Abstract
Background
Increasing life expectancies, burgeoning healthcare costs and an emphasis on the management of multiple health-risk behaviours point to a need to delineate health lifestyles in older adults.
Purpose
The aims of this study were to delineate health lifestyles of a cohort of older adults and to examine the association of these lifestyles with biological and psychological states and socio-economic indices.
Methods
Cluster analysis was applied to data derived from the self-reported 45 and Up cohort study (N = 96,276) of Australians over 45 years, regarding exercise, smoking, alcohol consumption, diet and cancer screening behaviours.
Results
Six lifestyle clusters emerged delineated by smoking, screening and physical activity levels. Individuals within health-risk dominant clusters were more likely to be male, living alone, low-income earners, living in a deprived neighbourhood, psychologically distressed and experiencing low quality of life.
Conclusions
Health lifestyle cluster membership can be used to identify older adults at greatest risk for physical and psychological health morbidity.
Keywords: Health behaviour, Cluster analysis, Cancer screening, Ageing, Audience segmentation
Inroduction
The benefits of preventive health behaviours (e.g. exercise, eating fruit and vegetables), and the adverse outcomes associated with risky health behaviours (e.g. smoking, excessive alcohol consumption), in terms of health and mortality have been the subject of considerable research [1]. Typically, these behaviours were examined separately, which may be an overly simplistic approach as there is evidence that they co-occur as lifestyle patterns within population sub-groups [2–4]. This co-occurrence appears to create synergistic effects, with increasing risk of premature mortality from cancer, cardiovascular disease and all-cause mortality beyond the expected additive effects of the separate behaviours [5–7]. Prior studies have demonstrated transfer effects, whereby health-promoting and health-harmful behaviours tend to be highly correlated within behaviour groupings, or clusters, but not between these groupings [8]. Furthermore, campaigns targeting just one health behaviour can have unintended consequences for modifying other health behaviours that co-occur [1]. Consequently, there is a growing emphasis on managing multiple health-risk behaviours as opposed to single risk factors, to increase the efficacy and lower costs of interventions across the population [9], an approach that has been encouraged by the World Health Organization [10].
Co-occurring health behaviours are described as “health lifestyles” [11] that are to some extent socially determined, through factors such as income and education, and have consequences for one’s ongoing health status. Understanding the components and correlates of health behaviour lifestyles is therefore important for: (1) identifying groups whose health lifestyles place them at greater risk for future ill health [12]; (2) designing holistic approaches to health promotion [2, 11]; and (3) targeting groups most likely to benefit from particular health campaigns or health services [13]. Since health lifestyles comprise a unique constellation of different attributes, cluster analysis is most suited for identifying different lifestyle groups within a given population [9] and for identifying characteristics of cluster membership that determine responses to health interventions. Lifestyle group membership has generally been related to self-rated health [2, 14], greater mortality risk [15], body mass index [16, 17], depression [14, 18, 19], quality of life [2] and to the differential effects of dietary interventions [20, 21]. Research employing cluster analysis of health lifestyles has typically focused on younger populations of adolescents and college students [12, 22, 23]. Much less is known about health lifestyle clusters across the full adult age range, but limited evidence suggests that the number and type of clusters vary across age groups [24], possibly reflecting different social and physical contexts or different rates of health behaviours across ages. Older people, for example, have higher rates of cancer screening than younger age groups and lower rates of smoking [25, 26].
In the context of rapidly ageing societies, increasing life expectancies and burgeoning costs of health care [27, 28], there is a need to understand how older people cluster in terms of their engagement in health-related behaviours in order to inform the design of age-appropriate health promotion interventions. Since adherence to a healthy lifestyle is associated with delayed onset of disability, slower functional decline and less cognitive impairment, information about health clusters is likely to be a key factor for promoting positive ageing [29, 30].
Only two studies have utilised a statistical clustering approach to specifically target older individuals: (1) N = 2,002 Germans over 50 years [4]; and (2) N = 5,880 Taiwanese over 60 years [14]. The German study focused on four health-related behaviours (i.e. smoking, alcohol consumption, exercise, diet) [4], identifying five clusters: (1) No risk behaviours; (2) Physically inactive individuals; (3) Fruit and vegetable avoiders; (4) Smokers with risk behaviours; and (5) Drinkers with risk behaviours. The Taiwanese study [14], included health check-ups, but not diet, and clustered behaviour across time separately for males and females, with gender affecting the number of cluster trajectories, but there was a relatively small healthy lifestyle grouping for both males and females. One further study (N = 4,165 older Koreans) [31], that did not use statistical clustering but divided the sample into 16 groups according to level of adherence to guidelines for smoking, drinking, physical activity and weight, found that only 11.7 % met recommendations for all four behaviours.
Whilst informative, these findings are based on relatively small samples of homogeneous populations, and may not generalise to health lifestyles of individuals in vastly heterogeneous and multicultural societies, such as Australia and the USA. One USA-based study [15] (N = 19,662) reported the existence of 12 health profiles amongst older (>50 years) adults based on permutations of only three health behaviours (smoking, drinking, physical activity). Smoking and heavy drinking were associated with the greatest mortality risk, with inactivity also associated with increased mortality. However, these groupings were based on an ad hoc approach, rather than employing a more statistically rigorous cluster analytic technique.
A notable limitation of the Korean-, US- and German-based studies was the exclusion of cancer screening, which is regarded as a key component of the preventive health approach, particularly for older adults [32]. Current guidelines in developed nations vary according to specific age recommendations to commence cancer screening, and the recommended between-screening intervals, but there is consensus that regular screening for cancers (e.g. bowel, breast) reduces both cancer-related mortality and morbidity [32, 33].
