Abstract
Purpose of review
To provide a summary and discussion of cockroach allergy and clinical trials of cockroach allergen immunotherapy.
Recent findings
Cockroach allergen exposure among sensitized children is increasingly recognized as a key factor contributing to asthma morbidity. Recent trials suggest that cockroach immunotherapy has promise as a treatment strategy with studies demonstrating immunomodulatory and clinical effects. However, a few obstacles need to be overcome to realize the full potential of this treatment modality as cockroach allergic patients often exhibit complex sensitization patterns to multiple cockroach-associated proteins and an immunodominant allergen has not been identified. These factors have made it difficult to produce standardized cockroach allergen extracts that are potent and provide the broad allergen profiles needed for optimal treatment. There have been important advances in the identification and cloning of cockroach allergens and several strategies are being developed to provide therapeutic cockroach allergen products with enhanced clinical efficacy.
Summary
Allergen immunotherapy has the capability of modulating the immune response to cockroach allergen and has potential as a valuable treatment modality. Further studies of the clinical efficacy along with the development of improved therapeutic products are needed to advance our knowledge and realize the full potential of this promising therapy.
Keywords: cockroach, immunotherapy, asthma, IgE
Introduction
Cockroaches are ubiquitous scavenger insects that can quickly become difficult to eradicate pests in any building with ingress and adequate shelter, food and water.1 The combination of cockroach exposure and allergic sensitization is a key factor contributing to asthma morbidity, particularly in urban areas where high-level cockroach allergen exposure is common and asthma burden is high. Although, cockroach exposure control strategies may be helpful, they are difficult to maintain and exposure may continue outside the home. In contrast, effective cockroach immunotherapy has the potential of modifying the course of asthma and providing sustained clinical benefit. A limited number of clinical trials using cockroach allergen extract have demonstrated improvement in both immunological and clinical parameters. Particularly in view of recent scientific advancements, cockroach immunotherapy has substantial potential as a useful treatment modality for sensitized patients with asthma.
The importance of cockroach sensitization and exposure in asthma
Cockroach exposure has been linked to subsequent cockroach sensitization and allergic respiratory symptoms. Among sensitized individuals, exposure is associated with high asthma morbidity, particularly among children living in urban environments.
Cockroach exposure is linked to sensitization and bronchospasm
Cockroach exposure at threshohold levels greater than 1–2 U/gram of allergen in settled dust increases the risk of allergic sensitization.2–4 Furthermore, immediate and late phase asthmatic reactions have been demonstrated in cockroach-sensitized asthmatics after bronchial challenge with the cockroach allergen.5
The role of cockroach allergen exposure in chronic asthma
In a landmark study by Rosenstreich et al., a strong association was discovered between asthma morbidity and the combination of cockroach sensitization with exposure to high levels of cockroach allergen in the homes of inner-city children with asthma.6 Specifically, sensitized children exposed to high levels of the allergen in the their bedroom had more hospitalizations, unscheduled medical visits, days of wheezing, and days with change in the care giver’s plans. Subsequent reports have confirmed these findings7,8 Furthermore, cockroach sensitization may have a greater effect on asthma morbidity than dust mite or pet allergy. 7
In addition to asthma morbidity, early exposure to cockroach in children with atopic parents may increase the risk of developing asthma. Compared to children without exposure, those exposed to cockroach allergens levels greater than 2U/g of settled dust were 35.9 times more likely to have asthma.9
Current management strategies for cockroach associated asthma
A multifaceted, stepwise approach to managing allergic asthma is recommended in the 2007 NHLBI Asthma Treatment Guidelines.10 This includes allergen avoidance, standard asthma pharmacotherapy and consideration of allergen immunotherapy in patients older than 5 years that require low to medium dose controller therapy.
