Abstract
Lineage-committed effector CD4+ T cells are generated at the peak of the primary response and are followed by heterogeneous populations of central and effector memory cells. Here we review the evidence that Th1 effector cells survive the contraction phase of the primary response and become effector memory cells. We discuss the applicability of this concept to the Th2, Th17, follicular helper cell, and induced regulatory T cell lineages. We also discuss how central memory cells are formed with an emphasis on the role that B cells play in this process.
The process of CD4+ memory T cell formation begins when the T cell antigen receptors (TCR) on naïve clones bind to major histocompatibility complex II-foreign peptide complexes (pMHCII) on antigen-presenting cells (APC)1 in secondary lymphoid organs. Signals through the TCR and APC-derived costimulatory molecules such as CD28 cause the naïve cells to divide and become effector cell lymphoblasts2, 3. Depending on the nature of cytokines produced by the innate immune system, these effector cells undergo a differentiation process that involves expression of specific transcription factors that control the capacity to produce certain lymphokines4. For, example effector cell differentiation in the presence of IL-12 promotes expression of T-bet, which commits the cells to the Th1 program of IFN-γ, but not IL-4 or IL-17 production; whereas differentiation in the presence of IL-4 promotes expression of GATA-3, which commits the cells to the Th2 program of IL-4, but not IFN-γ or IL-17 production. This differentiation also involves expression of homing receptors that facilitate the migration of effector cells to non-lymphoid sites of inflammation5 where these cells produce their cytokines to aid in antigen clearance. The number of effector cells peaks about a week into the response, at least in the case of antigens that are rapidly cleared from the body.
About 90% of the effector cells then die during the 1-2 week long contraction phase, leaving a residual population of long-lived cells. These cells, which are called memory cells, are predominantly quiescent but capable of intermittent self-renewal and long-term survival in the absence of the inducing pMHCII ligand6. Memory cells are heterogenous, however, and proposed to exist in at least two classes7. Effector memory cells (Tem) express homing receptors that facilitate migration to non-lymphoid sites of inflammation5 and produce a variety of microbicidal cytokines including IFN-γ, IL-4, and IL-5 within several hours of TCR stimulation. Central memory cells (Tcm) do not produce any of the prototypic effector cell lineage cytokines immediately after stimulation through the TCR, although they secrete IL-2 and proliferate extensively and acquire effector lymphokine production later. These cells express CD62L and CCR7, which are involved in migration through lymph nodes and mucosal lymphoid organs and positioning in the T cell areas of these organs8. It was therefore postulated that Tcm circulate through these locations, and would likely undergo secondary responses there7. This prediction was confirmed by studies in mice in which IL-2-producing CD4+ memory T cells were found primarily in the lymph nodes while IFN-γ-producing cells were located in the non-lymphoid organs5.
Here we will focus on two questions raised by these elegant models – what is the relationship between lineage-committed effector cells present at the peak of the response and the Tem that survive the contraction phase, and how are Tem and Tcm formed? We will review the evidence that some Th1, Th2, and Th17 effector cells become Tem and discuss B cells as drivers of the Tem/Tcm decision.
Evidence that lineage-committed effector cells become memory cells?
A key question in the immune memory field is how do the effector lymphoblasts present at the peak of the primary response relate to the quiescent memory cells that survive the contraction phase? A strong case can be made that some Th1 effector cells simply return to a quiescent state and become Th1 effector memory cells. Lohning and colleagues showed that highly purified in vitro differentiated Th1 cells derived from TCR transgenic naïve cells survived with a half-life of about 70 days after transfer into non-lymphophenic naïve recipients9. In addition, these investigators used cytokine capture flow cytometry to isolate IFN-γ-producing lymphocytic choriomeningitis virus (LCMV) pMHCII-specific TCR transgenic effector cells from adoptive hosts at the peak of acute infection, and showed that these cells survived with the same 25 day half-life after transfer in new recipients as did non-IFN-γ-producing effector cells. The reason that the in vivo-generated effector cells yielded memory cells with shorter lifespans than in vitro-generated cells was not clear, although excessive interclonal completion is a possibility. The memory cells derived from the IFN-γ-producing effector cells expressed the IL-7R, lacked CD62L expression, and rapidly produced IFN-γ but not IL-4 during a secondary response. Similarly, Harrington et al.10 used adoptive transfer of TCR transgenic LCMV pMHCII-specific CD4+ T cells from IFN-γ reporter mice to show that IFN-γ-producing effector cells from LCMV-infected mice gave rise to long-lived memory T cells. The memory cell population contained CD62Llow and CD62Lhigh subsets suggesting that both Tem and Tcm could be derived from IFN-γ-producing precursors in this case.