Sociodemographic factors are also regarded as key variables on which health lifestyles are clustered [11]. For example, age- and gender- distinguished clusters in the German [4] and Taiwanese [14] studies, whereby even amongst older adults, being younger and male decreased the odds of being in the healthy lifestyle category. Gender was likewise linked to clustering in studies of all-age samples from Ireland [2], Australia [34] and Belgium [35]. Having a marital partner was another factor associated with healthy lifestyle cluster membership in the German study [4]. Moreover, socio-economic status has consistently been associated with cluster membership with healthier/low risk lifestyle clusters more likely to emerge in higher socio-economic status groups [26]; however, reported socio-economic status typically uses only individual levels of income and education, neglecting other factors that might affect health status. For example, residential location influences health status through the effect of relative access to healthcare, fresh food, etc., with neighbourhood socio-economic status impacting all-cause mortality beyond the effect of an individual’s socio-economic status [36]. Work status (i.e. full-time vs. part-time vs. retired or not working) is also a potential economic factor to consider when undertaking clustering analyses of health lifestyles, especially in the over-50 age group for whom the transition into retirement is regarded as a significant life event [37]. In summary, the rapid ageing and increasing life expectancy seen in many countries has heightened the need to understand how to promote positive ageing both for the benefit of individuals who are living longer and to reduce burgeoning national spending on health care [28]. However, little is known of the health lifestyles of this ageing population. The initial aim of the current study was to extend earlier work by employing a cluster analytic approach to identify health behaviour lifestyle groups within a heterogeneous society, using a large Australian population cohort of older adults. These data were drawn from more than 92,000 people who were enrolled in the 45 and Up Study, the largest cohort panel in the Southern Hemisphere [38]. Addressing limitations of earlier work, we included cancer screening behaviour as a clustering factor, along with exercise, smoking, alcohol consumption, and diet. A second aim was to investigate the association between cluster membership and biological (body mass index and physical functioning) and psychological (self-rated quality of life and psychological distress) states, as well as a wide range of socio-economic variables (age, gender, income, marital status, education, neighbourhood location and work status). Consistent with the majority of prior research, it was hypothesised that cluster groupings would be characterised primarily by within-group similarities (i.e. healthy behaviours with other healthy behaviours; unhealthy behaviours with unhealthy behaviours). It was further hypothesised that unhealthy behaviour cluster membership would be associated with lower socio-economic status, greater psychological dysfunction and poorer physical functioning than healthy clusters.
Methods
Participants
Data from this study were derived from the baseline survey of the 45 and Up Study, which is a cohort panel of Australian residents in the state of New South Wales, conducted by the Sax Institute (see http://www.45andup.org.au/). Recruitment of those aged over 45 years was conducted via random sampling from the national health system (Medicare Australia), but with oversampling from rural areas and individuals aged 80 years and over. The final cohort represented 10 % of the total population in this age range [19]. All participants gave informed consent prior to their inclusion in this study. Data collection via a pen and paper self-report questionnaire commenced in 2006, and the analyses in this study were conducted on those recruited by the year 2008 (approximately 103,000). Those with missing data on the key health behaviours were removed, leaving a sample of 96,276. The average age was 62.9 years (SD = 11.11) and 52.1 % of the sample were females. The majority (77.3 %) were married or partnered, with 8.2 % divorced, 9 % widowed, and 5.5 % single. Individuals who were excluded from analyses due to missing data were, on average, 4.30 years older (t = 28.80, p < .001), more likely to be female (56 %; t = 6.90, p < .001) and had slightly lower levels of education and income (t = 26.60 and 12.25 respectively, p < .001).
Measures
Self-reported information from the baseline survey included engagement in five health behaviours: smoking, alcohol consumption, exercise, cancer screening and diet; seven sociodemographic factors: age, gender, education level, income, marital status, residential address and work status; two biological variables: body mass index and health-related disability; and two psychological variables: self-reported quality of life and psychological distress.
Health Behaviours
Participants indicated if they had ever smoked (1 = yes, 0 = no) and if they currently smoked (1 = yes, 0 = no). Individuals who had never smoked are coded as “no” in these variables. The number of alcoholic drinks in a week was used as the measure of alcohol consumption.
Exercise was measured via the Active Australia Survey [39], which asks participants how many times a week they engaged in each activity of vigorous exercise, moderate exercise and walking continuously, for at least 10 min. Good reliability and acceptable validity has been reported [39]. In Australia, current recommendations are that people in this age range exercise (walking or more vigorous activity) for at least 30 min on at least 3 days a week [40]. We calculated a dummy variable where 1 = met recommended guidelines and 0 = did not meet recommended guidelines.
Cancer screening was measured by asking participants if they had been screened at any time for bowel cancer (all participants), prostate cancer (males only) or breast cancer (females only). A dummy-coded variable was computed where 1 = had been screened for these cancers and 0 = never been screened. Note that at the time of data collection for this study, prostate cancer screening was recommended on a population-wide basis, hence this factor was incorporated in our measure of cancer screening.
Diet was assessed based on fruit and vegetable intake using items previously validated for use in this context [41]. Participants quantified the number of serves of vegetables and fruit they consumed each day, where one serve is equal to a half cup of cooked vegetables, one cup of salad, one piece of a medium-sized fruit, two pieces of small-sized fruit or one cup of diced fruit.
Sociodemographic Factors
Participants indicated their gender (1 = male, 0 = female), age, highest educational qualification (from 1 = less than 10 years schooling to 6 = University degree or higher) and marital status (1 = married or de facto, 0 = not partnered), derived from the original item that assessed whether the participant was separated, divorced, widowed or single. Household income referred to income in Australian dollars before tax from all sources including benefits, pensions and superannuation, with responses ranging from 1 (less than $5,000 per year) to 8 (more than $70,000 per year).
Residential location was coded using the 2006 Socio-Economic Indexes for Areas, a coding scheme developed by the Australian Bureau of Statistics [42] to summarise the socio-economic status conditions of people living in a specified area. The Index of Relative Socio-Economic Advantage and Disadvantage utilised was derived from a composite of census variables reflecting advantage and disadvantage (e.g. households with low incomes and people with tertiary education). The scores were recoded into deciles by the Australian Bureau of Statistics, with the lowest 10 % of scores given a value of 1 through to the top 10 % of scores which were given a value of 10.
Work status was derived from participant responses to an item regarding their work status (0 = disabled/sick; 1 = fully retired, studying only, looking after home/family only or currently unemployed; 2 = work part-time, partially retried, or in unpaid work only; 3 = work full-time).
Biological Variables
Body mass index (BMI) was calculated from self-reported weight and height using the standard formula [i.e. weight (kilograms) / height (square meters)]. Current physical function was measured using a 10-item scale derived from the Medical Outcomes Study - Physical Functioning scale (MOS-PF) [43]. Participants responded on a three-point scale (from 1 (yes, limited a lot) to 3 (no, not limited at all)) whether their health limited them in a range of physical activities (e.g. “Walking one flight of stairs”). Coefficient alpha for the present study was .92.
Psychological Factors
Self-reported quality of life was assessed by one item: “In general, how would you rate your quality of life?”, rated from 1 = poor to 5 = excellent. Psychological distress was assessed with the 10-item Kessler Psychological Distress Scale (K10; [44]), a commonly used measure of non-specific depression and anxiety [see [45] for additional reliability and validity information]. Participants indicated on a five-point response scale (1 = none of the time to 5 = all of the time) how often they felt conditions such as “tired out for no good reason,” “nervous” and “depressed” over the past 4 weeks. Coefficient alpha for the present study was .89.