Cockroach environmental control
Varying degrees of success have been reported using strategies to limit environmental cockroach exposure. A discussion of cockroach abatement strategies were recently summarized in a comprehensive Practice Parameter issues by a Joint Task Force of the three major U.S. professional allergy organizations .1 Importantly, strategies that effectively lower cockroach allergen levels appear to also result in clinical benefit. For instance, a controlled trial of a 1-year home-based, tailored environmental control and behavioral intervention targeting indoor allergens (including cockroach) and environmental tobacco smoke was completed inner-city children with asthma.11 Not only was the home-based intervention successful in reducing cockroach allergen, but the successful strategy was also linked to reduced cockroach-associated asthma morbidity.
Cockroach-specific allergen immunotherapy
The efficacy of allergen immunotherapy for asthma is well-supported by the literature12, but surprisingly few controlled studies have been published that specifically assess allergen-specific immunotherapy for cockroach-allergic patients.
Kang et al. completed a 5 year-long, trial of subcutaneous mixed cockroach (German, American and Oriental) immunotherapy among 28 cockroach sensitive asthma patients. The “active treatment” group received immunotherapy with cockroach extract (a cumulative dose of 65,600 protein nitrogen units over the 60 month trial) in addition to other inhalant allergens that elicited a significant wheal and flare on skin testing. The “control” group received immunotherapy with all relevant allergens except cockroach. Those receiving cockroach extract had reduced symptom and medication scores, an increase in cockroach-specific blocking antibody and blunted in vitro basophil histamine release after receiving 5 years of cockroach allergen.13. However, a limitation of this study was that although 11 of the 15 subjects in the active group completed the study, only 2 of 13 receiving control injections did so.
In 2011, Srivasta et al, completed a double-blind, placebo-controlled trial of American cockroach immunotherapy in patients with asthma, rhinitis or both.14 Forty-two patients completed 1 year of immunotherapy with a 1 ml volume maintenance dose of a lab-prepared aqueous extract containing 3 mg/ml of protein from American cockroach. Compared to placebo, after 1 year there was a significant reduction in symptoms, improvement in bronchial hyper-reactivity and increase in specific IgG4. After 2-years of immunotherapy there was significant reduction in symptoms and medication use as well as a reduction in specific IgE, and increase in cockroach specific IgG4.
A 2014 report by Wood et al. summarized information from 4 phase I/II pilot studies designed to provide safety and immunological data related to cockroach immunotherapy. 15 Three of the four studies focused on the sublingual administration route and were completed by the multicenter Inner City Asthma Consortium. Although direct clinical responses were not assessed, biomarkers including cockroach-specific IgE, cockroach-specific IgG4, and antigen-IgE complex binding to B cells (FAB), were measured as a reflection of the biologic activity of treatment. The studies are briefly summarized below and key characteristics for each study are listed in Table 1.
Table 1.
Summary of the four Inner City Asthma Consortium cockroach immunotherapy studies. Reproduced with permission from [15]
| SCSS | BioCSI | BioCSI2 | SCITCO | |
|---|---|---|---|---|
| Treatment | SLIT | SLIT | SLIT | SCIT |
| Design | Open label, single site |
DBPC, multi- center |
DBPC, low and higher dose, multi-center |
Open label, single site |
| CR antigen dose |
Bla g 2 – 4.2 µg Bla g 1 – 50 µg |
Bla g 2 – 4.2 µg Bla g 1 – 50 µg |
Low: Bla g 2 – 4.2 µg Bla g 1 – 50 µg High: Bla g 2 – 16.8 µg Bla g 1 – 202 µg |
Bla g 2 – 6 µg Bla g 1 – 120 µg |
| Primary outcome |
Adverse events | Δ in CR-specific IgE |
Δ in CR-specific IgE |
Adverse events |
| Treatment Duration |
14 days | 6 months | 3 months | 6 months |
| Participant Ages |
Children and adults |
Adults | Children | Adults |
| Sample Size | 27 | 54 | 99 | 10 |
The Sublingual Cockroach Safety Study (SCSS) and the Cockroach Subcutaneous Immunotherapy in Cockroach-Sensitive Adults (SCITCO), were open-label safety studies. In the SCSS study, nine adults, nine children age 8–17 years and nine 5–7 year-old children with perennial allergic rhinitis completed a single-day, supervised 8-dose escalation to a sublingual maintenance dose of 0.42 ml (3685 BAU) of commercially available glycerinated extract (Greer Pharmaceuticals, Lenoir NC) then 13 additional days receiving a single maintenance dose. Mild adverse reactions (primarily mouth and throat itching) were common but no severe reactions were apparent. SCITCO included 10 adults using the same German cockroach extract subcutaneously during an 11–18 week dosage escalation schedule (~2 doses weekly) to a maintenance dose of 0.6 ml (5142 BAU). This was followed by weekly maintenance injections to complete the 6 month trial. No treatment-related serious or severe adverse events were reported.
