Abstract
Background
Platelet transfusion is an essential part of the treatment of a variety of conditions such as thrombocytopenia and qualitative platelet disorders. As indicated in previous reports, during in vitro storage, platelets undergo morphological and physiological changes collectively known as the platelet storage lesion. Apoptosis is a programmed process of cell death, which has been considered as an important cause of platelet storage lesion under the common storage conditions in standard blood banks. Platelets are anucleate blood cells, but contain significant amounts of microRNA (miRNA, miR), which may play an important role in the regulation of gene expression. Drawing on previously published reports on cell apoptosis, we selected 49 miRNA for analysis to explore whether miRNA are of importance during the storage of platelets.
Materials and methods
We used quantitative real-time polymerase chain reaction analysis to determine the levels of expression of miRNA in apheresis platelets at different times of storage. Bioinformatics analysis was applied to explore target genes and the main functions of the selected miRNA.
Results
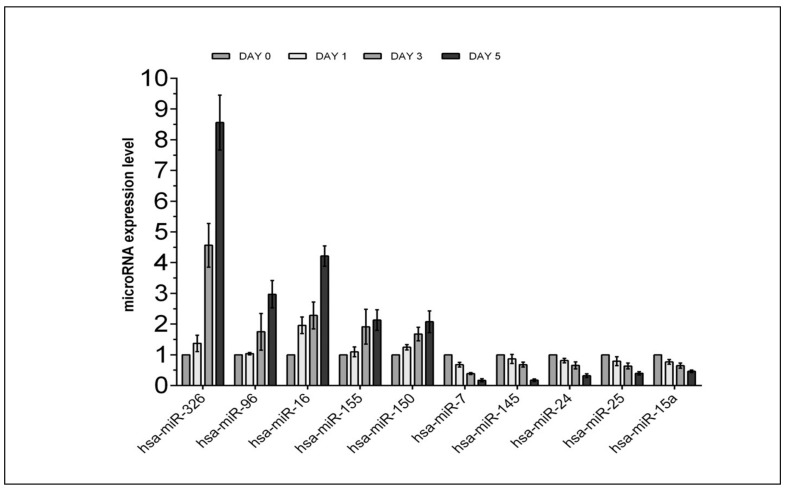
Our observations suggest that apheresis platelets contain large amounts of apoptosis-associated miRNA. The levels of expression of 25 miRNA remained high and ten of these miRNA showed different expression from that at day 0. Of these ten miRNA, hsa-miR-326, hsa-miR-96, hsa-miR-16, hsa-miR-155 and hsa-miR-150 were up-regulated, while hsa-miR-7, hsa-miR-145, hsa-miR-24, hsa-miR-25 and hsa-miR-15a were down-regulated. The markedly increased expression of hsa-miR-326 in all platelets is noteworthy (p<0.001).
Discussion
Since Bcl-xl and Bak1, members of the Bcl-2 family, are the targets of hsa-miR-326, our findings suggest that hsa-miR-326 may be involved in platelet apoptosis during storage.
Keywords: miRNA, apheresis platelet, apoptosis, qRT-PCR
Introduction
Human platelets are released into the circulation from the megakaryocytes of bone marrow as cytoplasmic fragments and play an important role in the development of cardiovascular diseases, inflammation and some tumours. Abnormalities in the number, adhesion, activation and aggregation of platelets are the key factors in thrombocytosis and haemorrhage, common conditions associated with high morbidity and mortality. Platelet transfusion is especially important in the treatment of a variety of conditions such as thrombocytopenia and qualitative platelet disorders. Platelets are anucleate cells and during vitro storage they undergo morphological and physiological changes collectively known as the platelet storage lesion1,2. The platelet storage lesion seriously affects the quality of stored platelets, and even causes them to be ineffective in vivo after transfusion. Investigations have, therefore, been focused on the mechanisms underlying the storage lesion1,2.
Apoptosis is a programmed process of cell death, which has been considered as an important cause of the platelet storage lesion under normal storage conditions in standard blood banks3,4. Studies have revealed that although platelets are anucleate, they contain numerous messenger RNA (mRNA) and undergo signal-dependent translational regulation5 and can synthesize some proteins6,7. Research on how mRNA participates in the regulation of gene expression and its function in platelets is the key to understanding the molecular mechanisms of platelets.
