Abstract
Background
Chronic Achilles tendinopathy is responsible for a severe reduction in physical performance and persistent pain. There is currently a number of therapeutic options and the local administration of growth factors is an emerging treatment strategy. In particular, platelet-rich plasma (PRP) is a widely used way to provide a local regenerative stimulus for tendon healing. The aim of this study was to document the mid-term results obtained after treating recalcitrant Achilles tendinopathy with injections of high concentrate, leucocyte-rich PRP.
Materials and methods
Twenty-seven patients (mean age: 44.6 years; 22 men and 5 women) affected by chronic mid-portion Achilles tendinopathy (7 bilateral, for a total of 34 tendons), refractory to previous treatments, were enrolled. Patients were treated with three ultrasound-guided intra-tendinous injections of PRP at 2-week intervals. Patients were prospectively evaluated at baseline, and then at 2, 6, and up to a mean of 54.1 months of follow-up (minimum 30 months), using the following tools: Blanzina, VISA-A, EQ-VAS for general health, and Tegner scores.
Results
The VISA-A score showed a significant improvement: the baseline score of 49.9±18.1 increased to 62.9±19.8 at 2 months (p=0.002), with a further improvement at 6 months (84.3±17.1, p<0.0005), and stable results at 4.5 years (90.0±13.9). The EQ-VAS score also showed a similar positive trend. An evaluation of the activity level confirmed these findings, showing a significant improvement in the Tegner score over time (p=0.017 for the final evaluation). The longer duration of symptoms before treatment was associated with a slower return to sport (p=0.041).
Discussion
PRP injections produced good overall results for the treatment of chronic recalcitrant Achilles tendinopathy with a stable outcome up to a medium-term follow-up. Longer symptom duration was related with a more difficult return to sporting activity.
Keywords: PRP, growth factors, Achilles tendinopathy, injections, long term
Introduction
Achilles tendinopathy, in particular pathology of the mid-portion of the tendon, is one of the most common findings in a sport-active population, especially among long-distance runners, and is responsible for a severe reduction in physical performance and persistent pain over several years1,2. Despite being often considered a “sport-related injury”, studies have revealed that this pathology may also affect an older population with less involvement in sporting activities, thus explaining the social impact of Achilles tendinopathy3,4.
The treatment of this condition is a real challenge for orthopaedic surgeons and sports medicine physicians and it is not always possible to achieve a good outcome. A number of options5–7 are available, ranging from rehabilitation protocols to injective treatment and surgery. Among these, the local administration of growth factors is an emerging treatment strategy which aims at providing a regenerative stimulus in a tissue, the tendon, characterised by a poor healing ability8. In particular, platelet-derived growth factors are the most widely used way to provide a local regenerative stimulus for tendon healing9,10. Platelet granules are rich in important molecules involved in the process of responding to damage and might be beneficial in conditions characterised by chronic, degenerative processes11–15. Platelet-rich plasma (PRP) has been used to a variable degree in recent years in the field of musculoskeletal pathologies16 and, in part as a consequence of the use of ultrasonography for selective intra-lesion administration, it has become especially common in the management of tendinopathy. Despite its widespread use for the treatment of Achilles tendinopathy, only a few studies have described the clinical outcome after injection treatment with PRP, and most of them were of poor methodological quality. Up to now only one randomised controlled trial has compared the effectiveness of a single PRP injection against saline solution for the management of Achilles tendinopathy in 54 patients17,18. The authors of this study reported an improvement in both groups of patients, without statistical difference in terms of clinical results between patients treated with PRP or saline, both at 6 and 12 months of follow-up. Although these findings might discourage clinicians from using PRP to treat Achilles tendinopathy, there are some important limitations in the study design that need to be taken into account before sentencing the ineffectiveness of PRP. First of all it should be taken into consideration that, in the case of saline injections, needling itself might be a treatment, involving mechanical stimulation and bleeding of the degenerated tendon tissue, and therefore the good results reported after saline injections cannot be ascribed only to a placebo effect. Furthermore, the mean age of the patients in the study was notably higher than that of the usual sport-active population, which is normally treated with this regenerative approach; older subjects might be less responsive to the biological effects of PRP. Another point to consider is that a single injection of PRP was administered, whereas other authors have preferred to use repeated PRP injections to guarantee a better and long-lasting action of the platelet-derived growth factors. Another, potentially major, weakness of the study is that the PRP was not activated; the researchers relied on in situ activation provided by the contact of the PRP with autologous collagen. This choice might be questioned because platelet gel formation is delayed and tendon contraction might squeeze the liquid PRP away from the injection site. Self-activation might, therefore, be too slow or insufficient to guarantee the local release of adequate amounts of growth factors. In view of these remarks, and also considering the positive clinical results documented in other trials, this study represents an important landmark in the field, but the question about the real role of PRP in the management of Achilles tendinopathy still remains open and worthy of further investigation.
Another key aspect to consider is that there are several different PRP formulations, which differ in terms of cell type content, platelet concentration, storage modalities, activation methods and protocols for therapeutic application19. If a particular type of PRP has proven to be less effective in treating Achilles tendinopathy, it does not exclude other PRP formulations from being more suitable and beneficial for this particular pathology. In fact, the goal of current research is to establish whether there are some particular characteristics of PRP and some specific features of tendon lesions that might represent clear indications for safe, effective use of PRP in tendinopathy. For this purpose it is essential to provide data about different PRP formulations used to treat Achilles tendinopathy, especially in the case of long-term studies, since such data might demonstrate not only the safety and possible efficacy of PRP, but also the stability of the clinical outcome.
The aim of this study was, therefore, to evaluate the therapeutic effects of repeated injections of laboratory-made, high-concentrate, leucocyte-rich PRP, administered to promote the healing of chronic, recalcitrant Achilles tendinopathy, documenting the quality and duration of clinical improvement in a large cohort of patients up to a mid-term follow-up.
