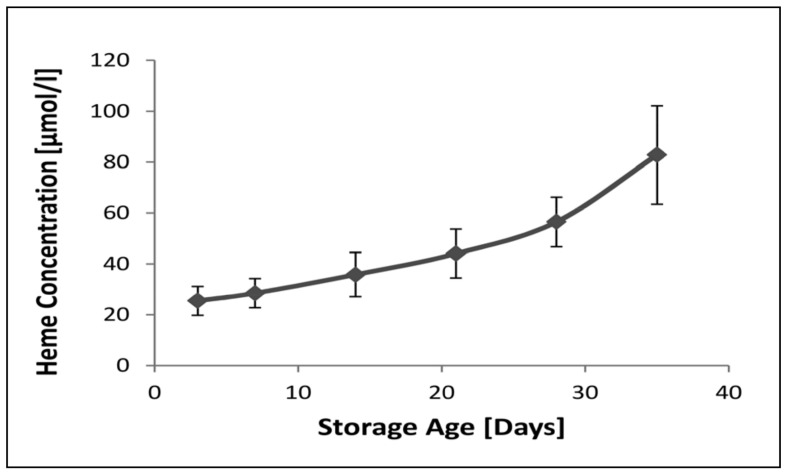
The level of extracellular haem as a function of storage age is shown in Figure 1. It can be seen that there was already a large amount of haem present in the extracellular fluid of the paediatric packs on day 3. The level then rose gradually up to 21 days’ storage and then more rapidly to the maximal storage age of 35 days.
Figure 1.
The concentration of haem in the extracellular milieu of stored paediatric packed cell units as a function of storage age.
Results are presented as the mean±SD of ten sets of paediatric packs. There were no significant differences between levels measured between days 3 and 14. Thereafter the levels increased significantly (p<0.05) with storage age. ANOVA with Duncan’s multiple comparison test.
Non-transferrin-bound iron in haem solutions
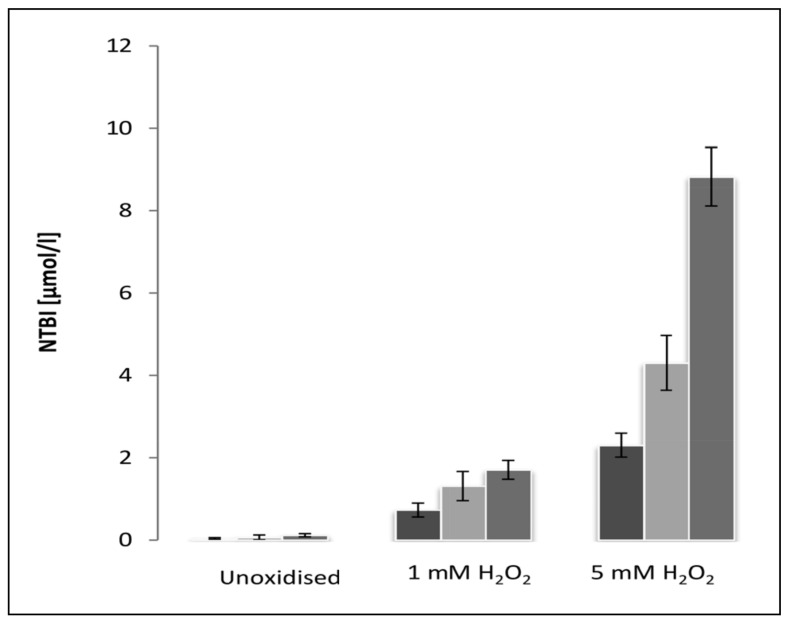
The amount of NTBI measured in solutions containing 10 μM, 20 μM and 50 μM is shown in Figure 2. Very little NTBI was detected in haem solutions not exposed to H2O2. However, there was a significant increase in the amount of NTBI following exposure to 1 mM and 5 mM H2O2. This indicates that the NTBI assay is incapable of removing iron from haem which has not been exposed to H2O2, but that the iron released from oxidatively modified haem is detectable as NTBI.
Figure 2.