Analytic Approach
Clusters were identified using the TwoStep Cluster analysis procedure (SPSS 19.0) allowing for the identification of natural groupings in large datasets containing categorical and continuous variables. Initially, cases were scanned sequentially and arranged into different pre-clusters based upon a distance measure derived from the decrease in log-likelihood. Pre-clusters were then grouped using a hierarchical clustering algorithm that created a range of clustering solutions, which were reduced by utilising the Bayesian Information Criterion (BIC) to find the optimum solution. Cases (n = 820) that did not fit well into any formed cluster were identified as outliers and excluded from further analyses.
To determine the stability of the cluster solution, the original data were split into two random halves and the TwoStep cluster analysis repeated. MANOVAs were then conducted to determine whether there was any variation within clusters between each split half for each health behaviour.
One-way ANOVAs and cross tabulations were used to investigate between-cluster differences in sociodemographic, biological and psychological variables. Two multinomial regression analyses (one for the sociodemographic variables and one for the biological/psychological variables) then assessed the relative impact of these variables on predicting cluster membership.
Results
Descriptive statistics for all study variables are reported in Table 1. Only 7 % of the sample were current smokers but a further 36 % had previously smoked (henceforth called “ex-smokers”). The majority (85 %) had undertaken a health screening test but only 42 % engaged in recommended exercise levels.
Table 1.
Means and standard deviations of all continuous study variables and percentages of categorical variables
| Continuous Variables | Mean | SD | Categorical variables | Frequency | Percent | |
|---|---|---|---|---|---|---|
| Alcohol | 7.1 | 9.9 | Smoking | Never smoked | 58,006 | 56.3 |
| Diet | 5.8 | 3.4 | Smoked in past | 37,374 | 36.3 | |
| Age | 63.1 | 11.2 | Current smokers | 7,660 | 7.4 | |
| Income | 6.1 | 2.3 | Cancer screening | Screened | 87,389 | 84.8 |
| SEIFA | 6.4 | 2.7 | Never screened | 15,632 | 15.2 | |
| BMI | 26.8 | 4.8 | Exercise | Above recommended | 42,417 | 42.1 |
| Physical functioning | 2.6 | 0.5 | Below recommended | 58,450 | 57.9 | |
| K10 | 1.5 | 0.6 | Gender | Male | 49,316 | 47.9 |
| Quality of life | 3.7 | 1.0 | Female | 53,726 | 52.1 | |
| Marital status | Married/de facto | 76,852 | 76.7 | |||
| Not Living with partner | 23,340 | 23.3 | ||||
| Work status | Working full-time | 23,388 | 23.1 | |||
| Disabled/sick | 3,714 | 3.7 | ||||
| Not working | 43,040 | 42.5 | ||||
| Working part-time | 31,083 | 30.7 | ||||
SEIFA Socio-Economic Indexes for Areas, BMI Body mass index, K10 Kessler Psychological Distress Scale
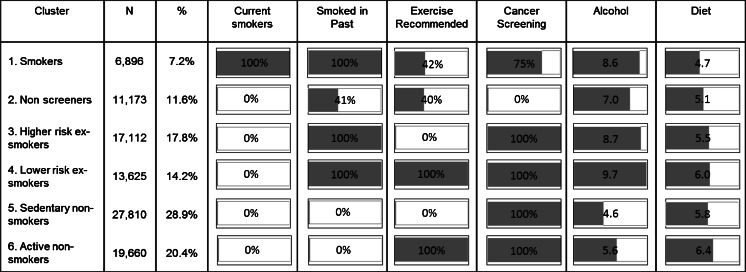
Cluster Analysis
Six clusters emerged from the TwoStep Cluster analysis, with the same groupings emerging when the analysis was repeated on two random half samples. Figure 1 shows the size and percentage of each cluster and the distribution of health behaviours in each. Cluster 1 “Smokers” was the smallest. Less than half (42 %) of the smokers exercised at recommended levels and 25 % had never undertaken cancer screening. They had a relatively high intake of alcohol and had the lowest fruit and vegetable intake. Cluster 2 “non-screeners” was also a smaller group, 41 % of whom were ex-smokers. Only 40 % of the non-screeners engaged in recommended levels of exercise and although just below average in amount of alcohol consumed, they were also below average fruit and vegetable consumers.
Fig. 1.
Distribution of health behaviours in each cluster (with smoking, exercise and cancer screening expressed as a percent of cluster membership and alcohol and diet as a mean for the cluster membership). Active non-smokers is the reference category used for multinomial regression analyses
Of the remaining four clusters, two were ex-smokers and two contained people who had never smoked. Cluster 3 “higher risk ex-smokers” did undertake cancer screening but they did not exercise at recommended levels. Their level of alcohol consumption was above the sample mean and fruit and vegetable intake was below the mean. Cluster 4 “lower risk ex-smokers” engaged in recommended levels of exercise, undertook cancer screening, and consumed above average amounts of fruit and vegetables. However, they were the highest consumers of alcohol. Cluster 5 “sedentary non-smokers” was the largest group. They did not exercise, had average fruit and vegetable intake, but engaged in cancer screening and consumed the lowest amount of alcohol of all groups. Cluster 6 “active non-smokers” had the most positive profile in terms of health behaviours. They typically engaged in recommended levels of exercise, undertook cancer screening, ate more fruit and vegetables than other groups and consumed relatively less alcohol.
Cluster Relationship to Sociodemographic Variables
Results of a multinomial regression analysis using active non-smokers as a reference group indicate that sociodemographic factors were a significant predictor of group membership (see Analysis 1 in Table 2). Chi-squared statistics derived from the likelihood ratio test (see Table 3), which compares a reduced model not containing a sociodemographic variable with the full model, also reveal that all factors were significant at p < 0.001.
Table 2.