Materials and methods
Patients
The clinical experimentation was approved by the Hospital Ethics Committee and informed consent was obtained from all patients. Twenty-seven patients (mean age: 44.6±10.6 years; mean body mass index: 25.6±4.0; 22 men, 5 women) affected by chronic mid-portion Achilles tendinopathy were enrolled in this trial. Inclusion criteria were: history (>3 months) of exercise-associated pain, pain or tenderness on palpation and imaging findings (magnetic resonance imaging [MRI]or ultrasound [US] scans) of degenerative changes in the Achilles’ tendon. Seven of the patients enrolled had bilateral tendinopathy, thus 34 tendons were treated in total. All tendons had previously undergone unsuccessful conservative or surgical management (Table I).
Table I.
Previous treatments of the patients included in the study.
| Type of treatment | Number of patients |
|---|---|
| Rehabilitation programme (minimum 12 weeks) | 27 (7 bilateral) |
| Laser therapy | 18 (4 bilateral) |
| External shock wave therapy | 3 |
| TENS therapy | 8 (2 bilateral) |
| Ultrasound therapy | 10 (3 bilateral) |
| Corticosteroids injections | 10 (4 bilateral) |
| Surgical treatment | 4 (1 bilateral): multiple longitudinal tenotomies and excision of degenerated areas |
Technique for preparing the platelet-rich plasma
A 150 mL venous blood sample was harvested for each treatment cycle for each tendon. The sample was centrifuged twice (the first time at 1,480 rpm for 6 minutes to separate erythrocytes, and the second time at 3,400 rpm for 15 minutes to concentrate platelets) to produce four small units of 5 mL of PRP each. One unit was sent to the laboratory for quality control, one was used immediately for the first treatment, and the other two units were stored at −30 °C. On average, the total number of platelets per milliliter in the PRP was five times greater than that in the whole blood. The platelet concentrate also contained leucocytes, which had been concentrated 1.2 times with respect to the normal blood values. The treatment cycle consisted of three intra-tendinous injections of 5 mL PRP at 2-week intervals. For the second and third treatments the samples were thawed in a dry-thermostat at 37 °C for 30 minutes, immediately prior to application. Before every injection, 10% of calcium chloride (Ca2+=0.22 mEq×dose) was added to activate the PRP.
Treatment and evaluation protocol
The patient was placed prone with his or her ankle in a neutral position. After sterile dressing and under US control (Acuson Antares Ultrasound System, Premium Edition; CH6-2 Convex Array Probe; Siemens AG, Munich, Germany), a single skin injection with a 22 G needle was performed and then the PRP was injected directly into the lesion site with multiple penetrations of the tendon.
After each injection the patient was sent home with instructions on limiting the use of the leg for at least 24 hours and to use cold therapy/ice on the treated area for pain. During this period the use of non-steroidal anti-inflammatory drugs was forbidden. After the second injection the patients were instructed to start a rehabilitation programme based on eccentric exercises to be carried out for 12 weeks. A gradual return to sporting activity was then promoted, by encouraging patients to perform stretching and eccentric exercises on a constant basis as a part of their training to prevent relapse.
Patients were prospectively evaluated at baseline, after 2 and 6 months, and then up to a mean of 54.1±19.7 months’ follow-up (minimum evaluation at 30 months). The following evaluation tools were used: Blanzina20, VISA-A21, EQ-VAS for general health, and Tegner22 scores. With regard to the scores used, the Blanzina score give a clinical classification of the severity of the tendinopathy (ranging from grade I to grade IV, which represents the worst clinical condition), VISA-A provides a subjective functional evaluation of the Achilles’ tendon, and the Tegner score indicates the patient’s usual level of sporting activity.
The patients’ overall satisfaction and time to return to sport were also reported. The treatment was considered to have failed in patients who later underwent injection or surgical treatment and the last scores before the operation were reported for the analysis at the subsequent follow-ups.
Statistical methods
All continuous data are expressed as a mean±standard deviation or mean and 95% confidence intervals; categorical variables are expressed as frequencies and percentages. The Kolmogorov-Smirnov test was performed to test the normality of continuous variables. The repeated measures general linear model (GLM) with post-hoc Sidak correction for multiple comparisons was performed to compare normally distributed scores at the different follow-up times. Friedman’s test followed by Wilcoxon’s pairwise post-hoc test was used to compare follow-up times of ordinal scores. The ANOVA test was used to assess the between-group differences of continuous, normally distributed and homoscedastic data, the Mann-Whitney test was used otherwise. Spearman’s rank correlation was used to assess correlations between scores and continuous data, whereas the Kendall-Tau correlation was used to assess influence between ordinal variables. Fisher’s χ2 test was applied to investigate the relationships between dichotomous variables, whereas Pearson’s χ2 test evaluated by Monte Carlo methods was performed to investigate the relationships between grouping variables. Kaplan-Meier analysis was used to assess the return to sport. For all tests p values <0.05 were considered statistically significant.
All statistical analyses were performed using SPSS v.19.0 (IBM Corp., Armonk, NY, USA).
Results
No major complications related to the injections or severe adverse events were observed during the treatment and follow-up period, and an overall improvement in all the scores was recorded over time.