The Biomarker based Cockroach Sublingual Immunotherapy Study (BioCSI), was a double-blind, placebo-controlled, randomized trial in 54 cockroach allergic adults with perennial allergic rhinitis and/or asthma. Similar to the SCSS safety study, those on active therapy received a maintenance dose of 0.42 ml (3685 BAU) of glycerinated German cockroach extract for 6 months. A nearly 2-fold higher post-treatment increase in specific IgE (a finding associated with clinical efficacy in trial with other allergens) was observed in the active compared to the placebo treated subjects (P<0.0001). However, post-treatment CR specific IgG4, blocking antibody response and cockroach skin tests responses, were not significantly different between active and placebo groups.
A second randomized trial of sublingual therapy, BioCSI2, was completed among 89 cockroach sensitized children between 5–17 years with perennial allergic rhinitis or mild asthma over a 3 month period. In addition to a placebo arm and treatment group receiving 0.42 ml of glycerinated extract, a third treatment arm included a 4-fold higher cockroach extract dose (0.84 ml twice daily). For the primary study outcome, 43% of those in the high dose group, 40% of those in the low dose and 11% receiving placebo exhibited a 3-fold increase in CR specific IgE. Both active treatments were associated with larger increases in cockroach specific IgE compared to placebo (P<0.01). Surprisingly, greater IgE responses were seen among the low dose group versus the high dose group (P=0.04). Furthermore, statistically significant increases in cockroach-specific IgG and IgG4 were observed in the high dose group but no changes in a blocking antibody assay were observed.
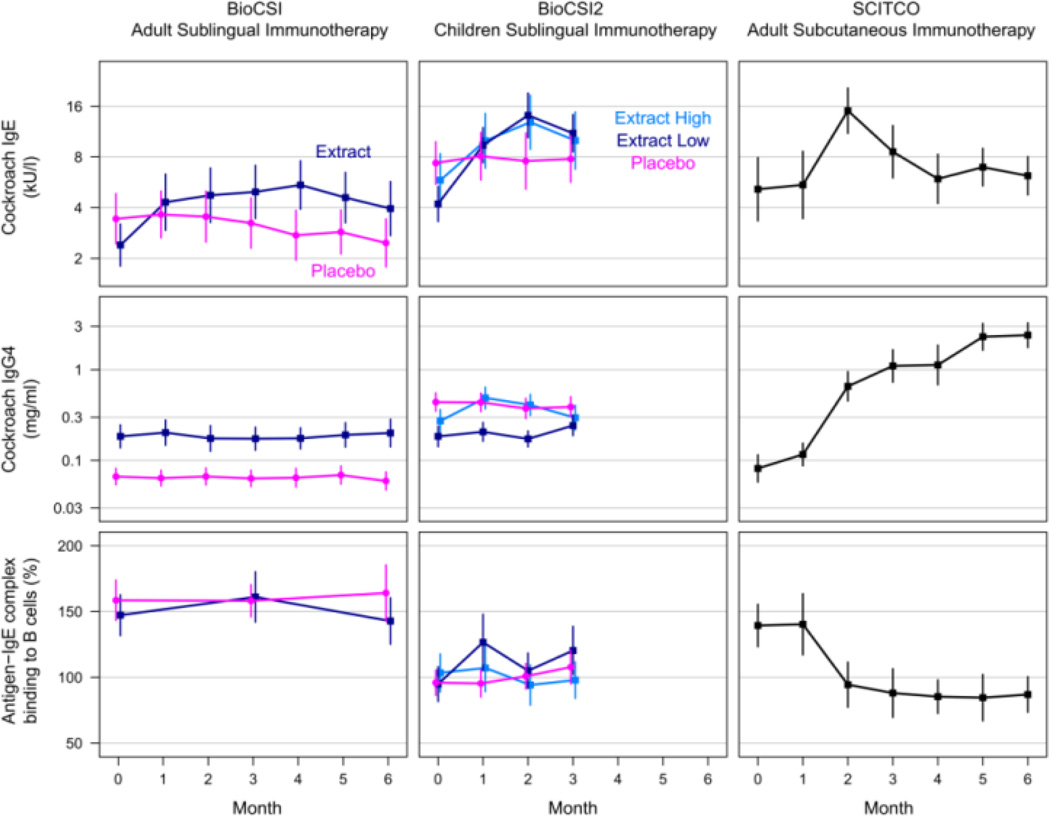
Biomarker responses from the two BIOCSI studies (sublingual administration) were compared to those observed in the adults that participated in the SCITCO subcutaneous safety study. (Figure 1) Subcutaneous therapy was associated with an increase from baseline in CR specific IgE (1.78-fold increase, p=0.02), IgG4 (12.95-fold increase, p<0.001), and blocking antibody (43% inhibition of B-cell binding, p<0.001). The IgE responses were comparatively similar in all three studies but only the subcutaneous route of administration was associated with a robust IgG4 response.
Figure 1.
Comparison of immune responses to two German cockroach sublingual Immunotherapy (BioCSI and BioCSI2 studies) to subcutaneous immunotherapy (SCITCO study) Reproduced with permission from [15]
Based on these observations and previous studies, the authors concluded that with currently available extracts, subcutaneous administration of cockroach immunotherapy was more immunologically active and would be more likely to be clinically effective. Furthermore, the results supported further study regarding a potential therapeutic role for German cockroach immunotherapy.
Unique features of cockroach allergy that may impact the efficacy of immunotherapy