Thus, both studies lead to the conclusion that some Th1-like effector cells can give rise to Th1 memory cells with the properties ascribed by Sallusto and Lanzavecchia11 to Tem. It is not clear how many of the cells in the effector Th1 population became memory cells and whether this conversion was stochastic or determined by a small subset of dedicated precursors. In addition, the results of these studies must be viewed with some caution since they relied on adoptive transfer of TCR transgenic T cells12, 13, which has been shown to influence memory cell generation and survival due excessive clonal competition14-18.
To avoid the issues related to adoptive transfer of TCR transgenic T cells, our laboratory studied effector to memory cell conversion by tracking endogenous polyclonal CD4+ T cells specific for a pMHCII derived from Listeria monocytogenes bacteria using a pMHCII tetramer-based cell enrichment method19. Intravenous infection with an attenuated strain of L. monocytogenes, which was cleared within several days, induced two effector cell populations of roughly equal size in the spleen and lymph nodes; one T-bet+ and one T-betlow 20. The T-bet+ cells lacked RORγt and CCR7 and rapidly produced IFN-γ but not canonical Th2 or Th17 cytokines when stimulated in vivo with pMHCII. These effector cells were therefore indistinguishable from Th1 cells. The T-betlow effector cells expressed CCR7 and produced none of the canonical lineage-defining cytokines immediately (although they could produce IFN-γ later) and therefore resembled Tcm despite being present during the effector phase of a Th1-driven response. Following the contraction phase and loss of 90% of the effector cells, the resulting memory cell population again consisted of equal subsets of T-bet+ CCR7− and T-betlow CCR7+ cells. These results are consistent with a model in which 10% of the cells within Th1 and non-Th1 effector cell populations survived the contraction phase and became Th1 effector memory cells and some other type of memory cells, respectively. Other groups reported similar results in other infection models where naive CD4+ T cells from pMHCII-specific TCR transgenic mice were tracked after transfer into normal recipients and infection21, 22.
Both T-bet+ CCR7− and T-betlow CCR7+ L. monocytogenes pMHCII-specific memory T cell populations declined slowly over time with a half-life of 50 days, while maintaining an approximately 50:50 ratio for almost a year20. The stability of this ratio suggests that the Th1 effector memory cells did not convert into Tcm. Rather, both subsets behaved as meta-stable non-convertible populations. The relative stability of these populations may be related to slow IL-15-driven homeostatic proliferation23, although this process must be exceeded by death since both memory cell populations slowly decayed. LCMV pMHCII-specific CD4+ memory T cells induced by acute infection also declined slowly over time24. Although pMHCII-specific CD4+ T cells induced by chronic viral infection were numerically stable, it is difficult to argue that these were memory cells since the relevant pMHCII was always present25. These results suggest that the type of stable pMHC-independent memory described for CD8+ T cells6 is difficult if not impossible to achieve for CD4+ T cells. This difference may be related to the efficiency of IL-15 sensing, with CD8+ memory T cells having the advantage due to expression of more IL-15 receptors26, or more memory stem cells27.
There is also evidence that Th2 effector cells can become Tem after the contraction phase of the immune response. In vitro differentiated Th2 cells derived from TCR transgenic naïve cells survived long term after transfer into non-lymphophenic naïve recipients9. Using an MHCII tetramer containing a peptide from a Leishmania major protein and an IL-4-GFP reporter mouse, Stetson and colleagues demonstrated that IL-4-competent cells are generated early after parasite infection28. Some of the parasite pMHCII-specific IL-4 competent effector cells may have become memory cells because such cells remained long after the parasite was eliminated by antibiotic treatment29. These long-lived Th2-like cells fit the definition of Tem based on low expression of CD62L and high expression of several gut homing molecules including α4β7 integrin. These findings have been confirmed in several other parasite infections including Trichuris muris30. Although these results are consistent with Th2 effector cells surviving the contraction phase to become Th2 memory cells, a detailed phenotypic study of a polyclonal parasite pMHCII-specific population throughout the expansion, contraction, and memory cell phases after infection is needed to cement this conclusion.