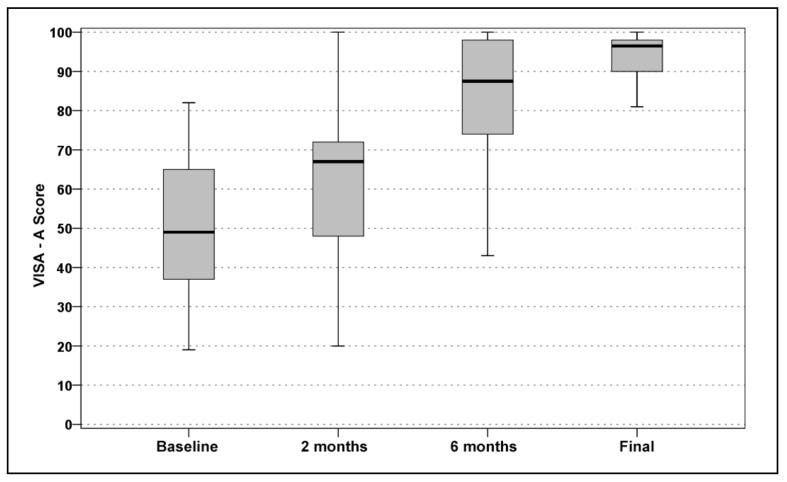
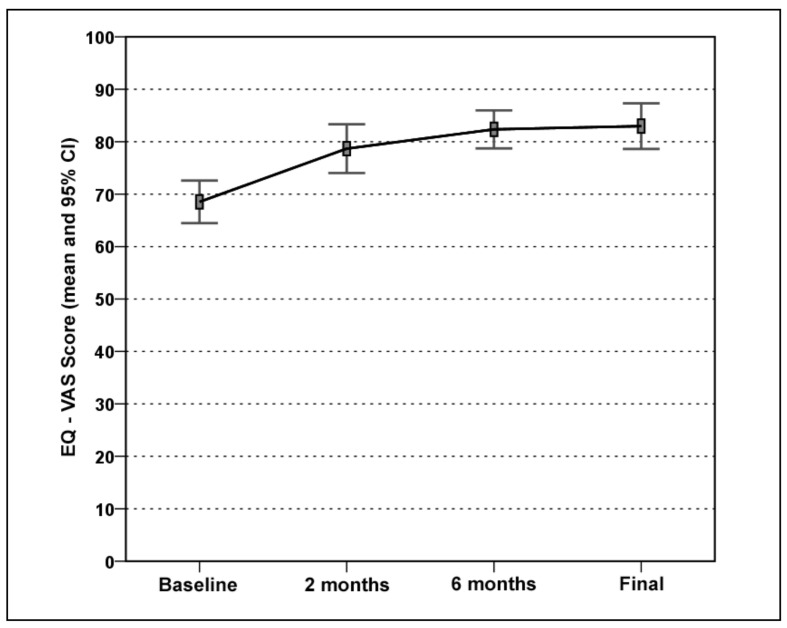
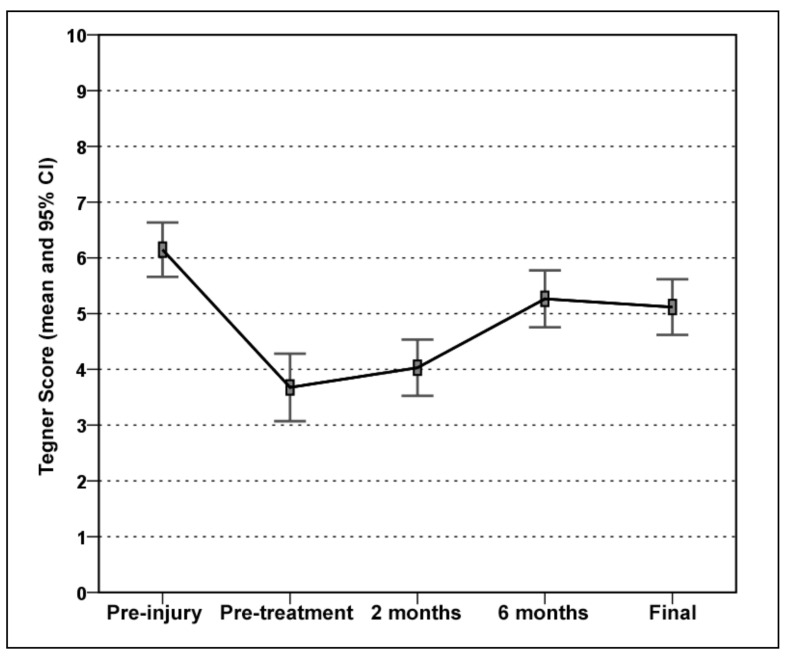
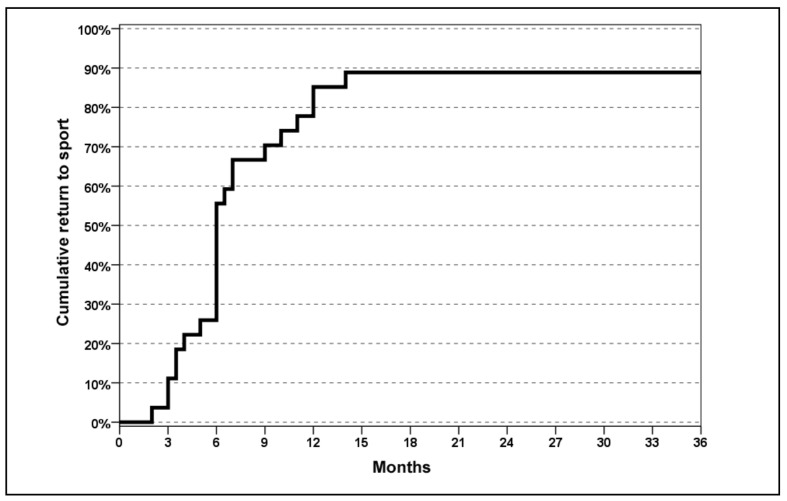
The VISA-A score improved significantly (global p<0.0005). In detail, the baseline score of 49.9±18.1 increased to 62.9±19.8 at 2 months (p=0.002), with a further improvement at 6 months (84.3±17.1; p<0.0005), and remained stable: 90.0±13.9 at 4.5 years (Figure 1). The EQ-VAS score increased from 68.6±11.6 at baseline to 78.7±13.3 at 2 months, 82.4±10.4 at 6 months, and 83.0±12.5 at 4.5 years, with a significant improvement at 2 months (p=0.001) and then stable results over time (Figure 2). Tendons were classified before the treatment as Blanzina grade IIIa, IIIb, and II in 21, 6, and 7 cases, respectively; at the 6 months’ evaluation 14 grade 0, 12 grade I, 3 grade II, 1 grade IIIa, and 4 grade IIIb scores were recorded; finally, at the last 4.5 evaluation at 4.5 years there were 24 grade 0, 2 grade I, 5 grade II, 0 grade IIIa, and 3 grade IIIb scores, with a significant improvement at every assessment (p<0.0005 vs the baseline evaluation at both 6 months and at the final evaluation, and with a tendency to further improvement from 6 months to 4.5 years: p=0.06). The evaluation of the activity level confirmed these findings, with the Tegner score showing a significant improvement over time: 6.2±1.4 before the onset of symptoms, 3.7±1.7 before treatment, 4.0±1.4 at 2 months, 5.3±1.5 at 6 months, and 5.1±1.4 at 4.5 years’ follow-up (p=n.s. at 2 months, p=0.001 at 6 months, and p=0.017 at 4.5 years with respect to the pre-treatment level, but without achieving the level prior to the onset of symptoms: p<0.0005; Figure 3). Moreover, 89% of the patients returned to sport (Figure 4) and 93% of the patients were satisfied and would repeat the treatment if needed. Finally, the treatment failed in three patients (1 bilateral): one patient was treated with corticosteroid injection before the 6 months’ evaluation and the other two patients sought surgical intervention, in both cases about 1 year after the PRP treatment.