Although further studies are needed, the trials discussed above demonstrate treatment –related immune alterations. However, targeting relevant cockroach allergens for individual patients is made imprecise since underlying immune responses to environmental cockroach exposure appear to be variable and complex Furthermore, several characteristics of currently available cockroach extracts may limit their potential to provide optimal clinical efficacy.
Immune responses to cockroach allergens are highly variable
Over 4,000 cockroach species have been identified. However, most allergen-related research has been focused on the common household invaders, Blattella germanica (the German cockroach) and Periplaneta Americana (the American cockroach).16 Prominent sources of environmental cockroach allergen exposure include fecal matter containing dried secretions from the insect’s digestive system (frass) and the shed or desiccated remains of the insect. Inhalation results in exposure to multiple proteins that are capable of eliciting IgE immune responses in humans. There is a substantial degree of homology and variable IgE crossreactivity between recognized German cockroach allergens (designated Bla g 1 through Bla g 8 and Bla g 11) and some homologous groups from the American cockroach (Per a 1, 3, 6, 7, 9 and Per a 10).17 Satinover et al measured specific IgE responses to 5 recombinant allergens [rBla g 1, 2, 4, 5 and Per a 7 (the American cockroach homolog of Bla g 7)] among 118 adults.18 Although there was a moderately high prevalence of IgE antibody to rBla g 2 (54.4%) and rBla g 5 (37.4%), the overall patterns of binding to the 5 proteins were unique for each individual. Furthermore, individual IgE responses also vary in regard to multiple conformational allergenic epitopes contained within a single allergen such as Bla g 2.19
Limitations of current cockroach allergen extracts
Allergen extracts contain a complex mixture of major and minor allergens. Major allergens represent components to which the majority of sensitized patients develop allergic sensitivity, whereas minor allergens are recognized by limited numbers of patients. Prior reports have identified Bla g 2 and Bla g 5 as potential major allergens.18,20 However, other allergens may approach this threshold in certain populations. For instance, skin testing with recombinant allergens demonstrated Per a 7 as the most common cockroach-related sensitizing protein among Brazilian citizens with allergy and asthma. 21
Some well-characterized allergen extracts can be standardized by quantitation of a strongly immunodominant allergen that is highly correlated to the prevalence of sensitization (e.g. cat with Fel d 1). A similar allergen has not been demonstrated for cockroach allergy. The lack of an immunodominant allergen and the complex patterns of IgE response to multiple cockroach allergens have contributed to the difficulty of producing standardized extracts with contents that would promote efficacy for a high percentage of heterogeneously sensitized patients.