The case for Th17 effector cells entering the memory cell pool is less clear. Although L. monocytogenes pMHCII-specific Th17 effector cells expressing RORγt+ but not T-bet were generated after intranasal infection, the number of RORγt+ cells declined after the contraction phase at a faster rate than Th1 effector memory cells induced by intravenous infection20. The RORγt+ cells did not express CD27, which is associated with short lifespan31. It is therefore possible that the RORγt+ effector cells died20.
It is also possible that the effector cells simply lost expression of RORγt. A recent study showed that T cells that produced IL-17 early during acute fungal infection lost the capacity to produce IL-17 over time32. In addition, there is mounting evidence for plasticity within the Th17 lineage. Numerous studies have demonstrated that in vitro-derived Th17 cells can turn into IFN-γ producers after transfer into mice33-36. Additionally, T-bet+ RORγt+ clones generated by injection of a peptide from myelin basic protein36, 37 reverted to Th1 cells in vivo. A population of IFN-γ and IL-17 double-producing T cells has also been identified in human peripheral blood38-40. The existence of these bipolar T cells suggests that certain priming conditions can generate effector cells that keep the Th1 or Th17 options open before committing to a final memory cell lineage. The fact that the Th1 lineage was the final choice may be explained by inhibition of RORγt by T-bet41. This idea is supported by the fact that conversion of Th1 cells to Th17 cells has not been reported to date.
The evidence that Treg cells can enter the memory pool is scarce. Although natural Treg cells comprised about 5% of the cells in a bacterial pMHCII-specific naïve T cell population, Foxp3+ cells were not present in the memory cell population formed after infection42. Foxp3+ Tbet+ cells can be generated in vivo under strong Th1 inflammatory conditions and after persistent infection with Mycobacterium43. The cells may, however, lose Foxp3 before becoming memory cells. Evidence from a fate-mapping reporter mouse indicates that some Th1 effector cells or Tem expressed Foxp3 in the past44.
Tfh cells marked by expression of CXCR5, ICOS, PD-1 and low levels of CCR7 can also been found at the peak of clonal expansion during immune responses in which germinal centers form45, 46. Indeed, Tfh formation is dependent on pMHCII presentation by B cells47 probably in germinal centers, and we find that Tfh cells persist only as long as germinal centers persist (unpublished data). Thus, it is possible that Tfh cells do not become memory cells. On the other hand, CXCR5+ CCR7+ cells lacking PD-1 and ICOS have been identified in humans and postulated to be resting Tfh memory cells48-50. As discussed below, however, it is also possible that these are Tcm that express CXCR5.
Tcm generation
As mentioned above, some Th1 effector cells generated in L. monocytogenes-infected mice appear to become Th1 effector memory cells. These infections, however, also induced CCR7+ effector cells, and subsequently memory cells that express low levels of T-bet and lack other lineage-defining transcription factors20. Could the early cells be Tcm precursors and the later cells their Tcm progeny? The fact that the T-betlow memory cells induced by this infection produce IL-2 (unpublished data) is consistent with this possibility. Another prediction would be that these cells should have the flexibility to give rise to diverse types of effectors cells if they are Tcm. Along these lines, Mosmann and colleagues have described uncommitted Th primed precursor cells, which are generated in response to immunization with soluble proteins antigens and produce IL-2 but not IFN-γ or IL-4, and have the subsequent capacity to produce these lymphokines when exposed to polarizing cytokines51. The finding that L. monocytogenes infection induces effector cells that give rise to both Tem and non-Tem (Tcm?) raises the question of how naïve T cells decide which path to follow. Several observations indicate that strong transient TCR signaling favors Tem formation. Activation of naïve TCR transgenic T cells under conditions where they are very abundant in adoptive recipients generates predominantly Tcm, perhaps due to reduced TCR signaling as a consequence of interclonal competition14-18. In addition, naïve T cells that enter a draining lymph node late in the primary response and are activated by dwindling pMHCII complexes primarily become Tcm52. Also, reduction of the metabolic activity of effector cells by inhibition of the mTOR pathway increased the number of CD8+ Tcm generated by acute viral infection53,54, whereas increased activity of T-bet or Blimp-1 increased effector cell generation55. Furthermore, intracellular fluorescent dye labeling experiments showed that the more effector cells divide, the more likely they are to lose CD62L56. These results suggest that strong stimulation is needed for commitment to one of the Tem lineages, while weaker stimulation favors the generation of less committed Tcm. In a case of persistent pMHCII presentation, weaker TCR signaling is needed for Tcm and Tem formation, likely by preventing T cell exhaustion57.