Figure 1.
VISA-A scores at baseline, 2 months, 6 months and at the mean final evaluation of 4.5 years’ follow-up.
Figure 2.
EQ-VAS scores at baseline, 2 months, 6 months and at the mean final evaluation of 4.5 years’ follow-up.
Figure 3.
Tegner score prior to the tendon injury, before treatment, at 2 months, at 6 months and at the mean final evaluation of 4.5 years’ follow-up.
Figure 4.
Time to return to sport and percentage of patients returning to sport after PRP treatment.
Further analysis was undertaken to evaluate which patients’ characteristics might influence the treatment outcome. Among these, age, sex, body mass index, unilateral or bilateral Achilles’ tendon involvement, and previous conservative or surgical treatment did not influence the results in this series. Longer duration of symptoms before treatment was correlated with a slower return to sport (rho= 0.376, p=0.041). Similarly, a poorer Blanzina score before treatment was correlated with a lower VISA-A score at 2 months (tau= −0.464, p=0.001) and a tendency to return to sport later (tau= 0.282, p=0.071).
Discussion
The main findings of the present study are that patients treated with repeated PRP injections for Achilles tendinopathy obtained overall good results and that the clinical improvement was stable and maintained up to a mid-term follow-up. Clinical improvement was significantly slower in patients with a higher pre-treatment symptom level, and the return to sport was more difficult in patients with a longer history of symptoms.
Various mechanisms of action may have played a role in determining the good clinical outcome reported: (i) the dry needling stimulus responsible for internal bleeding and consequently an inflammatory response which might start a repair process in the degenerated area of the lesion; (ii) the direct biological stimulus of the platelet-derived growth factors stimulating gene expression of matrix molecules, collagen production and tendon cell proliferation; and (iii) the activation of circulation-derived cells11, promoted by PRP releasate, which play a crucial role in the tissue healing process12.
Regardless of the real contribution of each of these mechanisms of action, in animal studies the overall effects of applying PRP were improvement in tendon callus strength and stiffness 13,14 and a better histological pattern23–26.
With regards to the use of PRP for the treatment of tendinopathy in humans, the currently available clinical evidence is drawn mainly from low-quality trials reporting overall good clinical outcomes, in the short-term, after PRP injections (Table II). The first study was a case report about the management of a partial Achilles’ tendon rupture in a 34-year old basketball player treated with three intra-tendinous injections of 5 mL PRP at 1 week intervals27. The athlete was able to return to sporting activities 64 days after the original trauma, and by 75 days was playing a full game. MRI and ultrasonography performed before and after the treatment showed a marked improvement in tendon signal and tissue structure. Gaweda et al.28 injected PRP in 14 patients (15 tendons in total) with non-insertional Achilles tendinopathy. A marked and significant improvement was recorded, and ultrasonography revealed normalisation of the peritendineum, a reduction in tendon thickness, and a reduction in hypoechoic lesions. Power-Doppler studies showed an initial increase in tendon vascularity up to 3 months and then a reduction at the final follow-up. Finoff et al.29 treated chronic tendinopathy with US-guided needle tenotomy and PRP injections. The study focused on tendinopathy in numerous sites, in both the upper and lower extremities: 14 Achilles’ tendons were treated and the follow-up evaluation was carried out at mean of 14 months. The investigators found a significant decrease in pain and concomitant functional recovery. No correlation was found between clinical outcome and parameters such as age, body mass index, smoking status, site of tendinopathy, duration of symptoms or PRP platelet concentration. Owens et al.30 retrospectively reviewed a small cohort of ten patients all treated with intra-tendinous PRP injections: an improvement was found in each clinical score used but MRI evaluation did not reveal a better appearance in most tendons treated, which remained similar to the state prior to treatment. A study by Monto et al.31 confirmed the positive clinical outcome in the aforementioned studies in a larger series of patients: they treated 30 patients affected by chronic tendinopathy refractory to at least 6 months of traditional non-operative management. Each patient received a single US-guided injection of autologous PRP. The clinical results were positive, with a significant improvement with respect to the first evaluation, and this improvement was confirmed up to the final follow-up at 24 months. In this series, even MRI/US control scans revealed signs of tendon healing in 27 out of 29 patients. The rate of return to full occupational activity and sport confirmed the trend revealed by the clinical score and imaging appearance. Deans et al.32 published their results of treating 26 patients with recalcitrant Achilles tendinopathy with one injection of autologous-conditioned plasma combined with exercise and therapeutic ultrasonography. At the short-term evaluation at 6 weeks, the patients were found to have had a significant clinical improvement. Subsequently, Mautner et al.33 published a retrospective, multicentre, cross-sectional study analysing clinical outcomes of 180 patients treated for different tendinopathies. The Achilles’ tendon was the third most common site treated (27 tendons) and the results were impressive, since 100% of the patients reported a “moderate improvement to complete resolution” on a Likert-scale: the group with Achilles tendinopathy responded best to PRP treatment. Interestingly, this trial included patients treated in four different medical centres, each employing its particular PRP formulation and therapeutic protocol. In spite of the study’s limitations, the overall good clinical outcome suggests that platelet-derived growth factors can be considered among the treatment options for Achilles tendinopathy. Another trial by Ferrero et al.34 showed good results at the 6-month evaluation in 24 patients treated with a single injection of PRP. Besides the good clinical outcome, follow-up US scans were also performed and revealed a widespread improvement in the fibrillar echo texture of the tendon and reduced hypervascularity as shown by power Doppler.
