Abstract
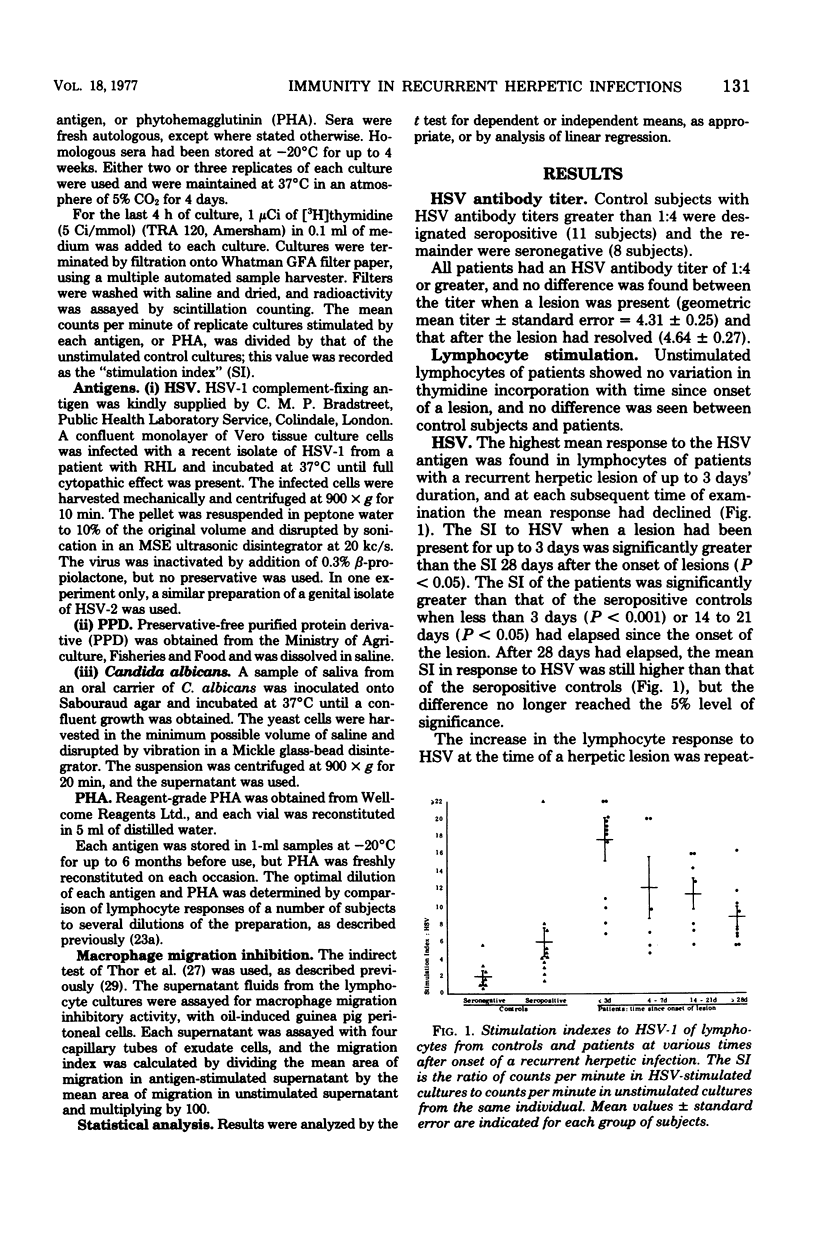
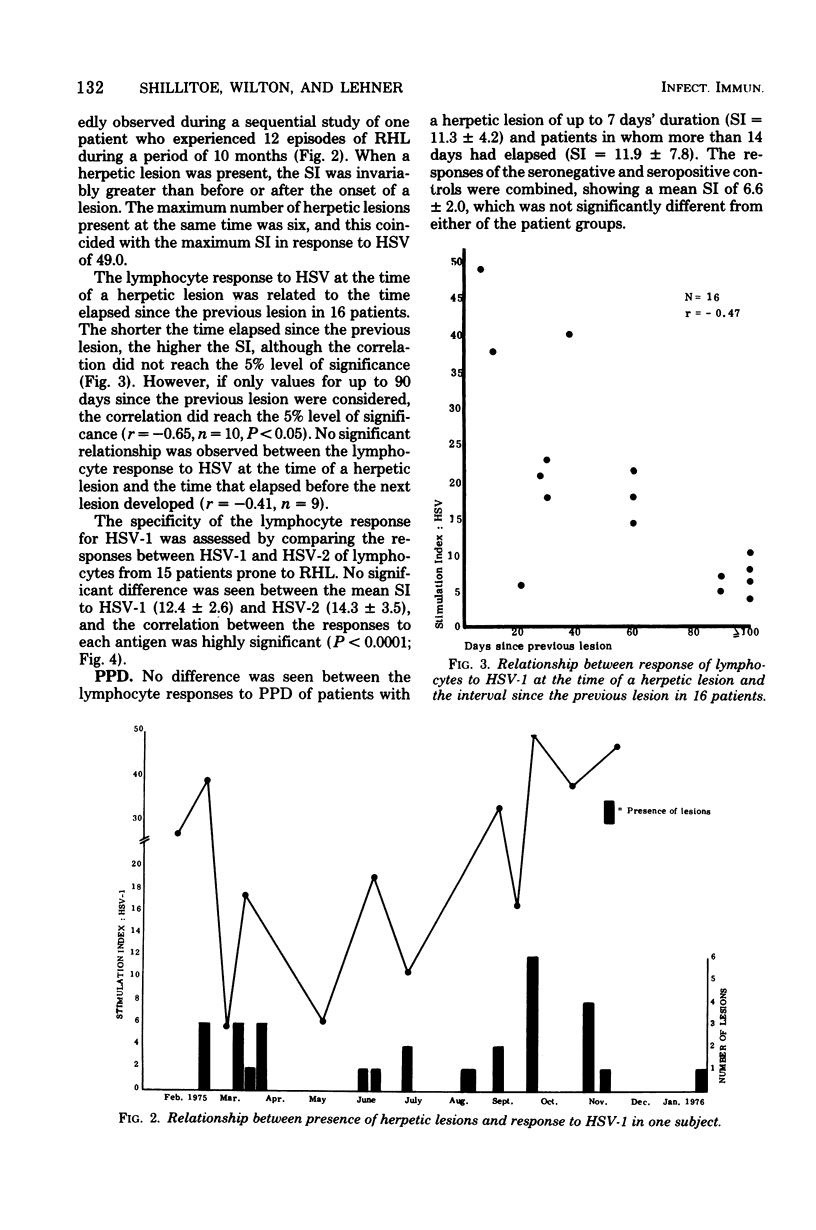
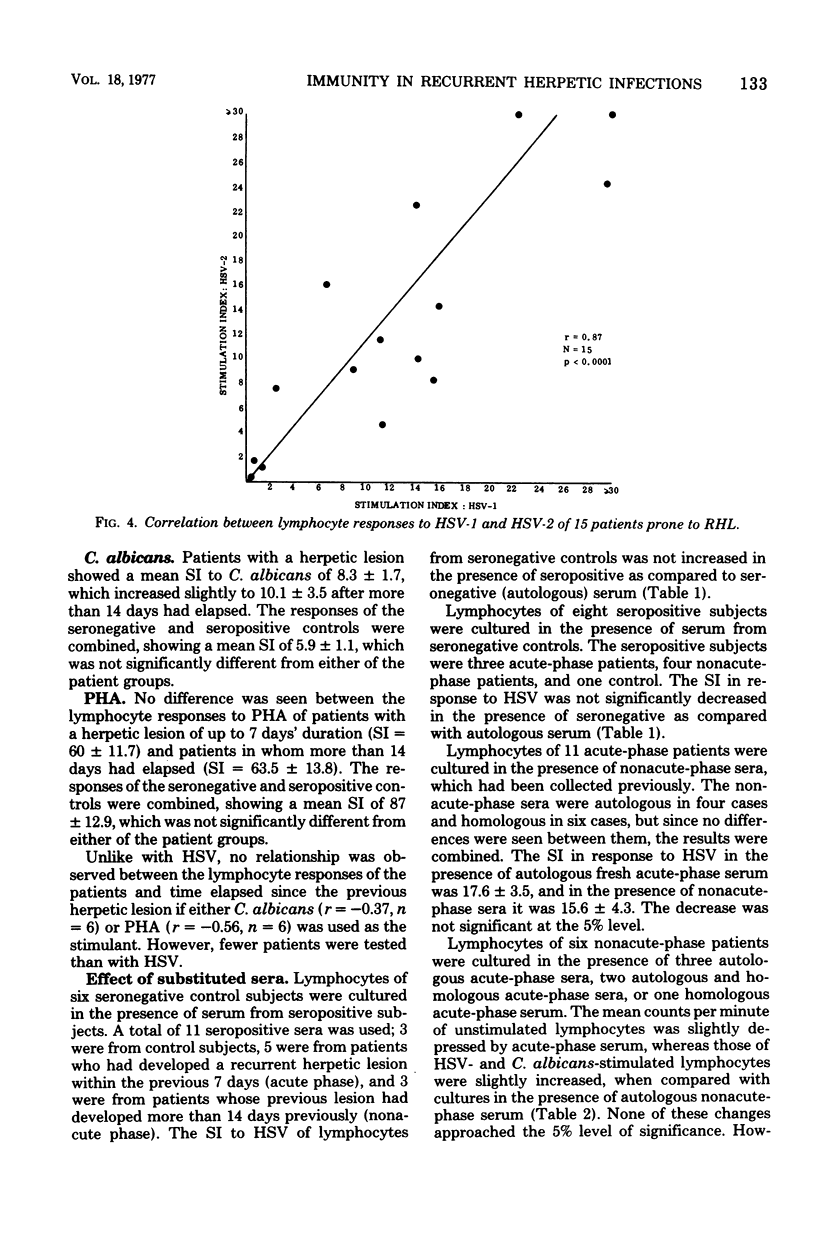
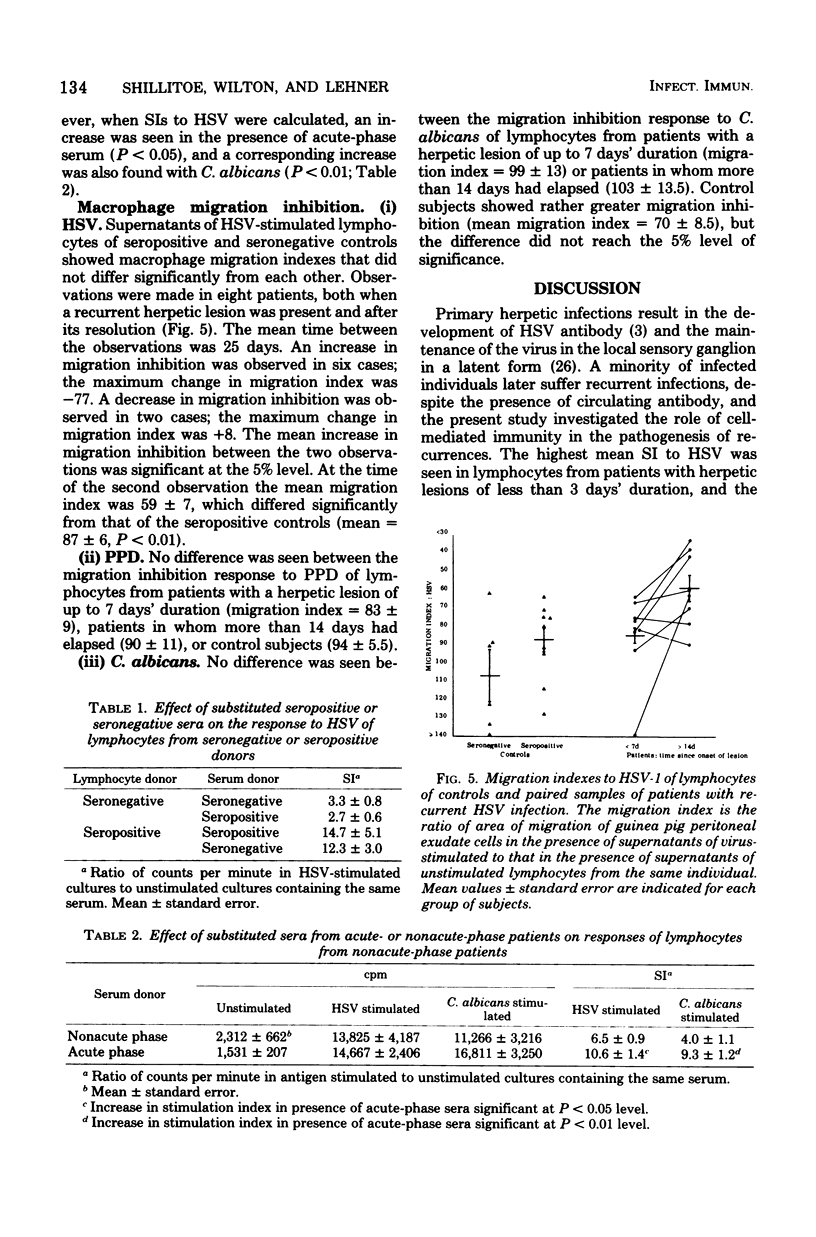
Lymphocyte responses to herpes simplex virus (HSV) were studied in 23 patients with recurrent herpes labialis and in 19 control subjects. Lymphocytes of seropositive, but not seronegative, controls responded to HSV by thymidine incorporation, and the supernatant fluids inhibited the migration of guinea pig macrophages. Lymphocytes from patients with a recurrent herpetic lesion responded to HSV by significantly greater thymidine incorporation than seropositive controls, but supernatants did not show an increased macrophage migration inhibition response. During the 28 days after the onset of a lesion, the thymidine incorporation to HSV fell to the level of the seropositive controls, and supernatants then induced an increased inhibition of macrophage migration. Lymphocyte responses to Candida albicans, purified protein derivative, or phytohemagglutinin did not fluctuate according to the presence of a lesion and did not differ from those of the controls. Lymphocyte responses to HSV were unaffected by culture in the presence of serum from seronegative or seropositive controls, or from patients with or without a herpetic lesion. It is suggested that in patients with recurrent herpes labialis a periodic defect of the migration inhibition response might have allowed the recurrent infection to develop, and that the increased thymidine incorporation stimulated by HSV in vitro is a result of antigenic stimulation from the lesion.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BRADSTREET C. M., TAYLOR C. E. Technique of complementfixation test applicable to the diagnosis of virus diseases. Mon Bull Minist Health Public Health Lab Serv. 1962 May;21:96–104. [PubMed] [Google Scholar]