MicroRNA (miRNA, miR) are small, highly conserved, non-protein-coding RNA molecules. Studies have shown that they can regulate gene expression at the post-transcriptional level and play an important role in gene expression regulation, including cell differentiation, cell proliferation and metabolism. There is also recent evidence that miRNA plays an important role in the process of cell apoptosis8. MiRNA can regulate mRNA translation through recognition of binding sites of imperfect complementarity, and through pairing of the miRNA. Recently, Ple et al.9 reported results of high-throughput sequencing showing that human platelets express more than 492 miRNA. Kannan et al.10 consider that platelets use miRNA as translational regulators and play a crucial role in platelet apoptosis during storage and it is known that platelets have a complex regulatory network involving miRNA11. On the basis of the e hypothesis that miRNA in platelets could act as translational regulators and play a crucial role in platelet apoptosis during storage, in this study we used quantitative real-time polymerase chain reaction (qRT-PCR) analysis to determine the expression of miRNA in stored apheresis platelets; we also predict the target genes of the miRNA and discuss the correlations between the miRNA and their target genes.
Materials and methods
Platelet preparation and storage for microRNA extraction
Apheresis platelets were collected from five healthy blood donors (3 males and 2 females) in Wenzhou Central Blood Station and Ningbo Central Blood Station, Zhejiang Province, China. The platelets were filtered through leucocyte-depletion filters for platelets (Nanjing Shuangwei Biotechnology Co. Ltd, Nanjing, China) within 12 hours to obtain samples with a volume of 15–20 mL, a platelet count ranging from 2.0–2.5×109/mL and a leucocyte count <5×106/mL. Each sample was divided into four parts and stored in platelet storage bags at 22±2 °C. Fresh apheresis platelets were used as day 0 platelets. The units were also tested on days 1, 3 and 5 of storage. In order to separate white blood cells from the platelets further, the platelets were centrifuged at 400g for 10 minutes in 1.5 mL Eppendorf centrifuge tubes. Generally the white blood cell contamination in platelets was approximately 0.01%, which is negligible. The samples were then subjected to a haemogram analysis using an automated cell counter (Sysmex kx 2100, Sysmex Corporation, Hyogo, Japan) to determine that the sample contained sufficient numbers of platelets for RNA extraction and was free from red blood cells, white blood cells, and other cell debris, in order to ensure that the RNA analysed was truly from platelets. The sample was enriched using a platelet function centrifuge and the isolated RNA was subjected to miRNA profiling.
MicroRNA extraction
We used a miRNA isolation kit (Beijing CoWin Bioscience Co, Ltd, Beijing, China) for the purification of total platelet RNA, according to the manufacturer’s instructions. The RNA concentration was estimated with a Nanodrop spectrophotometer (ND1000; Saveen & Werner, Limhamn, Sweden). The sample was kept at −70 °C until the reverse-transcription step.
Analysis of apoptosis-associated microRNA by quantitative real-time polymerase chain reaction
For reverse transcription of total RNA, including platelet miRNA, we used the miRNA cDNA Kit (Beijing CoWin Bioscience Co, Ltd) according to the manufacturer’s instructions. Total RNA was treated with Escherichia coli poly-A polymerase to generate a poly-A tail at the 3′-end of each miRNA. Following polyadenylation, the miRNA first strand cDNA was synthesized using the poly (T) adapter (GCGAGCACAGAATTAATACGACTCACTATAGGTTTTTTTTTTTTVN) at 42 °C for 1 hour. To measure the expression of mature miRNA, the miRNA-first strand cDNA was determined by qRT-PCR analysis, using the miRNA Real-Time PCR Assay Kit (Beijing CoWin Bioscience Co, Ltd) and a StepOnePlus™ Real-Time PCR System (Applied Biosystems, Foster City, CA, USA). Mature miRNA sense sequences (miRBase, http://microrna.sanger.ac.uk/) of tested miRNA were used as forward PCR primers (Table I). The universal reverse primer was 5′-GCGAGCACAGAATTAATACGACTC-3′. Results were normalized to 5s rRNA. The 5s RNA forward primer was 5′-TACGGCCATACCACCCTGAA-3′ and the reverse primer was 5′-TAACCAGGCCCGACCCTGCT-3′. PCR cycling conditions were 95 °C for 10 minutes followed by 40 cycles of the following steps: 95 °C for 15 seconds and 60 °C for 1 minute. The qRT-PCR data were normalized using the 2-ΔΔCt method [ΔΔCt=(Cttarget−Ct5s) days 1, 3, 5−(Cttarget–Ct5s) day 0]. Melting curve analysis was performed to test the specificity and quality of the qRT-PCR amplifications. The data were processed using StepOne™ software v2.2.2 (Applied Biosystems).