Table II.
Synopsis of the trials dealing with PRP treatment for Achilles tendinopathy.
| Authors, journal and year | Level of evidence | Disease | PRP preparation method | Leucocytes | Activation method | Protocol | Combined treatments (or other notes) | Patients | Follow-up | Results |
|---|---|---|---|---|---|---|---|---|---|---|
| Filardo et al. Orthopedics 2010 | Case report | Achilles partial rupture | Double centrifugation (laboratory-made) | Leucocyte-rich PRP | Ca-chloride | 3 weekly injections of 5 mL PRP | Needling | 1 | 18 months | Return to full sport practice in 75 days |
| Gaweda et al. Int J Sports Med 2010 | Case series | Achilles tendinopathy | Single centrifugation (PRP Kit - Curasan AG, Kleinostheim, Germany) | N.A. | N.A. | 1 injection of 3 mL PRP | Needling | 14 | 18 months | Significant reduction in pain and recovery of function |
| De Vos et al. JAMA 2010 + De Jonge et al. Am J Sports Med 2011 | Randomised trial | Achilles tendinopathy | Single centrifugation (Platelet Separation System - Biomet Inc, Warsaw, USA) | N.A. | No | 1 injection of 4 mL PRP | Local anaesthesia Needling | 54 (27 PRP vs 27 saline) | 12 months | PRP treatment was not superior to saline injection. Similar findings at US evaluation at each follow-up |
| Finoff et al. PM R 2011 | Case series | Achilles tendinopthay (plus other sites tendinopathies) | Single centrifugation (Magellan Autologous Platelet Separator System - Arteriocyte Medical System Inc, Cleveland, USA - or GPS III Platelet Separation System - Biomet Inc) | N.A. | No | 1 injection of 2.5–3.5 mL PRP | Local anaesthesia US-guided needling | 14 | 14 months | Positive clinical results but no evidence of better tissue repair at US evaluation |
| Owens et al. Foot Ankle Int 2011 | Case series | Achilles tendinopathy | Single centrifugation (Symphony System- DePuy Orthopaedics Inc., Warsaw, USA) | N.A. | No | 1 injection of 6 mL PRP | Injection administered in OR | 10 | 24 months | Modest clinical improvement but not MRI changes over time |
| Monto et al. Foot Ankle Int 2012 | Case series | Achilles tendinopathy | Single centrifugation (Accelerate platelet concentration system - Exactech Inc, Gainesville, USA) | N.A. | No | 1 injection of 4 mL PRP | Local anaesthesia Needling | 30 | 24 months | 28 patients reported a satisfactory outcome up to the final evaluation |
| Ferrero et al. J Ultrasound 2012 | Case series | Achilles tendinopathy | Single centrifugation (Laboratory-made) | N.A. | N.A. | 2 injections of 6 mL of PRP at a mean interval of 3 weeks | Local anaesthesia Needling | 24 | 6 months | Significant clinical improvement and also better appearance in tendon fibrillar structure, associated with reduced hypervascularity at power Doppler. |
| Deans et al. J Foot Ankle Surgery 2012 | Case series | Achilles tendinopathy | Single centrifugation (ACP double syringe system, Arthrex Inc, Naples, USA) | N.A. | No | 1 injection of PRP; 2 patients received a second injection after 6 weeks | No US-guidance No local anaesthesia | 26 | 6 weeks | 80% of patients reported improved symptoms whereas 20% had slightly worse symptoms. |
| Mautner et al. PM R 2013 | Retrospective study | Achilles tendinopathy (plus other sites tendinopathies) | N.A. | N.A. | N.A. | 1 PRP injection if 80% global i m p r o v e m e n t ; a second or even more injections performed in case of poorer results | N.A. | 27 | 6 months | 100% of Achilles’ tendon patients reported “moderate to complete resolution of symptoms”. |
N.A.: not assessed, meaning that the study does not provide specific information on the particular aspect considered in the table.