- Böyum A. Isolation of mononuclear cells and granulocytes from human blood. Isolation of monuclear cells by one centrifugation, and of granulocytes by combining centrifugation and sedimentation at 1 g. Scand J Clin Lab Invest Suppl. 1968;97:77–89. [PubMed] [Google Scholar]
- DASCOMB H. E., ADAIR C. V., ROGERS N. Serologic investigations of herpes simplex virus infections. J Lab Clin Med. 1955 Jul;46(1):1–11. [PubMed] [Google Scholar]
- Douglas R. G., Jr, Couch R. B. A prospective study of chronic herpes simplex virus infection and recurrent herpes labialis in humans. J Immunol. 1970 Feb;104(2):289–295. [PubMed] [Google Scholar]
- Fireman P., Friday G., Kumate J. Effect of measles vaccine on immunologic responsiveness. Pediatrics. 1969 Feb;43(2):264–272. [PubMed] [Google Scholar]
- Fujibayashi T., Hooks J. J., Notkins A. L. Production of interferon by immune lymphocytes exposed to herpes simplex virus-antibody complexes. J Immunol. 1975 Nov;115(5):1191–1193. [PubMed] [Google Scholar]
- Gange R. W., de Bats A., Park J. R., Bradstreet C. M., Rhodes E. L. Cellular immunity and circulating antibody to herpes simplex virus in subjects with recurrent herpex simplex lesions and controls as measured by the mixed leukocyte migration inhibition test and complement fixation. Br J Dermatol. 1975 Nov;93(5):539–544. doi: 10.1111/j.1365-2133.1975.tb02246.x. [DOI] [PubMed] [Google Scholar]
- Glezen W. P., Fernald G. W., Lohr J. A. Acute respiratory disease of university students with special reference to the etiologic role of Herpesvirus hominis. Am J Epidemiol. 1975 Feb;101(2):111–121. doi: 10.1093/oxfordjournals.aje.a112077. [DOI] [PubMed] [Google Scholar]