Published reports describing commercially available extracts using natural cockroach source materials suggest that they have inconsistent content of components such as Bla g 2 and relatively low and variable potency when compared to reference standards from the Center for Biological Evaluation and Research.22,23 For instance, the biological potencies of three glycerinated German cockroach extracts from U.S. manufacturers were compared, using both in vitro testing and intradermal skin test titration methods, among cockroach sensitized adults.23 Although the three extracts could be considered roughly bioequivalent on the basis of similar intradermal skin dilution testing, the estimated potency varied 4.9 fold between the most and least potent extract. In addition, the mean potency of the extracts was 3300 BAU/ml, somewhat lower than that of commonly used standardized extracts (5000 to 100,000 BAU/ml.)
Recombinant techniques may improve the efficacy of cockroach immunotherapy
Although of relatively low potency, immunotherapeutic doses are possible with current German cockroach allergen extracts.23 However, consensus and expert opinion remains that “very low dose immunotherapy is not effective, and high doses are more effective than moderate doses”.24 This suggests that more potent, targeted products for cockroach-based immunotherapy would provide incremental benefit.
Molecular cloning techniques allowing production of large amounts of single recombinant allergens have led to advances in the identification and characterization of relevant cockroach allergens. Cloning and other scientific advancements are also refining diagnostic methods and leading the way to improved therapeutics.25
Recombinant allergens
Since sensitized patients exhibit complex IgE-binding patterns to multiple cockroach allergens, careful characterization of an individual’s sensitization profile may be critical. It follows that vaccines might be more effective when containing adequate therapeutic amounts of all, or a majority of the allergens to which an individual is sensitized. Recombinant allergens have played a pivotal role, and continue to promote the discovery or confirmation of new cockroach allergens.26 Theoretically, recombinant allergens can correct the primary limitations of crude cockroach extracts by providing individual proteins for potent and targeted treatment that can be tailored to a patient’s specific immune response.
Recombinant peptides
Similar technology has fostered the identification of allergen-derived peptide epitopes capable of being presented to T cells in the context of common Class II HLA molecules on antigen presenting cells. The subsequent T cell response may involve Th2 cytokine production thus promoting the generation of IgE. However, other peptides may induce opposing or dampened Th2 responses by stimulating Th1 or regulatory cytokine responses, respectively.27 Oseroff et al. characterized T cell responses to epitopes derived from several Bla g allergens among 34 adults with allergic rhinitis or asthma who were also cockroach sensitive.28 Responses to 25 distinct antigenic peptide epitopes were identified. Similar to allergenic proteins, heterogeneous individual patterns of response were apparent. However, 5 peptides were responsible for greater than half the responses with 13 peptides accounting for 90% of the total. A particularly intriguing finding from this study was that an individual’s T cell responses to specific Bla g allergens could be differentially polarized toward production of Th1 or Th2 cytokines. These findings could have clear potential for individually tailored therapy with specific allergens and peptied that result in deviation away from Th2 responses.