As far as concerns randomised controlled trials, the only study available17,18 has already been discussed: although the results of PRP were unfavourable in comparison with those for saline, some major limitations of the study do not allow definitive conclusions to be drawn about this biological approach, and actually prompt further studies targeted at finding the best formulations, the optimum methods of application and the most precise indications. In fact, besides considerations regarding the level of evidence of the aforementioned studies, the most controversial aspect remains the marked inter-product variability and the different application strategies. PRP is an off-the-shelf product whose characteristics can vary greatly depending on the different production techniques adopted. The different types of cells and the variable concentrations provided by the different procedures and applied to the site of the lesion are fundamental aspects16 since growth factors are potent molecules, and small variations in their concentrations can produce very different effects35, some of which may even be detrimental in relation to the particular tissue in question36. Timing and number of injections are also important details that should be further investigated since they might have an influence on clinical outcome37. Other seminal aspects regard cellularity, since not only platelets but also leucocytes, monocytes, macrophages, and mast cells might be contained in platelet concentrates and play a role in the effects obtained in the injected tissue11–13. Furthermore, the storage procedure, if used, is thought to affect the amount and pattern of growth factors released: in particular freeze-thawing is considered by some authors to affect the performance of PRP negatively, although no clinical study up to now has revealed poorer clinical outcomes in case of freeze-thawing PRP38. The activation method may also influence the results, because activation may regulate the amount and speed of growth factor release and also the molecules used may themselves exert their own effect on the physiology of tissue healing and remodelling39, without forgetting that the time of gel formation might also determine the amount of releasate actually being delivered into the treated area, as previously discussed.
In the light of these remarks, it appears clear that different types of PRP have different biological properties and whereas some might offer good results others might be ineffective or even detrimental36 for the specific pathology. Unfortunately, the large number of factors involved makes comparisons of studies very difficult. There is a need to investigate specific PRP formulations, specific therapeutic protocols and specific phases of tendon disease and patients’ characteristics to determine whether they might be related to a better clinical outcome. Randomised controlled trials are required to shed light on what, based on the current evidence, is still a debated and controversial topic. Lastly, we must bear in mind that all trials concerning the use of PRP include a rehabilitation protocol after the cycle of injections, which might itself provide a contribution to symptom relief and functional improvement. This is also an important aspect to consider because an appropriate biomechanical stimulus after a biological treatment might increase the regenerative potential of PRP and enhance tendon tissue maturation synergically, as suggested by both preclinical and clinical studies13,40.
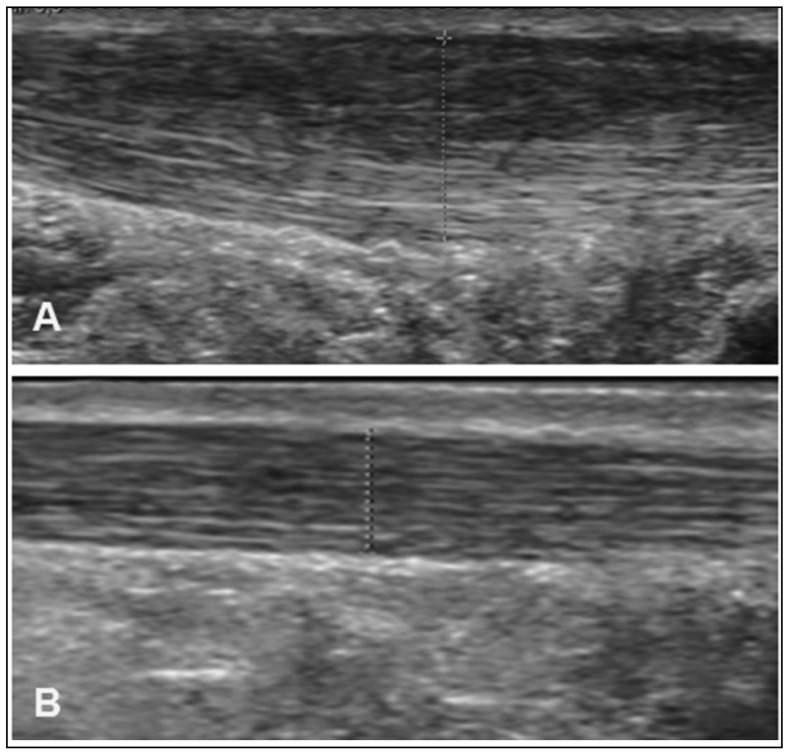
This study shows that the treatment of a high number of tendons with repeated PRP injections produced stable results over time, with a good outcome maintained up to 4.5 years’ follow-up (Figure 5). Another finding of this study was poorer results in patients with a longer history of symptoms and limited function.
Figure 5.
Comparison of US scans at the baseline evaluation (A) and after PRP treatment (B).
It can be seen that there is a reduction Achilles’ tendon thickness and an overall improvement of tendon echo-structure. The patient’s clinical scores were also satisfactory.
With respect to other applications of PRP in tendinopathy, such as patellar tendinopathy, the return to sport was slower41, but this might be related to the different biomechanical properties between the patellar and the Achilles’ tendon (which might also imply different aetiopathological pathways) or even the older age of the patients treated in this study.
The present study has some major limitations, such as the lack of a randomised control group and imaging evaluation. However, to the authors’ knowledge, this is the first study to report the mid-term outcome after a cycle of injections with a laboratory-made, high-concentrate, leucocyte-rich PRP. Even though these data cannot demonstrate the real potential of this particular PRP formulation, which would require level I studies, they show that the good short-term results observed in other studies on the Achilles’ tendon are confirmed in the mid-term follow-up, and that even patients affected by recalcitrant tendinopathy can benefit from this treatment approach with stable results over time.
Conclusions
Repeated intra-tendinous injections of autologous PRP produced good results in the treatment of chronic recalcitrant Achilles tendinopathy, with a stable clinical improvement maintained up to a mid-term follow-up. Longer duration of symptoms and lower pre-treatment functional level were responsible for a more difficult return to sporting activities.
Acknowledgements
We thank Pier Maria Fornasari and Milena Vaccari from the Immunohaematology and Transfusion Medicine Service, Rizzoli Orthopedic Institute, Bologna, Italy and Elettra Pignotti and Keith Smith, Task Force, Rizzoli Orthopedic Institute, Bologna, Italy.
Footnotes
The Authors declare no conflicts of interest.