- Haahr S., Rasmussen L., Merigan T. C. Lymphocyte transformation and interferon production in human mononuclear cell microcultures for assay of cellular immunity to herpes simplex virus. Infect Immun. 1976 Jul;14(1):47–54. doi: 10.1128/iai.14.1.47-54.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall C. B., Kantor F. S. Depression of established delayed hepersensitivity by mumps virus. J Immunol. 1972 Jan;108(1):81–85. [PubMed] [Google Scholar]
- Hirsch M. S., Zisman B., Allison A. C. Macrophages and age-dependent resistance to Herpes simplex virus in mice. J Immunol. 1970 May;104(5):1160–1165. [PubMed] [Google Scholar]
- Jacobs R. P., Aurelian L., Cole G. A. Cell-mediated immune response to herpes simplex virus: type-specific lymphoproliferative responses in lymph nodes draining the site of primary infection. J Immunol. 1976 Jun;116(6):1520–1525. [PubMed] [Google Scholar]
- Kurtz J. B. Specific IgG and IgM antibody responses in herpes-simplex-virus infections. J Med Microbiol. 1974 Aug;7(3):333–341. doi: 10.1099/00222615-7-3-333. [DOI] [PubMed] [Google Scholar]
- Lee J. C., Sigel M. M. A differential effect of IgM and IgG antibodies on the blastogenic response of lymphocytes to rubella virus. Cell Immunol. 1974 Jul;13(1):22–31. doi: 10.1016/0008-8749(74)90223-8. [DOI] [PubMed] [Google Scholar]
- Lehner T., Wilton J. M., Shillitoe E. J. Immunological basis for latency, recurrences and putative oncogenicity of herpes simplex virus. Lancet. 1975 Jul 12;2(7924):60–62. doi: 10.1016/s0140-6736(75)90499-7. [DOI] [PubMed] [Google Scholar]