The concentration of NTBI measured in haem solutions of 10 μmol/L (dark grey columns), 20 μmol/L (light grey columns) and 50 μmol/L (medium grey columns) following incubation for 30 minutes at room temperature without H2O2 (unoxidised), 1 mM H2O2 and 5 mM H2O2.
Results are expressed as the mean±SD of five experiments. There was very little NTBI in the non-oxidised group, and no significant differences with regard to haem concentration in this group. In the oxidised groups the level of NTBI increased with haem concentration (p<0.05). ANOVA with Duncan’s multiple comparison test.
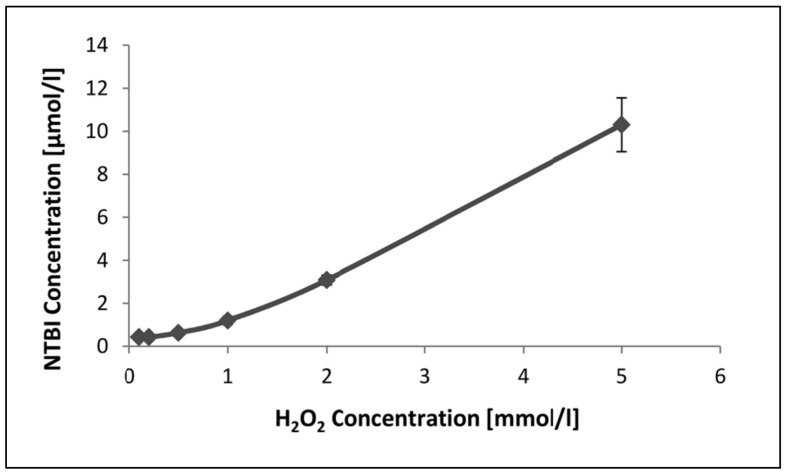
The measurement of NTBI released from 50 μM haem as a function of H2O2 concentration is shown in Figure 3. It can be seen that very little NTBI is detected at H2O2 concentrations below 1 mM, but thereafter the amount detected increases as H2O2 concentration rises. The limited effect of concentrations of H2O2 below 1 mM may indicate that haemin (used in this study) is more resistant to oxidative damage than haem20.
Figure 3.
The amount of NTBI released from 50 μM haem as a function of H2O2 concentration. Haem was incubated for 30 minutes at room temperature at the different concentrations of H2O2.
Results are expressed as the mean±SD of five experiments. NTBI concentration increased significantly (p<0.05) with H2O2 concentration beyond 1 mM. ANOVA with Duncan’s multiple comparison test.
Table I shows the influence of incubation time on NTBI measurements following incubation of haem with 1 mM or 5 mM H2O2. At 1 mM concentration the NTBI levels changed little with incubation time. At a concentration of 5 mM there was a considerable release of NTBI after 30 minutes’ incubation and this rose slowly as incubation time increased.
Table I.
The effect of time of incubation with hydrogen peroxide on the release of NTBI from haem.
| Time (min) | NTBI μmol/L | |
|---|---|---|
|
| ||
| 1 mM H2O2 | 5 mM H2O2 | |
| 30 | 1.301±0.166 | 8.326±0.120 |
| 60 | 1.787±0.084 | 9.347±0.467 |
| 90 | 1.748±0.119 | 11.049±0.159 |
| 120 | 1.673±0.056 | 12.051±0.297 |
The amount of NTBI released from 50 μM haem solutions as a function of incubation time. Solutions were incubated with either 1 mM H2O2 or 5 mM H2O2 for 30–120 minutes at room temperature. Results are expressed as the mean±SD of five separate experiments. There were no significant differences in the 1 mM group with the exception of times 30 and 60 minutes. The NTBI levels increased significantly (p<0.05) with incubation time in the 5 mM group. ANOVA with Duncan’s multiple comparison test.