References
- 1.Sobhani S, Dekker R, Postema K, Dijkstra PU. Epidemiology of ankle and foot overuse injuries in sports: a systematic review. Scand J Med Sci Sports. 2013;23:669–86. doi: 10.1111/j.1600-0838.2012.01509.x. [DOI] [PubMed] [Google Scholar]
- 2.Kujala UM, Sarna S, Kaprio J. Cumulative incidence of Achilles tendon rupture and tendinopathy in male former elite athletes. Clin J Sport Med. 2005;15:133–5. doi: 10.1097/01.jsm.0000165347.55638.23. [DOI] [PubMed] [Google Scholar]
- 3.de Jonge S, van den Berg C, de Vos RJ, et al. Incidence of midportion Achilles tendinopathy in the general population. Br J Sports Med. 2011;45:1026–8. doi: 10.1136/bjsports-2011-090342. [DOI] [PubMed] [Google Scholar]
- 4.Järvinen TA, Kannus P, Maffulli N, Khan KM. Achilles tendon disorders: etiology and epidemiology. Foot Ankle Clin. 2005;10:255–66. doi: 10.1016/j.fcl.2005.01.013. [DOI] [PubMed] [Google Scholar]
- 5.Sussmilch-Leitch SP, Collins NJ, Bialocerkowski AE, et al. Physical therapies for Achilles tendinopathy: systematic review and meta-analysis. J Foot Ankle Res. 2012;5:15. doi: 10.1186/1757-1146-5-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Malliaras P, Barton CJ, Reeves ND, Langberg H. Achilles and patellar tendinopathy loading programmes: a systematic review comparing clinical outcomes and identifying potential mechanisms for effectiveness. Sports Med. 2013;43:267–86. doi: 10.1007/s40279-013-0019-z. [DOI] [PubMed] [Google Scholar]
- 7.Al-Abbad H, Simon JV. The effectiveness of extracorporeal shock wave therapy on chronic Achilles tendinopathy: a systematic review. Foot Ankle Int. 2013;34:33–41. doi: 10.1177/1071100712464354. [DOI] [PubMed] [Google Scholar]
- 8.Kaux JF, Crielaard JM. Platelet-rich plasma application in the management of chronic tendinopathies. Acta Orthop Belg. 2013;79:10–15. [PubMed] [Google Scholar]
- 9.Cole BJ, Seroyer ST, Filardo G, et al. Platelet-rich plasma: where are we now and where are we going? Sports Health. 2010;2:203–10. doi: 10.1177/1941738110366385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Boswell SG, Cole BJ, Sundman EA, et al. Platelet-rich plasma: a milieu of bioactive factors. Arthroscopy. 2012;28:429–39. doi: 10.1016/j.arthro.2011.10.018. [DOI] [PubMed] [Google Scholar]
- 11.Kajikawa Y, Morihara T, Sakamoto H, et al. Platelet-rich plasma enhances the initial mobilization of circulation-derived cells for tendon healing. J Cell Physiol. 2008;215:837–45. doi: 10.1002/jcp.21368. [DOI] [PubMed] [Google Scholar]
- 12.De Mos M, Van der Windt AE, Jahr H, et al. Can platelet-rich plasma enhance tendon repair? A cell culture study. Am J Sports Med. 2008;36:1171–8. doi: 10.1177/0363546508314430. [DOI] [PubMed] [Google Scholar]
- 13.Virchenko O, Aspenberg P. How can one platelet injection after tendon injury lead to a stronger tendon after 4 weeks? Interplay between early regeneration and mechanical stimulation. Acta Orthop. 2006;77:806–812. doi: 10.1080/17453670610013033. [DOI] [PubMed] [Google Scholar]
- 14.Aspenberg P, Virchenko O. Platelet concentrate injection improves Achilles tendon repair in rats. Acta Orthop Scand. 2004;75:93–9. doi: 10.1080/00016470410001708190. [DOI] [PubMed] [Google Scholar]
- 15.Vannini F, Di Matteo B, Filardo G, et al. Platelet-rich plasma for foot and ankle pathologies: a systematic review. Foot Ankle Surg. 2014;20:2–9. doi: 10.1016/j.fas.2013.08.001. [DOI] [PubMed] [Google Scholar]
- 16.Kon E, Filardo G, Di Martino A, Marcacci M. Platelet-rich plasma (PRP) to treat sports injuries: evidence to support its use. Knee Surg Sports Traumatol Arthrosc. 2011;19:516–27. doi: 10.1007/s00167-010-1306-y. [DOI] [PubMed] [Google Scholar]
- 17.De Vos RJ, Weir A, van Schie HT, et al. Platelet-rich plasma injection for chronic Achilles tendinopathy: a randomized controlled trial. JAMA. 2010;303:144–9. doi: 10.1001/jama.2009.1986. [DOI] [PubMed] [Google Scholar]
- 18.de Jonge S, de Vos RJ, Weir A, et al. One-year follow-up of platelet-rich plasma treatment in chronic Achilles tendinopathy: a double-blind randomized placebo-controlled trial. Am J Sports Med. 2011;39:1623–9. doi: 10.1177/0363546511404877. [DOI] [PubMed] [Google Scholar]
- 19.Tschon M, Fini M, Giardino R, et al. Lights and shadows concerning platelet products for musculoskeletal regeneration. Front Biosci. 2011;3:96–107. doi: 10.2741/e224. [DOI] [PubMed] [Google Scholar]
- 20.Warden SJ, Kiss ZS, Malara FA, et al. Comparative accuracy of magnetic resonance imaging and ultrasonography in confirming clinically diagnosed patellar tendinopathy. Am J Sports Med. 2007;35:427–36. doi: 10.1177/0363546506294858. [DOI] [PubMed] [Google Scholar]
- 21.Robinson JM, Cook JL, Purdam C, et al. The VISA-A questionnaire: a valid and reliable index of the clinical severity of Achilles tendinopathy. Br J Sports Med. 2001;35:335–41. doi: 10.1136/bjsm.35.5.335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tegner Y, Lysholm J. Rating systems in the evaluation of knee ligament injuries. Clin Orthop. 1985;198:43–9. [PubMed] [Google Scholar]