- Nathan C. F., Remold H. G., David J. R. Characterization of a lymphocyte factor which alters macrophage functions. J Exp Med. 1973 Feb 1;137(2):275–290. doi: 10.1084/jem.137.2.275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Reilly R. J., Chibbaro A., Anger E., Lopez C. Cell-mediated immune responses in patients with recurrent Herpes Simplex infections. II. Infection-associated deficiency of lymphokine production in patients with recurrent herpes labialis or herpes progenitalis. J Immunol. 1977 Mar;118(3):1095–1102. [PubMed] [Google Scholar]
- Rasmussen L. E., Jordan G. W., Stevens D. A., Merigan T. C. Lymphocyte interferon production and transformation after Herpes simplex infections in humans. J Immunol. 1974 Feb;112(2):728–736. [PubMed] [Google Scholar]
- Rosenberg G. L., Farber P. A., Notkins A. L. In vitro stimulation of sensitized lymphocytes by herpes simplex virus and vaccinia virus. Proc Natl Acad Sci U S A. 1972 Mar;69(3):756–760. doi: 10.1073/pnas.69.3.756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenberg G. L., Notkins A. L. Induction of cellular immunity to herpes simplex virus: relationship to the humoral immune response. J Immunol. 1974 Mar;112(3):1019–1025. [PubMed] [Google Scholar]
- Rosenberg G. L., Snyderman R., Notkins A. L. Production of chemotactic factor and lymphotoxin by human leukocytes stimulated with Herpes simplex virus. Infect Immun. 1974 Jul;10(1):111–115. doi: 10.1128/iai.10.1.111-115.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenberg G. L., Wohlenberg C., Nahmias A. J., Notkins A. L. Differentiation of type 1 and type 2 herpes simplex virus by in vitro stimulation of immune lymphocytes. J Immunol. 1972 Aug;109(2):413–414. [PubMed] [Google Scholar]
- Russell A. S., Kaiser J., Lao V. S. Cell mediated immunity to herpes simplex in man. IV. A correlation of lymphocyte stimulation and inhibition of leukocyte migration. J Immunol Methods. 1976;9(3-4):273–279. doi: 10.1016/0022-1759(76)90202-7. [DOI] [PubMed] [Google Scholar]