Conclusions
Sensitization with exposure to cockroach allergen contributes to asthma morbidity. Cockroach immunotherapy modulates immune responses and appears to provide clinical benefit in a limited number of trials that support its potential usefulness in the management of cockroach-associated asthma. Although the lack of standardized extracts and complex immune reaction patterns to cockroach antigen represent barriers to realizing the full potential of cockroach desensitization, scientific advances including the use of recombinant allergen techniques are poised to further enhance our understanding of the complex antibody and cellular responses to cockroach antigen and eventually spur the development of improved immunotherapeutics.
Key points.
Cockroach exposure among sensitized patients has been identified as a key factor contributing to asthma morbidity, particularly among children in U.S. inner cities where high-level cockroach allergen exposure is common and asthma burden is high.
A limited number of trials of cockroach immunotherapy, using currently available aqueous crude cockroach extracts, have demonstrated improvement in immunological and clinical parameters.
The lack of an immunodominant allergen and complex patterns of IgE responses to multiple cockroach allergens have made if difficult to produce standardized allergenic extracts with content that would promote high efficacy for a large proportion of heterogeneously sensitized patients.
Molecular cloning techniques have allowed the production of single recombinant allergens and led to advances in the characterization of cockroach allergens and hold promise for leading to refined diagnostic methods and superior therapeutic agents.
Acknowledgements
E. Zoratti participated in clinical trials of cockroach immunotherapy with the NIAID funded Inner City Asthma Consortium (N01AI2008044) Other research support unrelated to this review: NIAID (P01 AI089473, R01AI051598), NHLBI (R01 HL113010, R01HL114981) and the Fund for Henry Ford Hospital.
Footnotes
G. Bassirpour and E. Zoratti report no conflicts of interest.
REFERENCES
- 1. Portnoy J, Chew GL, Phipatanakul W, et al. Environmental assessment and exposure reduction of cockroaches: A practice parameter. J Allergy Clin Immunol. 2013;132(4):802-8.e1–802-8.e25. doi: 10.1016/j.jaci.2013.04.061. This publication is an excellent resource is focused on the review of studies targeting the assessment and control of cockroach exposure and provides recommendations that are categorized and based on the strength of existing scientific data.
- 2.Chew GL, Perzanowski MS, Canfield SM, et al. Cockroach allergen levels and associations with cockroach-specific IgE. J Allergy Clin Immunol. 2008;121(1):240–245. doi: 10.1016/j.jaci.2007.08.024. [DOI] [PubMed] [Google Scholar]
- 3.Huss K, Adkinson NF, Jr, Eggleston PA, Dawson C, Van Natta ML, Hamilton RG. House dust mite and cockroach exposure are strong risk factors for positive allergy skin test responses in the childhood asthma management program. J Allergy Clin Immunol. 2001;107(1):48–54. doi: 10.1067/mai.2001.111146. [DOI] [PubMed] [Google Scholar]
- 4. Perzanowski MS, Chew GL, Divjan A, et al. Early-life cockroach allergen and polycyclic aromatic hydrocarbon exposures predict cockroach sensitization among inner-city children. J Allergy Clin Immunol. 2013;131(3):886–893. doi: 10.1016/j.jaci.2012.12.666. An intriguing report that highlights the potential importance of early life co-exposures (in this case polycyclic hydrocarbons) along with environmental cockroach allergen on the risk of sensitization.