Table I.
The upstream primers of 49 apoptosis-associated miRNA.
| Sanger Registry ID | Sanger Accession # | Entrez Gene ID | Primer |
|---|---|---|---|
| hsa-let-7a | MIMAT0000062 | 406883 | TGAGGTAGTAGGTTGTATAGTT |
| hsa-let-7b | MIMAT0000063 | 406884 | TGAGGTAGTAGGTTGTGTGGTT |
| hsa-let-7c | MIMAT0000064 | 406885 | TGAGGTAGTAGGTTGTATGGTT |
| hsa-let-7d | MIMAT0000065 | 406886 | AGAGGTAGTAGGTTGCATAGTT |
| hsa-let-7e | MIMAT0000066 | 406887 | TGAGGTAGGAGGTTGTATAGTT |
| hsa-let-7f | MIMAT0000067 | 406888 | TGAGGTAGTAGATTGTATAGTT |
| hsa-let-7g | MIMAT0000414 | 406890 | TGAGGTAGTAGTTTGTACAGTT |
| hsa-let-7i | MIMAT0000415 | 406891 | TGAGGTAGTAGTTTGTGCTGTT |
| hsa-miR-1 | MIMAT0000437 | 406905 | TGGAATGTAAAGAAGTATGTAT |
| hsa-miR-101 | MIMAT0000099 | 406893 | TACAGTACTGTGATAACTGAA |
| hsa-miR-10a | MIMAT0000253 | 406902 | TACCCTGTAGATCCGAATTTGTG |
| hsa-miR-133a | MIMAT0000427 | 406922 | TTTGGTCCCCTTCAACCAGCTG |
| hsa-miR-133b | MIMAT0000770 | 442890 | TTTGGTCCCCTTCAACCAGCTA |
| hsa-miR-142-3p | MIMAT0000434 | 406934 | TGTAGTGTTTCCTACTTTATGGA |
| hsa-miR-142-5p | MIMAT0000433 | 406934 | CATAAAGTAGAAAGCACTACT |
| hsa-miR-144 | MIMAT0000436 | 406936 | TACAGTATAGATGATGTACT |
| hsa-miR-145 | MIMAT0000437 | 406937 | GTCCAGTTTTCCCAGGAATCCCT |
| hsa-miR-148a | MIMAT0000243 | 406940 | TCAGTGCACTACAGAACTTTGT |
| hsa-miR-150 | MIMAT0000451 | 406942 | TCTCCCAACCCTTGTACCAGTG |
| hsa-miR-151-5p | MIMAT0004697 | 442893 | TCGAGGAGCTCACAGTCTAGT |
| hsa-miR-152 | MIMAT0000438 | 406943 | TCAGTGCATGACAGAACTTGG |
| hsa-miR-153 | MIMAT0000439 | 406944 | TTGCATAGTCACAAAAGTGATC |
| hsa-miR-155 | MIMAT0000646 | 406947 | TTAATGCTAATCGTGATAGGGGT |
| hsa-miR-15a | MIMAT0000068 | 406948 | TAGCAGCACATAATGGTTTGTG |
| hsa-miR-15b | MIMAT0000417 | 406949 | TAGCAGCACATCATGGTTTACA |
| hsa-miR-16 | MIMAT0000069 | 406950 | TAGCAGCACGTAAATATTGGCG |
| hsa-miR-182 | MIMAT0000259 | 406958 | TTTGGCAATGGTAGAACTCACACT |
| hsa-miR-184 | MIMAT0000454 | 406960 | TGGACGGAGAACTGATAAGGGT |
| hsa-miR-188 | MIMAT0004613 | 406964 | CTCCCACATGCAGGGTTTGCA |
| hsa-miR-193a | MIMAT0000459 | 406968 | AACTGGCCTACAAAGTCCCAGT |
| hsa-miR-193b | MIMAT0002819 | 574455 | AACTGGCCCTCAAAGTCCCGCT |
| hsa-miR-196a | MIMAT0000226 | 406972 | TAGGTAGTTTCATGTTGTTGGG |
| hsa-miR-197 | MIMAT0000227 | 406974 | TTCACCACCTTCTCCACCCAGC |
| hsa-miR-21 | MIMAT0000076 | 406991 | TAGCTTATCAGACTGATGTTGA |
| hsa-miR-210 | MIMAT0000267 | 406992 | CTGTGCGTGTGACAGCGGCTGA |
| hsa-miR-214 | MIMAT0000271 | 406996 | ACAGCAGGCACAGACAGGCAGT |
| hsa-miR-216b | MIMAT0004959 | 100126319 | AAATCTCTGCAGGCAAATGTGA |
| hsa-miR-218 | MIMAT0000275 | 407000 | TTGTGCTTGATCTAACCATGT |