Odds Ratios and 95 % confidence intervals for sociodemographic, biological and psychological variables
| Variable | 1. Smokers | 2. Non-screeners | 3. Higher risk ex-smokers | 4. Lower risk ex-smokers | 5. Sedentary non-smokers | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| OR | 95 % CI | OR | 95 % CI | OR | 95 % CI | OR | 95 % CI | OR | 95 % CI | |
| Analysis 1 | ||||||||||
| Sexa | 2.36** | (2.21, 2.51) | 4.60** | (4.35, 4.86) | 2.28** | (2.18, 2.39) | 2.26** | (2.15, 2.37) | 0.98 | (0.94, 1.02) |
| Age | 0.92** | (0.92, 0.93) | 0.95** | (0.95, 0.95) | 1.00 | (0.99, 1.00) | 0.99** | (0.99, 1.00) | 1.00 | (1.00, 1.00) |
| Marital statusb | 2.70** | (2.52, 2.90) | 1.71** | (1.60, 1.82) | 1.22** | (1.16, 1.30) | 1.21** | (1.14, 1.29) | 1.08* | (1.02, 1.13) |
| Education | 0.80** | (0.78, 0.81) | 0.93** | (0.92, 0.94) | 0.91** | (0.90, 0.92) | 0.96** | (0.95, 0.98) | 0.96** | (0.94, 0.97) |
| Work statusc | ||||||||||
| Disabled/sick | 2.44** | (2.01, 2.87) | 1.11 | (0.94, 1.31) | 1.88** | (1.62, 2.18) | 1.77** | (1.50, 2.09) | 1.13 | (0.98, 1.31) |
| Not working | 1.01 | (0.91, 1.11) | 0.70** | (0.65, 0.76) | 0.83** | (0.77, 0.90) | 1.15** | (1.06, 1.25) | 0.70** | (0.65, 0.75) |
| Working part-time | 0.78** | (0.72, 0.85) | 0.64** | (0.60, 0.69) | 0.73** | (0.68, 0.77) | 1.05 | (0.98, 1.12) | 0.68** | (0.64, 0.72) |
| Income | 0.92** | (0.91, 0.93) | 0.95** | (0.94, 0.96) | 0.99* | (0.98, 1.00) | 0.99* | (0.98, 1.00) | 1.00 | (0.99, 1.01) |
| SEIFA | 0.96** | (0.94, 0.97) | 1.01* | (1.00, 1.02) | 1.01* | (1.01, 1.02) | 0.99* | (0.98, 0.99) | 1.04** | (1.03, 1.04) |
| Overall adjusted model statistics: χ2 (45) = 11,851.37**, Negelkerke R 2 = .131; Cox and Snell R 2 = .126 | ||||||||||
| Analysis 2 | ||||||||||
| BMI | 1.00 | (1.00–1.01) | 1.03** | (1.03–1.04) | 1.06** | (1.05–1.06) | 1.03** | (1.02–1.03) | 1.03** | (1.03–1.04) |
| Physical functioning | 1.02 | (0.95–1.11) | 1.12* | (1.04–1.20) | 0.66** | (0.62–0.70) | 0.91* | (0.85–0.97) | 0.78** | (0.74–0.82) |
| Quality of life | 0.57** | (0.54–0.59) | 0.74** | (0.71–0.76) | 0.75** | (0.73–0.77) | 0.88** | (0.86–0.91) | 0.84** | (0.82–0.86) |
| K10 | 1.30** | (1.23–1.37) | 0.98 | (0.93–1.04) | 1.00 | (0.96–1.05) | 1.00 | (0.95–1.05) | 1.02 | (0.98–1.06) |
| Overall adjusted model statistics: χ2 (45) = 3,403.42**, Negelkerke R 2 = .044; Cox and Snell R 2 = .043 | ||||||||||
N (Analysis 1) = 87,878; N (Analysis 2) = 78,262; Odds ratios are adjusted to all other variables in the model, and the reference category is “Active non-smokers”
OR Odds Ratio, CI Confidence Interval, SEIFA Socio-Economic Indexes for Areas
* = p < .05 level; ** = p < .001 level
aMales (comparison group is females)
bNot living with a partner (comparison group is married/de-facto)
cWork status as specified in table (comparison group is working full-time)
Table 3.
Likelihood ratio test in multinomial logistic regressions
| Variable | −2 log-likelihood | χ2 | d.f |
|---|---|---|---|
| Analysis 1 | |||
| Sex | 278,106.3 | 5,377.0** | 5 |
| Age | 275,665.8 | 2,936.5** | 5 |
| Marital status | 273,682.9 | 953.7** | 5 |
| Education | 273,404.4 | 675.2** | 5 |
| Work status | 273,406.9 | 677.7** | 15 |
| Income | 272,912.1 | 182.9** | 5 |
| SEIFA | 273,020.8 | 291.6** | 5 |
| Analysis 2 | |||
| BMI | 242,333.7 | 160.1** | 5 |
| Physical functioning | 242,751.2 | 577.7** | 5 |
| Quality of life | 243,227.2 | 1,053.6** | 5 |
| K10 | 242,296.3 | 122.8** | 5 |
SEIFA Socio-Economic Indexes for Areas, BMI body mass index, K10 Kessler Psychological Distress Scale
**p < .001
The smokers cluster was the youngest and the two ex-smoker groups were the oldest (and not significantly different from each other). The non-screeners had the greatest number of males (68.8 %) while the two groups of those who had never smoked had the least number of males (37 and 37.6 %). Smokers had a higher proportion of single people (36 %) compared to the other five groups, where the percentage of singles ranged from 20 to 23.5 %.
Smokers also had the lowest socio-economic status as assessed by education, income and the Index of Relative Socio-Economic Advantage and Disadvantage. Non-screeners and the active non-smokers had the highest education (significantly different from all other groups) and the highest income (significantly different from all but sedentary non-smokers). However, the sedentary non-smokers had the highest average Index of Relative Socio-Economic Advantage And Disadvantage, with non-screeners living in the second lowest Index of Relative Socio-Economic Advantage And Disadvantage areas on average.
In terms of work status, the non-screeners had the highest proportion of full-time workers whilst the higher risk ex-smokers had the highest proportion of retirees and smokers had the highest proportion who described themselves as sick/disabled.
Cluster Relationship to Biological/Psychological Variables
Results of a second multinomial regression (Analysis 2 in Table 2) indicate that cluster membership was significantly related to the biological and psychological health status variables, and when the final model containing all outcomes was compared to a reduced model not containing a particular biological/psychological variable, the chi-squared statistics based upon -2 log-likelihood demonstrated that each outcome was significant at p < .001 (see Table 3). Active non-smokers had better outcomes on all indicators, with the lowest body mass index, best physical function, highest self-rated quality of life, and lowest psychological distress. Higher risk ex-smokers had the poorest biological markers (highest body mass index and lowest physical functioning), while the smokers had the poorest psychological markers (highest psychological distress and lowest quality of life).
Discussion
This study addressed the need to identify and describe health lifestyle clusters evident in older populations. Understanding the factors that promote healthy and positive ageing has become increasingly important in the face of longer life expectancies and the growing burden on national health budgets [27]. A second aim was to investigate the association between cluster membership and biological (body mass index and physical functioning) and psychological (self-rated quality of life and psychological distress) states, as well as a wide range of socio-economic variables (age, gender, income, marital status, education, neighbourhood location and work status).