- 23.Bosch G, van Schie HT, de Groot MW, et al. Effects of platelet-rich plasma on the quality of repair of mechanically induced core lesions in equine superficial digital flexor tendons: A placebo-controlled experimental study. J Orthop Res. 2010;28:211–7. doi: 10.1002/jor.20980. [DOI] [PubMed] [Google Scholar]
- 24.Lyras DN, Kazakos K, Verettas D, et al. The effect of platelet-rich plasma gel in the early phase of patellar tendon healing. Arch Orthop Trauma Surg. 2009;129:1577–82. doi: 10.1007/s00402-009-0935-4. [DOI] [PubMed] [Google Scholar]
- 25.Lyras D, Kazakos K, Verettas D, et al. Immunohistochemical study of angiogenesis after local administration of platelet-rich plasma in a patellar tendon defect. Int Orthop. 2010;34:143–8. doi: 10.1007/s00264-009-0728-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lyras DN, Kazakos K, Verettas D, et al. The influence of platelet-rich plasma on angiogenesis during the early phase of tendon healing. Foot Ankle Int. 2009;30:1101–6. doi: 10.3113/FAI.2009.1101. [DOI] [PubMed] [Google Scholar]
- 27.Filardo G, Presti ML, Kon E, Marcacci M. Nonoperative biological treatment approach for partial Achilles tendon lesion. Orthopedics. 2010;33:120–3. doi: 10.3928/01477447-20100104-31. [DOI] [PubMed] [Google Scholar]
- 28.Gaweda K, Tarczynska M, Krzyzanowski W. Treatment of Achilles tendinopathy with platelet-rich plasma. Int J Sports Med. 2010;31:577–83. doi: 10.1055/s-0030-1255028. [DOI] [PubMed] [Google Scholar]
- 29.Finnoff JT, Fowler SP, Lai JK, et al. Treatment of chronic tendinopathy with ultrasound-guided needle tenotomy and platelet-rich plasma injection. PM R. 2011;3:900–11. doi: 10.1016/j.pmrj.2011.05.015. [DOI] [PubMed] [Google Scholar]
- 30.Owens RF, Jr, Ginnetti J, Conti SF, Latona C. Clinical and magnetic resonance imaging outcomes following platelet rich plasma injection for chronic midsubstance Achilles tendinopathy. Foot Ankle Int. 2011;32:1032–9. doi: 10.3113/FAI.2011.1032. [DOI] [PubMed] [Google Scholar]
- 31.Monto RR. Platelet rich plasma treatment for chronic Achilles tendinosis. Foot Ankle Int. 2012;33:379–85. doi: 10.3113/FAI.2012.0379. [DOI] [PubMed] [Google Scholar]
- 32.Deans VM, Miller A, Ramos J. A prospective series of patients with chronic Achilles tendinopathy treated with autologous-conditioned plasma injections combined with exercise and therapeutic ultrasonography. J Foot Ankle Surg. 2012;51:706–10. doi: 10.1053/j.jfas.2012.06.009. [DOI] [PubMed] [Google Scholar]
- 33.Mautner K, Colberg RE, Malanga G, et al. Outcomes after ultrasound-guided platelet-rich plasma injections for chronic tendinopathy: a multicenter, retrospective review. PM R. 2013;5:169–75. doi: 10.1016/j.pmrj.2012.12.010. [DOI] [PubMed] [Google Scholar]
- 34.Ferrero G, Fabbro E, Orlandi D, et al. Ultrasound-guided injection of platelet-rich plasma in chronic Achilles and patellar tendinopathy. J Ultrasound. 2012;15:260–6. doi: 10.1016/j.jus.2012.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Torricelli P, Fini M, Filardo G, et al. Regenerative medicine for the treatment of musculoskeletal overuse injuries in competition horses. Int Orthop. 2011;35:1569–76. doi: 10.1007/s00264-011-1237-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kon E, Filardo G, Delcogliano M, et al. Platelet autologous growth factors decrease the osteochondral regeneration capability of a collagen-hydroxyapatite scaffold in a sheep model. BMC Musculoskelet Disord. 2010;11:220. doi: 10.1186/1471-2474-11-220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Batten ML, Hansen JC, Dahners LE. Influence of dosage and timing of application of platelet-derived growth factor on early healing of the rat medial collateral ligament. J Orthop Res. 1996;14:736–41. doi: 10.1002/jor.1100140509. [DOI] [PubMed] [Google Scholar]
- 38.Filardo G, Kon E, Pereira Ruiz MT, et al. Platelet-rich plasma intra-articular injections for cartilage degeneration and osteoarthritis: single- versus double-spinning approach. Knee Surg Sports Traumatol Arthrosc. 2012;20:2082–91. doi: 10.1007/s00167-011-1837-x. [DOI] [PubMed] [Google Scholar]
- 39.Borzini P, Mazzucco L. Tissue regeneration and in loco administration of platelet derivatives: clinical outcome, heterogeneous products, and heterogeneity of the effector mechanisms. Transfusion. 2005;45:1759–67. doi: 10.1111/j.1537-2995.2005.00600.x. [DOI] [PubMed] [Google Scholar]
- 40.Filardo G, Kon E, Della Villa S, et al. Use of platelet-rich plasma for the treatment of refractory jumper’s knee. Int Orthop. 2010;34:909–15. doi: 10.1007/s00264-009-0845-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Filardo G, Kon E, Di Matteo B, et al. Platelet-rich plasma for the treatment of patellar tendinopathy: clinical and imaging findings at medium-term follow-up. Int Orthop. 2013;37:1583–9. doi: 10.1007/s00264-013-1972-8. [DOI] [PMC free article] [PubMed] [Google Scholar]