Non-transferrin-bound iron as a percentage of available haem iron
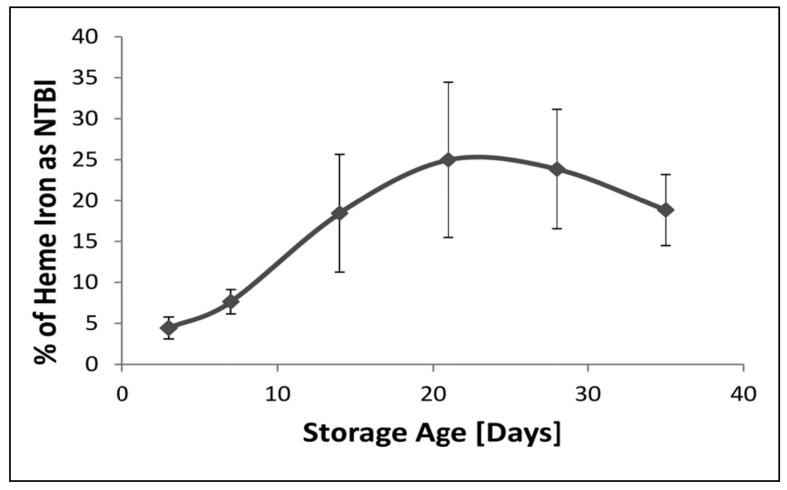
Assuming that 1 mol of haem contains 1 mol of iron, the percentage of available haem iron that was detected as NTBI in paediatric packs as a function of storage age is shown in Figure 4. It can be seen that the percentage of potentially available iron that was detected as NTBI was low during the first few days of storage, but then rose rapidly to a peak at 21 days. Thereafter it fell as the haem level rose rapidly. Despite this there remained a strong positive correlation between NTBI and haem (r=0.769, p<0.05; n=60).
Figure 4.
The percentage of available haem iron present as NTBI in the extracellular milieu of paediatric packed cell units as a function of storage age.
Results are expressed as the mean percentage±SD of ten sets of paediatric packed cell units. There were significant differences (p<0.05) between all time-points with the exception of between days 21 and 14, and days 21 and 28. ANOVA with Duncan’s multiple comparison test.
Discussion
Haem levels during storage
This study confirms the findings of Ozment et al.12 regarding the presence of haem in stored packed red blood cell units. The amount of haem found in this study was, however, greater than that seen in the previous study. We used the same assay procedure, so other factors need to be considered to account for this difference. The paediatric packed cell units used in this study had SAGM as the additive. In the previous study ADSOL3 or CPDA-1 was used. Although these additives have some similarities in composition, it would be useful to see whether haem levels are influenced by these factors. Small volume paediatric packed cell units were used in this study whereas adult packs were used in the previous one. There may be an unknown influence of volume or greater interaction with the plastic material in the paediatric packs. Finally, this study was conducted on fluid used in the previous study1 and stored at −80 °C for between 6 and 12 months. It is possible that storage-related breakdown of haemoglobin might have contributed to the higher levels of haem reported in this study. Despite these differences, the findings of both studies show that haem is present in the packs immediately on arrival at the blood bank (3 days post-donation), indicating that events occurring during donation and processing cause the build-up of haem in the extracellular phase of the packs. From this point the level rises slowly during storage until about day 21 before rising more abruptly. This pattern of change is consistent with a paradigm that involves an initial release of haem (possibly by oxidative damage to haemoglobin), followed by the release of iron from the oxidatively modified haem which then drives the generation of further reactive oxygen species to cause more haem release.
The relationship between haem and non-transferrin-bound iron
The source of the storage-related build-up of extracellular NTBI in paediatric packs has yet to be determined1. The iron-containing molecules within the paediatric packs are haem and haemoglobin. It is known that oxidatively modified haem may lose its iron15,16,21, but it is not clear whether the NTBI assay used in this, and previous studies, is capable of detecting iron released from the oxidatively modified haem, or indeed extracting the iron from non-oxidised haem. These studies showed that while not being able to detect iron from haem which has not been exposed to an oxidising agent, the assay detects iron from oxidatively modified haem. The percentage of available iron on the haem molecules that is detected by the NTBI assay is low in the first few days of storage when the degree of oxidative stress is lowest1 and then peaks after 21 days of storage. The percentage of available iron detected then falls as the total amount of haem rises exponentially (although the level of NTBI continues to rise1). This probably reflects differences in the ease with which reactive oxygen species can cause the release of haem from haemoglobin, and iron from haem. Oxidative damage to haemoglobin produces superoxide (ultimately H2O2), methaemoglobin15 and free iron22. Methaemoglobin is relatively unstable and will readily release the haem moiety from the haem pocket23. Further oxidation of the haem molecule leads to the release of free iron.