| hsa-miR-224 | MIMAT0000281 | 407009 | CAAGTCACTAGTGGTTCCGTT |
| hsa-miR-24 | MIMAT0000080 | 407012 | TGGCTCAGTTCAGCAGGAACAG |
| hsa-miR-25 | MIMAT0000081 | 407014 | CATTGCACTTGTCTCGGTCTGA |
| hsa-miR-28 | MIMAT0004502 | 407020 | CACTAGATTGTGAGCTCCTGGA |
| hsa-miR-326 | MIMAT0000756 | 442900 | CCTCTGGGCCCTTCCTCCAG |
| hsa-miR-337 | MIMAT0000754 | 442905 | CTCCTATATGATGCCTTTCTTC |
| hsa-miR-338 | MIMAT0000763 | 442906 | TCCAGCATCAGTGATTTTGTTG |
| hsa-miR-342 | MIMAT0000753 | 442909 | TCTCACACAGAAATCGCACCCGT |
| hsa-miR-371 | MIMAT0000723 | 442916 | AAGTGCCGCCATCTTTTGAGTGT |
| hsa-miR-7 | MIMAT0000252 | 407045 | TGGAAGACTAGTGATTTTGTTGT |
| hsa-miR-96 | MIMAT0000095 | 407053 | TTTGGCACTAGCACATTTTTGCT |
MicroRNA target gene prediction
Bioinformatic analysis was applied to explore target genes and the main functions of the miRNA identified. To obtain a higher degree of prediction verification, the targets were predicted by at least three prediction programmes for further data analysis (TargetScan 5.2, Miranda, PicTar, miRGen). The software provided information regarding miRNA sequences and target genes. The genes were then reorganized on the basis of statistical significance.
Statistical analyses
Results are expressed as mean ± standard deviation (SD). Statistical analyses were performed using GraphPad prism 5 software (GraphPad Software, La Jolla, CA, USA). Comparisons between groups were assessed using the Student’s t-test. Given the non-Gaussian distribution of the results, non-parametric Kruskal-Wallis ANOVA was used to compare the data from groups. Expression levels that differed by more than 2-fold between day 0 and day 5 were regarded as significant. For all results, p values <0.05 were considered statistically significant.
Results
Comparison of microRNA expression in apheresis platelets after different periods of storage qRT-PCR was used to identify 49 miRNA in apheresis platelets after different periods of storage under standard blood bank conditions. We analysed miRNA at baseline (day 0) and after 1, 3 and 5 days of storage. We found that platelets contain abundant amounts of apoptosis-associated miRNA (Table I). The expression of 25 miRNA consistently remained at high levels (Table II) in all samples at all time points, and ten miRNA had a more than two-fold difference in expression between day 0 and day 5 (Table II). In detail, hsa-miR-326, hsa-miR-96, hsa-miR-16, hsa-miR-155 and hsa-miR-150 were up-regulated, while hsa-miR-7, hsa-miR-145, hsa-miR-24, hsa-miR-25 and hsa-miR-15a were down-regulated (Figure 1). The increase of hsa-miR-326 was particularly marked.
Table II.