- 5.Kang B, Vellody D, Homburger H, Yunginger JW. Cockroach cause of allergic asthma. its specificity and immunologic profile. J Allergy Clin Immunol. 1979;63(2):80–86. doi: 10.1016/0091-6749(79)90196-9. [DOI] [PubMed] [Google Scholar]
- 6.Rosenstreich DL, Eggleston P, Kattan M, et al. The role of cockroach allergy and exposure to cockroach allergen in causing morbidity among inner-city children with asthma. N Engl J Med. 1997;336(19):1356–1363. doi: 10.1056/NEJM199705083361904. [DOI] [PubMed] [Google Scholar]
- 7.Gruchalla RS, Pongracic J, Plaut M, et al. Inner city asthma study: Relationships among sensitivity, allergen exposure, and asthma morbidity. J Allergy Clin Immunol. 2005;115(3):478–485. doi: 10.1016/j.jaci.2004.12.006. [DOI] [PubMed] [Google Scholar]
- 8.Wang J, Visness CM, Calatroni A, Gergen PJ, Mitchell HE, Sampson HA. Effect of environmental allergen sensitization on asthma morbidity in inner-city asthmatic children. Clin Exp Allergy. 2009;39(9):1381–1389. doi: 10.1111/j.1365-2222.2009.03225.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Litonjua AA, Carey VJ, Burge HA, Weiss ST, Gold DR. Exposure to cockroach allergen in the home is associated with incident doctor-diagnosed asthma and recurrent wheezing. J Allergy Clin Immunol. 2001;107(1):41–47. doi: 10.1067/mai.2001.111143. [DOI] [PubMed] [Google Scholar]
- 10.National Asthma Education and Prevention Program. Expert panel report 3 (EPR-3): Guidelines for the diagnosis and management of asthma-summary report 2007. J Allergy Clin Immunol. 2007;120(5 Suppl):S94–S138. doi: 10.1016/j.jaci.2007.09.043. [DOI] [PubMed] [Google Scholar]
- 11.Morgan WJ, Crain EF, Gruchalla RS, et al. Results of a home-based environmental intervention among urban children with asthma. N Engl J Med. 2004;351(11):1068–1080. doi: 10.1056/NEJMoa032097. [DOI] [PubMed] [Google Scholar]
- 12.Abramson MJ, Puy RM, Weiner JM. Injection allergen immunotherapy for asthma. Cochrane Database Syst Rev. 2010;8 doi: 10.1002/14651858.CD001186.pub2. (8):CD001186. doi(8):CD001186. [DOI] [PubMed] [Google Scholar]
- 13.Kang BC, Johnson J, Morgan C, Chang JL. The role of immunotherapy in cockroach asthma. J Asthma. 1988;25(4):205–218. doi: 10.3109/02770908809071367. [DOI] [PubMed] [Google Scholar]
- 14.Srivastava D, Gaur SN, Arora N, Singh BP. Clinico-immunological changes post-immunotherapy with periplaneta americana. Eur J Clin Invest. 2011;41(8):879–888. doi: 10.1111/j.1365-2362.2011.02480.x. [DOI] [PubMed] [Google Scholar]
- 15. Wood RA, Togias A, Wildfire J, et al. Development of cockroach immunotherapy by the inner-city asthma consortium. J Allergy Clin Immunol. 2014;133(3):846–52.e6. doi: 10.1016/j.jaci.2013.08.047. This manuscript includes the most recent clinical data related to cockroach immunotherapy. The report summarizes 4 studies designed to assess safety and the impact of sublingual and subcutaneous immunotherapy on immune biomarkers. The results demonstrate immunomodulatory effects of both sublingual and subcutaneous therapy and suggest that subcutaneous therapy is more immunologically active using commercially available cockroach extract.
- 16. Arruda LK, Pomes A. Every cockroach is beautiful to its mother. Int Arch Allergy Immunol. 2013;161(4):289–292. doi: 10.1159/000350207. A well written review focused on advancements in the identification of cockroach allergens.
- 17. Arruda LK, Barbosa MC, Santos AB, Moreno AS, Chapman MD, Pomes A. Recombinant allergens for diagnosis of cockroach allergy. Curr Allergy Asthma Rep. 2014;14(4) doi: 10.1007/s11882-014-0428-6. :428-014-0428-6. The importance of variable allergen sensitization profiles in cockroach allergic patients is emphasized in this review. The review describes the challenges of identifying clinically relevant cockroach allergens and summarizes studies that demonstrate the utility of recombinant allergens for the diagnosis of cockroach sensitization.