It is well known that oxidative stress, normal ageing and aerobic incubation leads to the release of free chelatable iron from haemoglobin within erythrocytes21,22. There is evidence that iron released within the erythrocyte can mediate oxidative damage to the cell membrane leading to haemolysis and the release of extracellular haemoglobin21. This may be further oxidised extracellularly to release haem and iron. Whatever mechanism is occurring, it happens rapidly during the donation and preparation procedure because on day 3 after donation there is already evidence of oxidative damage to the red blood cells and the release of haem and some iron1. One possible mechanism for the initial rapid changes might be auto-oxidation of haemoglobin. Intracellular antioxidants will protect events occurring within the cytosol, but the consequences of oxidation of membrane-bound haemoglobin are not well protected against20. This could cause the initial generation of iron which may go on to induce further oxidative damage within the cell. Once these events have been initiated a potential feed-forward process may occur leading to further oxidative damage as storage continues1. Such a scenario would be supported by the more rapid rise in malondialdehyde and haem seen during the latter stages of storage1,12.
The potential adverse consequences for the recipients of transfusion products (particularly those stored for more than 14 days) containing free iron and haem may contribute to transfusion-related morbidity in premature babies4–7. Iron, if not well sequestered on transferrin, can lead to the generation of reactive oxygen species and consequent oxidative damage to the tissues. Similarly free, circulating haem, if not well sequestered on haemopexin, also has pro-oxidant and pro-inflammatory activity24,25. As indicated in previous studies, premature babies are deficient in transferrin, transferrin-binding capacity and haemopexin26–28, increasing the likelihood of transfusion-mediated iron and haem overload contributing to the transfusion-mediated morbidity seen in these babies.
The potential iron load received by transfused premature babies has been alluded to earlier1. Normally, each baby receives 10–20% of their total blood volume over a 2–4 hour transfusion. Based on our figures1, blood stored for 14 days (NTBI≈6 μM) would add NTBI to give a final concentration of 0.6–1.2 μM if not sequestered or bound. Studies by Stark et al.3 provide figures of NTBI of around 0.1–0.2 μM 4 hours post-transfusion, indicating some level of sequestering or removal. The level had returned to pre-transfusion levels 24 hours after transfusion. A previous study by Dani et al.29 using an NTBI assay similar to ours, reported much higher NTBI levels (2.03 μM) within 3 hours of receiving blood (≈5% of total blood volume/hour over 3–4 hours). To achieve this solely from transfused NTBI would require that the blood used for transfusion be stored for near maximum time. This could add NTBI to a final concentration of 1.4–2.8 μM. More likely, the post-transfusion increase in NTBI occurs as a consequence of the addition of NTBI already present in the transfused blood plus any derived from post-transfusion haemolysis of the transfused erythrocytes. Hod et al.30 have provided evidence that most NTBI in adult recipients is derived from post-transfusional haemolysis rather than pre-formed NTBI in the transfusate, and in the study by Starke et al.3, the level measured in the blood exceeded that of the supernatant of the packed cell units, which would also suggest a contribution from post-transfusion haemolysis. Whatever the origin of the NTBI, Amin et al.31 showed that 50% of babies who received more than three erythrocyte transfusions were iron-overloaded at 35 weeks post-menstrual age, irrespective of when they received their transfusions. Investigations into the long-term outcome of these babies would help in clarifying the importance of transfusion-mediated iron load in premature babies.
Footnotes
Funding
This work was funded by The Northcott Devon Medical Foundation.
The Authors declare no conflict of interest.