List of expression levels of miRNA in apheresis platelets by qRT-PCR (ranked according to their abundance in apheresis platelets) and the change trend of expression levels (cut-off was 2-fold change compared with day 0).
| miR (expression in platelets) | Trend in level changes |
|---|---|
| hsa-miR-21 | No change |
| hsa-miR-142-3p | No change |
| hsa-miR-16 | Up |
| hsa-let-7f | No change |
| hsa-miR-15b | No change |
| hsa-let-7a | No change |
| hsa-miR-24 | Down |
| hsa-miR-15a | Down |
| hsa-let-7i | No change |
| hsa-let-7g | No change |
| hsa-miR-142-5p | No change |
| hsa-miR-151-5p | No change |
| hsa-miR-326 | Up |
| hsa-let-7d | No change |
| hsa-miR-150 | Up |
| hsa-miR-155 | Up |
| hsa-miR-96 | Up |
| hsa-miR-342 | No change |
| hsa-miR-337 | No change |
| hsa-miR-145 | Down |
| hsa-miR-133a | No change |
| hsa-miR-133b | No change |
| hsa-miR-101 | No change |
| hsa-miR-25 | Down |
| hsa-miR-7 | Down |
Figure 1.
Relative expression levels of selected miRNA during storage.
The miRNA in apheresis platelets are differentially expressed on days 1, 3 and 5 compared with on day 0, as determined by qRT-PCR. MiRNA whose expression changed at least 2-fold change are listed. Statistical analyses were performed using GraphPad prism 5 software. Results identified as significant were those that were more than 2-fold different between day 0 and day 5. The change for each miRNA represents the average found in five independent donors. Results are expressed as mean±standard deviation (SD) (*p<0.05 vs day 0).
Potential target mRNA of microRNA with significant expression changes
The miRNA whose expression changed significantly in all platelets under blood bank storage conditions were selected for target prediction analysis. Bioinformatics analysis indicated that hsa-miR-15a and hsa-miR-16 target Bcl-2, hsa-miR-24 and hsa-miR-25 target Bim, and hsa-miR-326 targets Bcl-xl and Bak. Bcl-2 and Bcl-xl belong to an anti-apoptotic family of proteins, while Bax, Bak and Bim are members of a pro-apoptotic family and are, therefore, relevant to apoptosis in platelets (Table III).
Table III.
Predicted target mRNA of selected miRNA which regulate apoptosis.
| miRNA | Symbol | Gene Description | Function |
|---|---|---|---|
| hsa-miR-7 | ETS2 | v-ets erythroblastosis virus E26 oncogene homolog 2 | apoptosis |
| EGFR | epidermal growth factor receptor | anti-apoptosis | |
| Raf1 | v-raf-1 murine leukemia viral oncogene homolog 1 | apoptosis | |
| PAK1 | p21 protein (Cdc42/Rac)-activated kinase 1 | anti-apoptosis | |
| TNK2 | tyrosine kinase, non-receptor, 2 | anti-apoptosis | |
| IGF1R | insulin-like growth factor 1 receptor | anti-apoptosis | |
| hsa-miR-15 | Bcl2 | B-cell CLL/lymphoma 2 | anti-apoptosis |
| CCND1 | cyclin D1 | anti-apoptosis | |
| CCNE | cyclin E1 | anti-apoptosis | |
| DEDD | death effector domain containing | apoptosis | |
| MYB | v-myb myeloblastosis viral oncogene homolog | anti-apoptosis | |
| AKT3 | v-akt murine thymoma viral oncogene homolog 3 | anti-apoptosis | |
| RPS6KB1 | ribosomal protein S6 kinase, 70kDa, polypeptide 1 | ant-apoptosis | |
| hsa-miR-16 | Bcl2 | B-cell CLL/lymphoma 2 | anti-apoptosis |
| CCND1 | cyclin D1 | anti-apoptosis | |
| CCNE | cyclin E1 | anti-apoptosis | |
| DEDD | death effector domain containing | apoptosis | |
| RECK | reversion-inducing-cysteine-rich protein with kazal motifs | apoptosis | |
| ZYX | zyxin | apoptosis | |
| MYB | v-myb myeloblastosis viral oncogene homolog | anti-apoptosis | |
| AKT3 | v-akt murine thymoma viral oncogene homolog 3 | anti-apoptosis | |
| RPS6KB1 | ribosomal protein S6 kinase, 70kDa, polypeptide 1 | ant-apoptosis | |
| hsa-miR-24 | E2F2 | E2F transcription factor 2 | apoptosis |
| Myc | v-myc myelocytomatosis viral oncogene homolog | anti-apoptosis | |
| Net1A | neuroepithelial cell transforming 1 | anti-apoptosis | |
| Bim | BCL2-like 11 (apoptosis facilitator) | apoptosis | |