- 18.Satinover SM, Reefer AJ, Pomes A, Chapman MD, Platts-Mills TA, Woodfolk JA. Specific IgE and IgG antibody-binding patterns to recombinant cockroach allergens. J Allergy Clin Immunol. 2005;115(4):803–809. doi: 10.1016/j.jaci.2005.01.018. [DOI] [PubMed] [Google Scholar]
- 19.Glesner J, Wunschmann S, Li M, et al. Mechanisms of allergen-antibody interaction of cockroach allergen bla g 2 with monoclonal antibodies that inhibit IgE antibody binding. PLoS One. 2011;6(7):e22223. doi: 10.1371/journal.pone.0022223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Arruda LK, Ferriani VP, Vailes LD, Pomes A, Chapman MD. Cockroach allergens: Environmental distribution and relationship to disease. Curr Allergy Asthma Rep. 2001;1(5):466–473. doi: 10.1007/s11882-001-0035-1. [DOI] [PubMed] [Google Scholar]
- 21. Barbosa MC, Santos AB, Ferriani VP, Pomes A, Chapman MD, Arruda LK. Efficacy of recombinant allergens for diagnosis of cockroach allergy in patients with asthma and/or rhinitis. Int Arch Allergy Immunol. 2013;161(3):213–219. doi: 10.1159/000346318. This study in a Brazilian population identifies Per a 7 as major cockroach allergen in this population and highlights the variablility in cockroach sensitization patterns that may be the result of diverse geographic exposure to cockroach and cross-reacting allergens.
- 22.Patterson ML, Slater JE. Characterization and comparison of commercially available german and american cockroach allergen extracts. Clin Exp Allergy. 2002;32(5):721–727. doi: 10.1046/j.1365-2222.2002.01397.x. [DOI] [PubMed] [Google Scholar]
- 23.Slater JE, James R, Pongracic JA, et al. Biological potency of german cockroach allergen extracts determined in an inner city population. Clin Exp Allergy. 2007;37(7):1033–1039. doi: 10.1111/j.1365-2222.2007.02751.x. [DOI] [PubMed] [Google Scholar]
- 24.Nelson HS. Chapter 87: Injection immunotherapy for inhalant allergens. In: Adkinson N, Bochner BS, Burks AW, et al., editors. eds. Middleton’s allergy principles and practice. 8th ed. Philadelphia, PA 19103-2899: Elsevier Saunders; 2014. pp. 1416–1437. [Google Scholar]
- 25. Pomes A, Arruda LK. Investigating cockroach allergens: Aiming to improve diagnosis and treatment of cockroach allergic patients. Methods. 2014;66(1):75–85. doi: 10.1016/j.ymeth.2013.07.036. A recent update focused on summarizing the advancements in the identification and molecular cloning of cockroach allergens and their potential for application in practice.
- 26. Khurana T, Collison M, Chew FT, Slater JE. Bla g 3: A novel allergen of german cockroach identified using cockroach-specific avian single-chain variable fragment antibody. Ann Allergy Asthma Immunol. 2014;112(2):140–145.e1. doi: 10.1016/j.anai.2013.11.007. This paper describes the application a new technique using cockroach-specific avian single-chain variable fragment antibodies as a method to isolate a novel allergen, Bla g 3, from whole-body cockroach allergen extract.
- 27.Schulten V, Oseroff C, Alam R, et al. The identification of potentially pathogenic and therapeutic epitopes from common human allergens. Ann Allergy Asthma Immunol. 2013;110(1):7–10. doi: 10.1016/j.anai.2012.10.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Oseroff C, Sidney J, Tripple V, et al. Analysis of T cell responses to the major allergens from german cockroach: Epitope specificity and relationship to IgE production. J Immunol. 2012;189(2):679–688. doi: 10.4049/jimmunol.1200694. This is the first publication to describe the spectrum of T cell responses to cockroach-derived allergens. Findings of substantial importance included identification of peptides that appeared to drive isolated Th1 responses and the fact that IgE production did not necessarily correlate with T cell responses to cockroach allergens.