References
- 1.Collard K, White D, Copplestone A. The influence of storage age on iron status, oxidative stress and antioxidant protection in paediatric packed cell units. Blood Transfus. 2013 Nov 29;:1–10. doi: 10.2450/2013.0142-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Marwah SS, Blann A, Harrison P, et al. Increased non-transferrin bound iron in plasma depleted SAG-M red blood cell units. Vox Sang. 2002;82:122–6. doi: 10.1046/j.1423-0410.2002.00153.x. [DOI] [PubMed] [Google Scholar]
- 3.Stark MJ, Keir AK, Andersen CC. Does non-transferrin bound iron contribute to transfusion related immune-modulation in preterms? Arch Dis Child. 2013;98:F424–9. doi: 10.1136/archdischild-2012-303353. [DOI] [PubMed] [Google Scholar]
- 4.Collard KJ, Godeck S, Holley JE, Quinn MW. Pulmonary antioxidant levels and oxidative damage in ventilated premature babies. Arch Dis Child. 2004;89:F412–6. doi: 10.1136/adc.2002.016717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Baer VL, Lambert DK, Henry E, et al. Among very-low-birth-weight neonates is red blood cell transfusion an independent risk factor for subsequently developing a severe intraventricular hemorrhage? Transfusion. 2011;51:1170–8. doi: 10.1111/j.1537-2995.2010.02980.x. [DOI] [PubMed] [Google Scholar]
- 6.El-Dib M, Narang S, Lee E, et al. Red blood cell transfusion, feeding and necrotizing enterocolitis in preterm infants. J Perinatol. 2011;31:183–7. doi: 10.1038/jp.2010.157. [DOI] [PubMed] [Google Scholar]
- 7.Romagnoli C. Risk factors and growth factors in ROP. Early Hum Devel. 2009;85:S79–82. doi: 10.1016/j.earlhumdev.2009.08.026. [DOI] [PubMed] [Google Scholar]
- 8.Gauvin F, Spinella PC, Lacroix J, et al. Association between length of storage of transfused red blood cells and multiple organ dysfunction syndrome in pediatric intensive care patients. Transfusion. 2010;50:1902–13. doi: 10.1111/j.1537-2995.2010.02661.x. [DOI] [PubMed] [Google Scholar]
- 9.Karam O, Tucci M, Bateman ST, et al. Association between length of storage of red blood cell units and outcome of critically ill children: a prospective observational study. Crit Care. 2010;14:R57–R64. doi: 10.1186/cc8953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gevi F, D’Alessandro A, Rinalducci S, Zolla L. Alterations of red blood cell metabolome during cold liquid storage of erythrocyte concentrates in CPD-SAGM. J Proteomics. 2012;76:168–80. doi: 10.1016/j.jprot.2012.03.012. [DOI] [PubMed] [Google Scholar]
- 11.D’Alessandro A, D’Amici GM, Vaglio S, Zolla L. Time-course investigation of SAGM-stored leukocyte-filtered red blood cell concentrates: from metabolism to proteomics. Haematologica. 2012;97:107–15. doi: 10.3324/haematol.2011.051789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ozment CP, Mamo LB, Campbell ML, et al. Transfusion-related biologic effects and free haemoglobin, heme, and iron. Transfusion. 2013;53:732–40. doi: 10.1111/j.1537-2995.2012.03837.x. [DOI] [PubMed] [Google Scholar]
- 13.Dumaswala UJ, Zhuo L, Jacobsen DW, et al. Protein and lipid oxidation of banked human erythrocytes: role of glutathione. Free Rad Biol Med. 1999;27:1041–9. doi: 10.1016/s0891-5849(99)00149-5. [DOI] [PubMed] [Google Scholar]
- 14.Chaudhury R, Katharia R. Oxidative injury as contributory factor for red cells storage lesion during twenty eight days of storage. Blood Transfus. 2012;10:59–62. doi: 10.2450/2011.0107-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Nagababu E, Rifkind JM. Heme degradation by reactive oxygen species. Antiox Redox Signal. 2004;6:967–78. doi: 10.1089/ars.2004.6.967. [DOI] [PubMed] [Google Scholar]