| DHFR | dihydrofolate reductase | anti-apoptosis | |
| hsa-miR-25 | Bim | BCL2-like 11 (apoptosis facilitator) | apoptosis |
| TRAIL | TNF-related apoptosis-inducing ligand | apoptosis | |
| FASLG | Fas ligand (TNF superfamily, member 6) | apoptosis | |
| TP53 | tumor protein p53 | apoptosis | |
| hsa-miR-96 | GPC3 | glypican 3 | apoptosis |
| FOXO1 | forkhead box O1 | apoptosis | |
| CASP2 | caspase 2, apoptosis-related cysteine peptidase | apoptosis | |
| BIRC4 | X-linked inhibitor of apoptosis | anti-apoptosis | |
| KRAS | v-Ki-ras2 Kirsten rat sarcoma viral oncogene homolog | anti-apoptosis | |
| hsa-miR-150 | MUC4 | mucin 4, cell surface associated | anti-apoptosis |
| EGR2 | early growth response 2 | apoptosis | |
| hsa-miR-155 | TAB2 | TGF-beta activated kinase 1/MAP3K7 binding protein 2 | anti-apoptosis |
| FADD | Fas-associated protein with death domain | apoptosis | |
| RIP-1 | small subunit (SSU) processome component, homolog | apoptosis | |
| IKK | inhibitor of kappa light polypeptide gene enhancer in B-cells | anti-apoptosis | |
| ET-1 | Endothelin-1 | anti-apoptosis | |
| SMAD5 | SMAD family member 5 | apoptosis | |
| FOXO3 | forkhead box O3 | apoptosis | |
| SOCS1 | suppressor of cytokine signaling 1 | anti-apoptosis | |
| hsa-miR-145 | c-MYC | V-Myc Myelocytomatosis Viral Oncogene Homolog | apoptosis |
| CDK4 | cyclin-dependent kinase 4 | anti-apoptosis | |
| FSCN1 | fascin homolog 1, actin-bundling protein | anti-apoptosis | |
| SOX2 | SRY (sex determining region Y)-box 2 | anti-apoptosis | |
| hsa-miR-326 | Bcl2L1 | BCL2-like 1 | anti-apoptosis |
| Notch1 | notch 1 | apoptosis | |
| BAK1 | BCL2-antagonist/killer 1 | apoptosis | |
| PKM2 | pyruvate kinase, muscle | anti-apoptosis |
Discussion
Platelet lacks nuclear DNA, but do contain various mRNA and can synthesize some apoptosis proteins given the existence of mitochondria and ribosomes, which come from macrophages. In recent years, research on the functions of platelet miRNA has provided new perspectives regarding the mechanism of platelet apoptosis. Platelets contain numerous miRNA; the process of maturation of miRNA is different in platelets than in nucleated cells. In platelets, the maturation of miRNA begins with the unspliced pre-miRNA, which mostly originates from the cytoplasm of macrophages. Platelets contain related regulatory proteins, Dicer and Argonaute 2 (Ago2), and can process pre-miRNA into mature miRNA. These miRNA may be relevant for post-transcriptional gene regulation in platelets, which are anucleate cells. In this study, we found ten miRNA whose levels of expression were significantly different (>2-fold increase or decrease) at day 5 from baseline (day 0) (Table II and Figure 1). A possible explanation for the differential expression of these miRNA might be that platelets simultaneously contain both pre-miRNA that are processed to mature miRNA and miRNA-degrading enzymes and/or an miRNA partitioning mechanism that promotes miRNA degradation11.
According to our data, the expression of hsa-miR-326 significantly increased in all platelets, while the level of expression of hsa-let-7b was low and there was no statistically significant difference during the storage process. These findings differ somewhat from those of Kannan et al.10 who used membrane arrays and found that let-7b remained at high levels, with a tendency to increase, during storage. There were no related reports of further study. In 2011, Barrey et al.12 verified the existence of pre-miRNA and mature miRNA in the mitochondria of human muscle cells. In the same year, Bandiera et al.13 found 13 miRNA expressed in the mitochondria of Hela cells, which they named mitomiR, and a large number of let-7b. We, therefore, speculate that let-7b is abundant in the mitochondria of platelets. Perhaps some mitomiR, including hsa-let-7b, were lost during our extraction process and influenced our findings.