Using data from one of the largest samples that has ever contributed to a cluster analysis of health behaviours, six clear clusters emerged: smokers, non-screeners, higher risk ex-smokers, lower risk ex-smokers, sedentary non-smokers and active non-smokers. The clustering of several unhealthy behaviours together, particularly in the smoker, non-screener, higher risk ex-smoker and sedentary non-smoker groupings, is consistent with other research demonstrating within-group similarities and transfer effects of unhealthy behaviours, but which did not include screening behaviours in their analyses [e.g. Laaksonen et al. (Finland; [46]); Héroux et al. (Canada; [47]); Hsu et al. (Taiwan; [14]); Tobias et al. (New Zealand; [48])]. Since smoking and heavy drinking have been shown to substantially elevate risk for mortality for middle-aged (51–65 years) and older (66+ years) adults, along with inactivity among non-smokers [15], the “unhealthy” behaviour clusters identified in the present study represent individuals who are at increased mortality risk, warranting future intervention to modify these health-damaging behaviours within the cluster groupings. The Higher Risk Ex-smoker clustering represents an example of healthy and unhealthy behaviours in combination, suggesting a compensatory effect similar to that described by Knäuper et al. [49], whereby individuals engaging a health-harmful activity such as high alcohol intake compensate somewhat for this unhealthy practice by adopting a health activity (i.e. screening, giving up smoking). While engagement in at least one healthy behaviour is promising, the ongoing adoption of unhealthy behaviours continues to place these individuals at increased risk for future ill health, warranting the need for interventions to increase the healthy, and decrease the unhealthy behaviours of these groups.
The characteristics of the clusters identified in the present study differed somewhat from the German study of older people [4], whose clusters were focused on the dimensions of physical activity, diet, smoking and alcohol intake. These differences may reflect the inclusion of cancer screening in the present study, which was found to be a significant factor that identified a unique cluster. Given its relevance for older individuals [32], understanding the characteristics of the non-screened group of individuals is an important contribution of the current study and should be used to inform future screening promotion approaches. The differences between the studies may also reflect broad cultural differences in health-related behaviours across nations. For example, there were lower rates of smoking (7 %) in the Australian sample compared to 15 % of smokers in both the German and Korean studies, and 17 % in Shaw and Agahi’s sample of US citizens [15]. However, in all samples, smokers were more likely to be male and younger. The current study also distinguished current from previous smokers. This distinction is important as while quitting smoking decreases risks for certain diseases (e.g. asthma and respiratory-related diseases; [50]), previous smoking still places individuals at increased risk of developing other chronic and life-threatening conditions [51–54].
As in other health cluster studies [2, 4, 34], and consistent with the concept of transfer from healthy behaviours to other healthy behaviours [8], one overall positive or “ideal” health behaviour cluster was found with individuals characterised by being physically active non-smokers who had a healthy diet, low to moderate alcohol intake and were cancer screeners. These findings are also consistent with limited findings suggesting increased positive health behaviours amongst individuals undergoing screening for disease risk [55]. However, the relative size (20 %) of this healthy cluster, while larger than the Korean sample [31] of older people (12 %) was somewhat smaller than in other studies, where for example, 25 % of older Germans [4], 34 % of older Americans [15], and approximately 29 % of Taiwanese [14] were in the healthy profile groups, as were 45 % of participants in the all-age Western Australian study [34]. Although past research indicates that older people may engage in fewer risky health behaviours [56], future research should aim to confirm if they also engage in lower rates of positive health behaviour.
As found in other health behaviour cluster studies [2, 4, 34, 35], the clusters differed significantly in terms of the sociodemographic profile of their members, highlighting the importance of considering such factors when addressing health behaviour. Consistent with Shaw and Agahi [15] and Lee et al. [31], women dominated the two non-smoking profiles with the lowest alcohol intake, and as found by Hsu et al. [14], education level was associated with cluster membership.
The results extend prior work by showing that not only do clusters differ by age, gender, marital status, education and income but they also differ in terms of work status and residential locality, which explained unique variance in membership even after controlling for all other factors. Retirement transition (through part-time work, for example) and full retirement (cessation of all paid work) are important milestones that are unique to the older population. The finding that the non-screeners cluster had the highest level of full-time workers (holding age constant) suggests that lack of time may be an issue related to their non-compliance. The results of this study also showed that living in a poorer, disadvantaged neighbourhood with larger numbers of unemployed and disabled people not only increased the likelihood of membership in less healthy behaviour clusters for those who were themselves less educated, had a lower income, or less work but also increased the likelihood of being in the less healthy clusters for the more highly educated, wealthy and fully employed who nevertheless lived in these disadvantaged areas. This association between neighbourhood socio-economic status and cluster membership regardless of an individual’s socio-economic status supports arguments that health interventions need to consider social context [57, 58].
Clusters were significantly different in terms of key physical and psychological indicators, with the active non-smokers having the most “positive” indicators of all groups, the higher risk ex-smokers having poor physical characteristics (higher body mass index and lower physical functioning) and the smokers having poor psychological characteristics (lower quality of life and higher distress). This reflects emerging evidence that in those who successfully quit smoking are psychologically healthier (i.e. on measures of overall quality of life, health-related quality of life and positive emotions), than their smoking counterparts [59].
Study Limitations
Although these results provide support for the expected benefits and problems associated with health lifestyles [10, 11], this was a cross-sectional study. Future research needs to investigate the longitudinal outcomes of cluster membership, and whether or not changing cluster membership can alter such outcomes.
A limitation of the current study is that, like many investigations of health behaviour, it relied on self-reported data. Whilst the risk of socially desirable or inaccurate responses exists, self-report data from the 45 and Up Study have been validated against actual measurements (e.g. of body mass index) and found to be highly correlated (r = .95) [60]. In addition, the measure of health behaviours was at a fairly broad level. For example, although one of few studies to distinguish ex-smokers from those who had never smoked, the quantity of smoking and number of years smoked was not considered, even though such information may provide a more fine-grained distinction between those in the smoking cluster. Likewise, the measure of dietary behaviour only assessed fruit and vegetable consumption whereas a more comprehensive analysis would have included fat and sugar intake, for example.
The study’s strengths include the large sample size, which enabled cross validation of the results. The broad representation of adults in this age group also contributes to confidence in the stability of the clusters. Nonetheless, the recent study by Hsu et al. [14] indicates that older people also follow different trajectories across time in terms of changing engagement in health behaviour. Future research tracking the stability of cluster membership is needed to understand the factors that trigger change.