- Shillitoe E. J., Tarro G., Lehner T. Cell-mediated immunity to herpes simplex virus types 1 and 2 antigens in leukoplakia and carcinoma in man. Oncology. 1976;33(4):192–195. doi: 10.1159/000225141. [DOI] [PubMed] [Google Scholar]
- Starr S. E., Karatela S. A., Shore S. L., Duffey A., Nahmias A. J. Stimulation of human lymphocytes by Herpes simplex virus antigens. Infect Immun. 1975 Jan;11(1):109–112. doi: 10.1128/iai.11.1.109-112.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steele R. W., Vincent M. M., Hensen S. A., Fuccillo D. A., Chapa I. A., Canales L. Cellular immune responses to Herpes simplex virus type 1 in recurrent herpes labialis: in vitro blastogenesis and cytotoxicity to infected cell line. J Infect Dis. 1975 May;131(5):528–534. doi: 10.1093/infdis/131.5.528. [DOI] [PubMed] [Google Scholar]
- Stevens J. G., Cook M. L. Latent herpes simplex virus in spinal ganglia of mice. Science. 1971 Aug 27;173(3999):843–845. doi: 10.1126/science.173.3999.843. [DOI] [PubMed] [Google Scholar]
- Thor D. E., Jureziz R. E., Veach S. R., Miller E., Dray S. Cell migration inhibition factor released by antigen from human peripheral lymphocytes. Nature. 1968 Aug 17;219(5155):755–757. doi: 10.1038/219755a0. [DOI] [PubMed] [Google Scholar]
- Vesikari T., Buimovici-Klein E. Lymphocyte responses to rubella antigen and phytohemagglutinin after administration of the RA 27/3 strain of live attenuated rubella vaccine. Infect Immun. 1975 Apr;11(4):748–753. doi: 10.1128/iai.11.4.748-753.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilton J. M., Ivanyi L., Lehner T. Cell-mediated immunity in Herpesvirus hominis infections. Br Med J. 1972 Mar 18;1(5802):723–726. doi: 10.1136/bmj.1.5802.723. [DOI] [PMC free article] [PubMed] [Google Scholar]