The intrinsic apoptosis pathway is regulated by the Bcl-2 family of proteins, which are divided into two groups14–16, the pro-apoptotic family and anti-apoptotic family. The pro-apoptotic family, including Bax and Bak, play an essential role in mediating the release of cytochrome c and trigger the apoptotic cascade17,18. Research shows that the anti-apoptotic family comprises five members: Bcl-2, Bcl-xl, Mcl-1, A1 and Bcl-w, and maintains cellular viability by preventing the activation of Bax and/or Bak14–16,19,20. Mason et al.19 found that older platelet contain less Bcl-xl than younger platelets; decreasing levels of Bcl-xl lead to a reduction in Bcl-xl-mediated inhibition of Bak and, therefore, induce platelet apoptosis. Their research identified Bcl-xl as a major regulator of platelet survival. Our data show that the expression levels of hsa-miR-326 increase significantly during storage and target the genes Bcl-xl and Bak1. We hypothesized that the increase of hsa-miR-326 may be related to the down-regulation of Bcl-xl gene expression and then restraint of Bak and/or Bax proteins, which have an important role in the onset and progress of platelet apoptosis.
Platelets not only play a central role in the maintenance of haemostasis and thrombotic disorders, but also contribute to diverse functions and conditions, such as inflammatory and immune responses, acute lung injury, tumour progression and metastasis21,22. Recently, Benoit Laffont et al.23 demonstrated that platelet microparticle-derived miR-223 can be delivered to endothelial cells and possibly other recipient cells of the circulatory system and regulate genes at both mRNA and protein levels; this provides a new research direction for the regulatory model of miRNA in platelets.
Platelet transfusion is important in the treatment of multiple conditions such as thrombocytopenia and qualitative platelet disorders. During storage, platelets undergo apoptosis, and finally, lose their viability, and even become ineffective in vivo after transfusion. Careful research on the regulatory network of miRNA in platelets during storage will provide some information to help the understanding of the regulatory mechanisms of platelet apoptosis and function.
Acknowledgements
We are grateful to Prof. Guoguang Wu for his suggestions regarding this paper. We also thank the Wenzhou Blood Centre and Ningbo Blood Centre for providing the platelet samples. This work was supported in part by grants from the Social Development Research Project Foundation of Wenzhou Science and Technology Bureau (NO.Y20110051 & No. Y20110079), and Zhejiang Provincial Natural Science Foundation (NO. Q12H200001).
Footnotes
Authorship contributions
Shifang Yu and Gang Deng contributed equally to this work. Shifang Yu, Qiang Li and Gang Deng conceived and designed the experiments; Shifang Yu, Gang Deng, Qiang Li and He Sun performed the experiments and evaluated the results; Dingliang Qian, Zuoting Xie and Dandan Huang contributed reagents, materials or analysis tools; Shifang Yu and Qiang Li wrote the manuscript. All authors read and approved the final version of the manuscript.
The Authors declare no conflict of interest.
References
- 1.Cauwenberghs S, van Pampus E, Curvers J, et al. Hemostatic and signaling functions of transfused platelets. Transfus Med Rev. 2007;21:287–94. doi: 10.1016/j.tmrv.2007.05.004. [DOI] [PubMed] [Google Scholar]
- 2.Prochazkova R, Andrys C, Hubackova L, Krejsek J. Markers of platelet activation and apoptosis in platelet concentrates collected by apheresis. Transfus Apher Sci. 2007;37:115–23. doi: 10.1016/j.transci.2007.03.015. [DOI] [PubMed] [Google Scholar]
- 3.Schubert P, Devine DV. Towards targeting platelet storage lesion-related signaling pathways. Blood Transfus. 2010;8(Suppl 3):s69–72. doi: 10.2450/2010.011S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Shrivastava M. The platelet storage lesion. Transfus Apher Sci. 2009;41:105–13. doi: 10.1016/j.transci.2009.07.002. [DOI] [PubMed] [Google Scholar]