Conclusions
This study lends important insight into the range of health lifestyle profiles or clusters evident in an older culturally heterogeneous population. Previous research had demonstrated the importance of considering multiple behaviours and cluster membership on health status and long-term survival. This study extended this earlier work by delineating lifestyle clusters that incorporated cancer screening behaviours (a key component of the preventive health approach currently advocated), a more fine-grained analysis of smoking incorporating both current and past smoking behaviours, as well as drinking, diet and physical activity variables that have typically been included in past investigations. Moreover, the role of socio-economic variables on cluster membership was more comprehensively assessed than in previous reports, incorporating work status and neighbourhood socio-economic status as factors beyond the typical range of socio-economic status indicators. Result indicated that clusters characterised by the most harmful behaviours are more likely to include men, those who are living alone and people with a lower income and who live in a deprived neighbourhood. Importantly, members of these clusters are also more likely to report having a lower quality of life and being more distressed.
From a public health perspective, these findings are important as they highlight the need to consider beyond the typical factors of smoking, drinking and physical exercise when characterising health behaviours for older adults, to incorporate factors such as screening behaviours that are critical components of preventive health approaches for this age group. Our study focused specifically on cancer screening behaviours, but future research could incorporate other forms of disease screening such as cholesterol monitoring. Inclusion of a greater range of health behaviours might also enable the identification of “gateway behaviours,” whereby intervention on a key behaviour effects positive change in other behaviours not directly subjected to intervention [61]. Within the context of a rapidly ageing population, this type of information can inform future campaigns to target multiple health behaviour changes, rather than focusing on individual risk factors [62]. Audience segmentation approaches utilising cluster analytic techniques will be crucial, since a message for young to older adults regarding the role of work status and screening behaviours on health would be very different from a message for older individuals who have long been retired.
Acknowledgments
This research was completed using data collected through the 45 and Up Study (www.saxinstitute.org.au). The 45 and Up Study is managed by the Sax Institute in collaboration with major partner Cancer Council NSW; and the following partners: the National Heart Foundation of Australia (NSW Division); NSW Ministry of Health; beyondblue; Ageing, Disability and Home Care, Department of Family and Community Services; the Australian Red Cross Blood Service; and UnitingCare Ageing. We thank the many thousands of people participating in the 45 and Up Study
Authors’ Statement of Conflict of Interest and Adherence to Ethical Standards
Authors Griffin, Sherman, Jones, and Bayl-Smith declare that they have no conflict of interest. All procedures, including the informed consent process, were conducted in accordance with the ethical standards of the responsible committee on human experimentation (institutional and national) and with the Helsinki Declaration of 1975, as revised in 2000.
References
- 1.Spring B, Moller AC, Coons MJ. Multiple health behaviours: Overview and implications. J Public Health (Oxf). 2012;34:i3–i10. doi: 10.1093/pubmed/fdr111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Conry MC, Morgan K, Curry P, et al. The clustering of health behaviours in Ireland and their relationship with mental health, self-rated health and quality of life. BMC Public Health. 2011;11(1):692. doi: 10.1186/1471-2458-11-692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Liang W, Shediac-Rizkallah MC, Celentano DD, Rohde C. A population-based study of age and gender differences in patterns of health-related behaviors. Am J Prev Med. 1999;17(1):8–17. doi: 10.1016/S0749-3797(99)00040-9. [DOI] [PubMed] [Google Scholar]
- 4.Schneider S, Huy C, Schuessler M, Diehl K, Schwarz S. Optimising lifestyle interventions: identification of health behaviour patterns by cluster analysis in a German 50+ survey. Eur J Public Health. 2009;19(3):271–7. doi: 10.1093/eurpub/ckn144. [DOI] [PubMed] [Google Scholar]
- 5.Kvaavik E, Batty GD, Ursin G, Huxley R, Gale CR. Influence of individual and combined health behaviors on total and cause-specific mortality in men and women: the United Kingdom health and lifestyle survey. Arch Intern Med. 2010;170(8):711–718. doi: 10.1001/archinternmed.2010.76. [DOI] [PubMed] [Google Scholar]
- 6.McCullough ML, Patel AV, Kushi LH, et al. Following cancer prevention guidelines reduces risk of cancer, cardiovascular disease, and all-cause mortality. Cancer Epidemiol Biomarkers Prev. 2011;20(6):1089–1097. doi: 10.1158/1055-9965.EPI-10-1173. [DOI] [PubMed] [Google Scholar]
- 7.Tamakoshi A, Tamakoshi K, Lin Y, Yagyu K, Kikuchi S. Healthy lifestyle and preventable death: Findings from the Japan Collaborative Cohort (JACC) Study. Prev Med. 2009;48(5):486–92. doi: 10.1016/j.ypmed.2009.02.017. [DOI] [PubMed] [Google Scholar]
- 8.Lippke S, Nigg CR, Maddock JE. Health-Promoting and Health-Risk Behaviors: Theory-Driven Analyses of Multiple Health Behavior Change in Three International Samples. Int J Behav Med. 2012;19:1–13. doi: 10.1007/s12529-010-9135-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Prochaska JO, Velicer WF, Redding C, et al. Stage-based expert systems to guide a population of primary care patients to quit smoking, eat healthier, prevent skin cancer, and receive regular mammograms. Prev Med. 2005;41(2):406–416. doi: 10.1016/j.ypmed.2004.09.050. [DOI] [PubMed] [Google Scholar]
- 10.Prochaska JO, Evers KE, Castle PH, et al. Enhancing multiple domains of well-being by decreasing multiple health risk behaviors: A randomized clinical trial. Popul Health Manag. 2012;15(5):276–286. doi: 10.1089/pop.2011.0060. [DOI] [PubMed] [Google Scholar]
- 11.Abel T. Measuring health lifestyles in a comparative analysis: Theoretical issues and empirical findings. Soc Sci Med. 1991;32(8):899–908. doi: 10.1016/0277-9536(91)90245-8. [DOI] [PubMed] [Google Scholar]