- 16.Maitra D, Byun J, Andreana PR, et al. Reaction of haemoglobin with HOCl: mechanism of heme destruction and free iron release. Free Rad Biol Med. 2011;51:374–86. doi: 10.1016/j.freeradbiomed.2011.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Collard K, White D, Copplestone A. The effect of maximum storage on iron status, oxidative stress and antioxidant protection in paediatric packed cell units. Blood Transfusion. 2013;11:419–25. doi: 10.2450/2012.0046-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kime R, Gibson A, Yong W, et al. Chromatographic method for the determination of non-transferrin-bound iron suitable for use on the plasma and bronchoalveolar lavage fluid of preterm babies. Clin Sci. 1996;91:633–9. doi: 10.1042/cs0910633. [DOI] [PubMed] [Google Scholar]
- 19.Paffetti P, Perrone S, Longini M, et al. Non-protein bound iron detection in small samples of biologic fluids and tissues. Biol Trace Elem Res. 2006;112:221–32. doi: 10.1385/BTER:112:3:221. [DOI] [PubMed] [Google Scholar]
- 20.Nagababu E, Mohanty JG, Bhamidipaty S, et al. Role of the membrane in the formation of heme degradation products in red blood cells. Life Sci. 2010;86:133–48. doi: 10.1016/j.lfs.2009.11.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Comporti M, Signorini C, Buonocore G, et al. Iron release, oxidative stress and erythrocyte ageing. Free Rad Biol Med. 2002;32:568–76. doi: 10.1016/s0891-5849(02)00759-1. [DOI] [PubMed] [Google Scholar]
- 22.Signorini C, Ferrali M, Ciccoli L, et al. Iron release, membrane protein oxidation and erythrocyte ageing. FEBS Lett. 1995;362:165–70. doi: 10.1016/0014-5793(95)00235-2. [DOI] [PubMed] [Google Scholar]
- 23.Kanias T, Acker JP. Biopreservation of red blood cells-the struggle with haemoglobin oxidation. FEBS J. 2010;277:343–56. doi: 10.1111/j.1742-4658.2009.07472.x. [DOI] [PubMed] [Google Scholar]
- 24.Jeney V, Balla J, Yachie A, et al. Pro-oxidant and cytotoxic effects of circulating heme. Blood. 2002;100:879–87. doi: 10.1182/blood.v100.3.879. [DOI] [PubMed] [Google Scholar]
- 25.Kumar S, Bandyopadhyay U. Free heme toxicity and its detoxification systems in human. Toxicol Lett. 2005;157:175–88. doi: 10.1016/j.toxlet.2005.03.004. [DOI] [PubMed] [Google Scholar]
- 26.Silvers KM, Gibson AT, Russell JM, et al. Antioxidant activity, packed cell transfusions, and outcome in premature infants. Arch Dis Child. 1998;78:F214–9. doi: 10.1136/fn.78.3.f214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Evans PJ, Evans R, Kovar IZ, et al. Bleomycin-detectable iron in the plasma of premature and full-term neonates. FEBS Lett. 1992;303:210–12. doi: 10.1016/0014-5793(92)80521-h. [DOI] [PubMed] [Google Scholar]
- 28.Kanakoudi F, Drossou V, Tzmouli V, et al. Serum concentrations of 10 acute phase proteins in healthy term and preterm infants from birth to age 6 months. Clin Chem. 1995;41:605–8. [PubMed] [Google Scholar]
- 29.Dani C, Martelli E, Beretini G, et al. Effect of blood transfusions on oxidative stress in preterm infants. Arch Dis Child Fetal Neonatal Ed. 2004;89:F408–11. doi: 10.1136/adc.2003.037085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hod EA, Brittenham GM, Billote GB, et al. Transfusion of human volunteers with older, stored red blood cells produces extravascular hemolysis and circulating non-transferrin-bound iron. Blood. 2011;118:6675–82. doi: 10.1182/blood-2011-08-371849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Amin SB, Scholer L, Srivastava M. Pre-discharge iron status and its determinants in premature infants. J Maternal Fetal Neonatal Med. 2012;25:2265–9. doi: 10.3109/14767058.2012.685788. [DOI] [PubMed] [Google Scholar]