- 5.Zimmerman GA, Weyrich AS. Signal-dependent protein synthesis by activated platelets: new pathways to altered phenotype and function. Arterioscler Thromb Vasc Biol. 2008;28:s17–24. doi: 10.1161/ATVBAHA.107.160218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Weyrich AS, Schwertz H, Kraiss LW, Zimmerman GA. Protein synthesis by platelets: historical and new perspectives. J Thromb Haemost. 2009;7:241–6. doi: 10.1111/j.1538-7836.2008.03211.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Denis MM, Tolley ND, Bunting M, et al. Escaping the nuclear confines: signal-dependent pre-mRNA splicing in anucleate platelets. Cell. 2005;122:379–91. doi: 10.1016/j.cell.2005.06.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lima RT, Busacca S, Almeida GM, et al. MicroRNA regulation of core apoptosis pathways in cancer. Eur J Cancer. 2011;47:163–74. doi: 10.1016/j.ejca.2010.11.005. [DOI] [PubMed] [Google Scholar]
- 9.Ple H, Landry P, Benham A, et al. The repertoire and features of human platelet microRNAs. PLoS One. 2012;7:e50746. doi: 10.1371/journal.pone.0050746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kannan M, Mohan KV, Kulkarni S, Atreya C. Membrane array-based differential profiling of platelets during storage for 52 miRNAs associated with apoptosis. Transfusion. 2009;49:1443–50. doi: 10.1111/j.1537-2995.2009.02140.x. [DOI] [PubMed] [Google Scholar]
- 11.Landry P, Plante I, Ouellet DL, et al. Existence of a microRNA pathway in anucleate platelets. Nat Struct Mol Biol. 2009;16:961–6. doi: 10.1038/nsmb.1651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Barrey E, Saint-Auret G, Bonnamy B, et al. Pre-microRNA and mature microRNA in human mitochondria. PLoS One. 2011;6:e20220. doi: 10.1371/journal.pone.0020220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bandiera S, Ruberg S, Girard M, et al. Nuclear outsourcing of RNA interference components to human mitochondria. PLoS One. 2011;6:e20746. doi: 10.1371/journal.pone.0020746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Strasser A, Cory S, Adams JM. Deciphering the rules of programmed cell death to improve therapy of cancer and other diseases. EMBO J. 2011;30:3667–83. doi: 10.1038/emboj.2011.307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Youle RJ, Strasser A. The BCL-2 protein family: opposing activities that mediate cell death. Nat Rev Mol Cell Biol. 2008;9:47–59. doi: 10.1038/nrm2308. [DOI] [PubMed] [Google Scholar]
- 16.Dowling MR, Josefsson EC, Henley KJ, et al. Platelet senescence is regulated by an internal timer, not damage inflicted by hits. Blood. 2010;116:1776–8. doi: 10.1182/blood-2009-12-259663. [DOI] [PubMed] [Google Scholar]
- 17.Kluck RM, Bossy-Wetzel E, Green DR, Newmeyer DD. The release of cytochrome c from mitochondria: a primary site for Bcl-2 regulation of apoptosis. Science. 1997;275:1132–6. doi: 10.1126/science.275.5303.1132. [DOI] [PubMed] [Google Scholar]
- 18.Li P, Nijhawan D, Budihardjo I, et al. Cytochrome c and dATP-dependent formation of Apaf-1/caspase-9 complex initiates an apoptotic protease cascade. Cell. 1997;91:479–89. doi: 10.1016/s0092-8674(00)80434-1. [DOI] [PubMed] [Google Scholar]
- 19.Mason KD, Carpinelli MR, Fletcher JI, et al. Programmed anuclear cell death delimits platelet life span. Cell. 2007;128:1173–86. doi: 10.1016/j.cell.2007.01.037. [DOI] [PubMed] [Google Scholar]
- 20.Zhou F, Yang Y, Xing D. Bcl-2 and Bcl-xL play important roles in the crosstalk between autophagy and apoptosis. FEBS J. 2011;278:403–13. doi: 10.1111/j.1742-4658.2010.07965.x. [DOI] [PubMed] [Google Scholar]
- 21.Semple JW, Italiano JE, Jr, Freedman J. Platelets and the immune continuum. Nat Rev Immunol. 2011;11:264–74. doi: 10.1038/nri2956. [DOI] [PubMed] [Google Scholar]
- 22.Smyth SS, McEver RP, Weyrich AS, et al. Platelet functions beyond hemostasis. J Thromb Haemost. 2009;7:1759–66. doi: 10.1111/j.1538-7836.2009.03586.x. [DOI] [PubMed] [Google Scholar]
- 23.Laffont B, Corduan A, Ple H, et al. Activated platelets can deliver mRNA regulatory Ago2*microRNA complexes to endothelial cells via microparticles. Blood. 2013;122:253–61. doi: 10.1182/blood-2013-03-492801. [DOI] [PubMed] [Google Scholar]