- 12.Ottevaere C, Huybrechts I, Benser J, et al. Clustering patterns of physical activity, sedentary and dietary behavior among European adolescents: The HELENA study. BMC Public Health. 2011;11:328. doi: 10.1186/1471-2458-11-328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Clatworthy J, Buick D, Hankins M, Weinman J, Horne R. The use and reporting of cluster analysis in health psychology: a review. Br J Health Psychol. 2005;10(3):329–358. doi: 10.1348/135910705X25697. [DOI] [PubMed] [Google Scholar]
- 14.Hsu H-C, Luh D-L, Chang W-C, Pan L-Y. Joint trajectories of multiple health-related behaviors among the elderly. Int J Public Health. 2013;58(1):109–120. doi: 10.1007/s00038-012-0358-9. [DOI] [PubMed] [Google Scholar]
- 15.Shaw BA, Agahi N. A prospective cohort study of health behavior profiles after age 50 and mortality risk. BMC Public Health. 2012;12:803. doi: 10.1186/1471-2458-12-803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Charreire H, Casey R, Salze P, et al. Leisure-time physical activity and sedentary behavior clusters and their associations with overweight in middle-aged French adults. Int J Obes (Lond). 2010;34(8):1293–1301. doi: 10.1038/ijo.2010.39. [DOI] [PubMed] [Google Scholar]
- 17.Zabinski MF, Norman GJ, Sallis JF, Calfas KJ, Patrick K. Patterns of sedentary behavior among adolescents. Health Psychol. 2007;26(1):113–120. doi: 10.1037/0278-6133.26.1.113. [DOI] [PubMed] [Google Scholar]
- 18.Paxton RJ, Valois RF, Watkins KW, Huebner ES, Drane JW. Associations between depressed mood and clusters of health risk behaviors. Am J Health Behav. 2007;31(3):272–283. doi: 10.5993/AJHB.31.3.5. [DOI] [PubMed] [Google Scholar]
- 19.Verger P, Lions C, Ventelou B. Is depression associated with health risk-related behaviour clusters in adults? Eur J Public Health. 2009;19(6):618–624. doi: 10.1093/eurpub/ckp057. [DOI] [PubMed] [Google Scholar]
- 20.Reedy J, Haines PS, Campbell MK. The influence of health behavior clusters on dietary change. Prev Med. 2005;41(1):268–275. doi: 10.1016/j.ypmed.2004.11.005. [DOI] [PubMed] [Google Scholar]
- 21.O’Halloran P, Lazovich D, Patterson RE, et al. Effect of health lifestyle pattern on dietary change. Am J Health Promot. 2001;16(1):27–33. doi: 10.4278/0890-1171-16.1.27. [DOI] [PubMed] [Google Scholar]
- 22.Burke V, Milligan RA, Beilin LJ, et al. Clustering of health-related behaviors among 18-year-old Australians. Prev Med. 1997;26(5):724–733. doi: 10.1006/pmed.1997.0198. [DOI] [PubMed] [Google Scholar]
- 23.Dodd LJ, Al-Nakeeb Y, Nevill A, Forshaw MJ. Lifestyle risk factors of students: a cluster analytical approach. Prev Med. 2010;51(1):73–77. doi: 10.1016/j.ypmed.2010.04.005. [DOI] [PubMed] [Google Scholar]
- 24.Van Nieuwenhuijzen M, Junger M, Velderman MK, et al. Clustering of health-compromising behavior and delinquency in adolescents and adults in the Dutch population. Prev Med. 2009;48(6):572–578. doi: 10.1016/j.ypmed.2009.04.008. [DOI] [PubMed] [Google Scholar]
- 25.Kadiyala S. Are U.S. cancer screening test patterns consistent with guideline recommendations with respect to the age of screening initiation. BMC Health Serv Res. 2009;9:185. doi: 10.1186/1472-6963-9-185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Pierce JP, Messer K, White MM, Cowling DW, Thomas DP. Prevalence of heavy smoking in California and the United States, 1965-2007. JAMA. 2011;305(11):1106–1112. doi: 10.1001/jama.2011.334. [DOI] [PubMed] [Google Scholar]
- 27.Armstrong BK, Gillespie JA, Leeder SR, Rubin GL, Russell LM. Challenges in health and health care for Australia. Med J Aust. 2007;187(9):485–489. doi: 10.5694/j.1326-5377.2007.tb01383.x. [DOI] [PubMed] [Google Scholar]
- 28.Rechel B, Doyle Y, Grundy E, McKee M. How can health systems respond to population ageing? Copenhagen: World Health Organization; 2009. Available at: http://www.euro.who.int/__data/assets/pdf_file/0004/64966/E92560.pdf. Accessed October 16, 2012.
- 29.Ford ES, Bergmann MM, Boeing H, Li C, Capewell S. Healthy lifestyle behaviors and all-cause mortality among adults in the United States. Prev Med. 2012;55(1):23–27. doi: 10.1016/j.ypmed.2012.04.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Peel NM, McClure RJ, Bartlett HP. Behavioral determinants of healthy aging. Am J Prev Med. 2005;28(3):298–304. doi: 10.1016/j.amepre.2004.12.002. [DOI] [PubMed] [Google Scholar]
- 31.Lee Y, Back JH, Kim J, Byeon H, Kim S, Ryu M. Clustering of multiple healthy lifestyles among older Korean adults living in the community. Geriatr Gerontol Int. 2012;12(3):515–23. doi: 10.1111/j.1447-0594.2011.00788.x. [DOI] [PubMed] [Google Scholar]
- 32.Smith RA, Cokkinides V, Brooks D, Saslow D, Brawley OW. Cancer screening in the United States, 2010: A review of current American Cancer Society guidelines and issues in cancer screening. CA Cancer J Clin. 2010;60(2):99–119. doi: 10.3322/caac.20063. [DOI] [PubMed] [Google Scholar]
- 33.Department of Health and Ageing (Australia). Cancer Screening Programmes. 2006 June 7. Available at: http://www.cancerscreening.gov.au/. Accessed October 17, 2012.
- 34.French S, Rosenberg M, Knuiman M. The clustering of health risk behaviors in a Western Australian adult population. Health Promot J Austr. 2008;19(3):203–209. doi: 10.1071/he08203. [DOI] [PubMed] [Google Scholar]
- 35.De Bourdeaudhuij I, van Oost P. A cluster-analytical approach toward physical activity and other health related behaviors. Med Sci Sports Exerc. 1999;31(4):605–612. doi: 10.1097/00005768-199904000-00019. [DOI] [PubMed] [Google Scholar]
- 36.Wilkinson RG, Pickett KE. Income inequality and population health: A review and explanation of the evidence. Soc Sci Med. 2006;62(7):1768–1784. doi: 10.1016/j.socscimed.2005.08.036. [DOI] [PubMed] [Google Scholar]
- 37.Shultz KS, Wang M. Psychological perspectives on the changing nature of retirement. Am Psychol. 2011;66(3):170–179. doi: 10.1037/a0022411. [DOI] [PubMed] [Google Scholar]
- 38.Banks E, Redman S, Jorm L, et al. Cohort profile: the 45 and up study. Int J Epidemiol. 2008;37(5):941–947. doi: 10.1093/ije/dym184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Australian Institute of Health and Welfare. The active Australia survey: A guide and manual for implementation, analysis and reporting. Canberra: AIHW; 2003. Available at: http://www.aihw.gov.au/WorkArea/DownloadAsset.aspx?id=6442454895. Accessed October 16, 2012.
- 40.Department of Health and Ageing (Australia). Physical Activity Guidelines. 2013 May 17. Available at: http://www.health.gov.au/internet/main/publishing.nsf/Content/health-pubhlth-strateg-phys-act-guidelines. Accessed September 16, 2013.
- 41.Roddam AW, Spencer E, Banks E, Beral V, Reeves G, Appleby P, Barnes I, Whiteman DC, Key TJ. Reproducibility of a short semi-quantitative food group questionnaire and its performance in estimating nutrient intake compared with a 7-day diet diary in the Million Women Study. Public Health Nutr. 2005;8(2):201–13. doi: 10.1079/PHN2004676. [DOI] [PubMed] [Google Scholar]
- 42.Australian Bureau of Statistics. 20330.0.55.001 - Census of Population and Housing: Socio-Economic Indexes (SEIFA), Australia - Data only, 2006. 2008 Mar 26. Available at: http://www.abs.gov.au/AUSSTATS/abs@.nsf/DetailsPage/2033.0.55.0012006. Accessed June 29, 2012
- 43.Hays RD, Liu H, Spritzer K, Cella D. Item response theory analyses of physical functioning items in the medical outcomes study. Med Care. 2007;45(5):S32–S38. doi: 10.1097/01.mlr.0000246649.43232.82. [DOI] [PubMed] [Google Scholar]
- 44.Kessler RC, Andrews G, Colpe LJ, et al. Short screening scales to monitor population prevalences and trends in non-specific psychological distress. Psychol Med. 2002;32(6):959–976. doi: 10.1017/S0033291702006074. [DOI] [PubMed] [Google Scholar]
- 45.Sunderland M, Slade T, Stewart G, Andrews G. Estimating the prevalence of DSM-IV mental illness in the Australian general population using the Kessler Psychological Distress Scale. Aust N Z J Psychiatry. 2011;45(10):880–9. doi: 10.3109/00048674.2011.606785. [DOI] [PubMed] [Google Scholar]
- 46.Laaksonen M, Práttalä R, Karisto A. Patterns of unhealthy behaviour in Finland. Eur J Public Health. 2001;11(3):294–300. doi: 10.1093/eurpub/11.3.294. [DOI] [PubMed] [Google Scholar]
- 47.Héroux M, Janssen I, Lee D, Sui X, Hebert JR, Blair SN. Clustering of unhealthy behaviors in the aerobics center longitudinal study. Prev Sci. 2012;13(2):183–195. doi: 10.1007/s11121-011-0255-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Tobias M, Jackson G, Yeh L-C, Huang K. Do healthy and unhealthy behaviours cluster in New Zealand? Aust N Z J Public Health. 2007;31(2):155–163. doi: 10.1111/j.1753-6405.2007.00034.x. [DOI] [PubMed] [Google Scholar]
- 49.Knäuper B, Rabiau M, Cohen O, Patriciu N. Compensatory health beliefs: Scale development and psychometric properties. Psychol Heath. 2004; 19(5): 607–624.
- 50.Frank P, Morris J, Hazell M, Linehan M, Frank T. Smoking, respiratory symptoms and likely asthma in young people: evidence from postal questionnaire surveys in the Wythenshawe Community Asthma Project (WYCAP) BMC Pulm Med. 2006;6:10. doi: 10.1186/1471-2466-6-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Costenbader KH, Feskanich D, Mandl LA, Karlson EW. Smoking intensity, duration, and cessation, and the risk of rheumatoid arthritis in women. Am J Med. 2006; 119(6): 503–511. [DOI] [PubMed]
- 52.Helmersson J, Larsson A, Vessby B, Basu S. Active smoking and a history of smoking are associated with enhanced prostaglandin F2α, interleukin-6 and F2-isoprostane formation in elderly men. Atherosclerosis. 2005; 181(1): 201–207. [DOI] [PubMed]
- 53.Higuchi LM, Khalili H, Chan AT, Richter JM, Bousvaros A, Fuchs CS. A prospective study of cigarette smoking and the risk of inflammatory bowel disease in women. Am J Gastroenterol. 2012;107(9):1399–1406. doi: 10.1038/ajg.2012.196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Kenfield SA, Stampfer MJ, Rosner, BA, Colditz GA. Smoking and smoking cessation in relation to mortality in women. JAMA. 2008; 299(17): 2037–2047. [DOI] [PMC free article] [PubMed]
- 55.Deutekom M, Vansenne F, McCaffery K, Essink-Bot M-L, Stronks K, Bossuyt PMM. The effects of screening on health behaviour: A summary of the results of randomized controlled trials. J Public Health (Oxf). 2011;33(1):71–79. doi: 10.1093/pubmed/fdq050. [DOI] [PubMed] [Google Scholar]
- 56.Siahpush M, Borland R. Socio-demographic variations in smoking status among Australians aged ≥18: Multivariate results from the 1995 National Health Survey. Aust N Z J Public Health. 2001;25(5):438–442. doi: 10.1111/j.1467-842X.2001.tb00289.x. [DOI] [PubMed] [Google Scholar]
- 57.Bosma H, van de Mheen HD, Borsboom GJ, Mackenbach JP. Neighborhood Socioeconomic Status and All-Cause Mortality. Am J Epidemiol. 2001;153(4):363–371. doi: 10.1093/aje/153.4.363. [DOI] [PubMed] [Google Scholar]
- 58.Gary-Webb TL, Baptiste-Roberts K, Pham L, et al. Neighborhood socioeconomic status, depression, and health status in the Look AHEAD (Action for Health in Diabetes) study. BMC Public Health. 2011;11:349. doi: 10.1186/1471-2458-11-349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Piper ME, Kenford S, Fiore MC, Baker TB. Smoking cessation and quality of life: Changes in life satisfaction over 3 years following a quit attempt. Ann Behav Med. 2012;43(2):262–270. doi: 10.1007/s12160-011-9329-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Ng SP, Korda R, Clements M, et al. Validity of self-reported height and weight and derived body mass index in middle-aged and elderly individuals in Australia. Aust N Z J Public Health. 2011;35(6):557–563. doi: 10.1111/j.1753-6405.2011.00742.x. [DOI] [PubMed] [Google Scholar]
- 61.Nigg CR, Burbank P, Padula C, Dufresne R, Rossi JS, Velicer WF, Laforge RG, Prochaska JO. Stages of change across ten health risk behaviors for older adults. The Gerontologist. 1999;39:473–482. doi: 10.1093/geront/39.4.473. [DOI] [PubMed] [Google Scholar]
- 62.Prochaska JO. Multiple Health Behavior Research represents the future of preventive medicine. Prev Med. 2008;46(3):281–285. doi: 10.1016/j.ypmed.2008.01.015. [DOI] [PubMed] [Google Scholar]