B cells may also play a role in the appearance of different memory T cell lineages during the primary response. Acute viral infection of B cell-deficient mice had no effect on effector cell generation but resulted in a dramatic reduction in the generation of CD4+ memory T cells by a mechanism that does not depend on secreted antibody58. This result suggests that the generation of all memory T cells, Tem and Tcm, depends on B cells in this infection. In contrast, we found that T-bet+ (Th1) but not T-betlow (Tcm?) memory cells formed in B cell-deficient mice after acute intravenous infection with attenuated strain of L. monocytogenes (unpublished data). These results are reminiscent of findings in mice or humans lacking ICOS. Memory T cell generation is altered in the absence of ICOS59 or ICOSL such that Th1 formation is increased60-62 while a population of CXCR5+ memory cells (78) is decreased48, 50, 63. These ICOS-dependent CXCR5+ memory cells may be the descendants of Tfh, which also depend on ICOS64. This link, however, has not been proven.
Model of Tcm generation
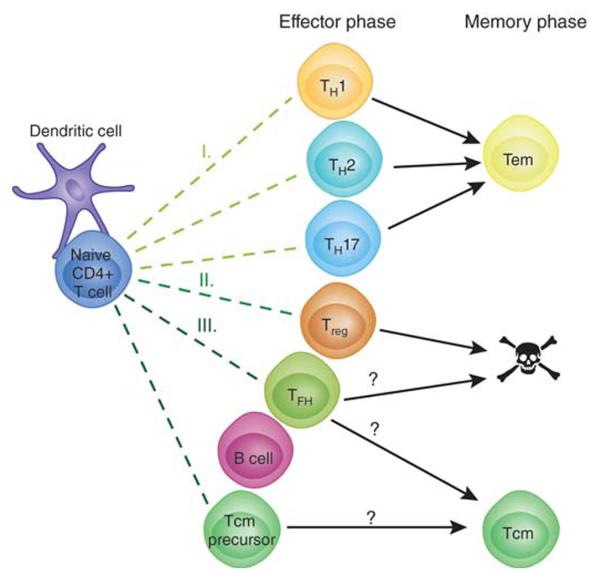
Based on these results we propose the following model of Tcm generation. Naïve T cells are first stimulated by pMHCII presentation by dendritic cells and then proliferate to form effector cells under the influence of the cytokines from the innate immune system (Fig. 1). Some of these early effector cells, perhaps those that do not interact with B cells, commit to a lineage (e.g. Th1) and some of these survive to become lineage-committed Tem (I). Treg cells may also be induced but fail to survive or change into Th1 effector memory cells (II). Other early effector T cells interact with B cells and receive signals through ICOS (III). Some these cells become Tfh effector cells that survive as long as antigen and pMHCII-presenting germinal center B cells are present. After the germinal center reaction ends, some of the Tfh become quiescent memory cells while retaining some Tfh markers such as CXCR5 and losing others such as PD-1. In this case the nonlineage committed Tcm-like CXCR5+ memory cells observed in several systems are the descendants of Tfh20-22. Alternatively, some early effector cells become CXCR5+ Tfh and others CXCR5+ Tcm precursors, both in response to ICOS signals from B cells. The Tfh effector cells then all die as the germinal center reaction ends while the Tcm survive. In this case, Tcm derive from different effector cells than Tfh. The two variants of this model can be tested in mice lacking the Bcl-6 transcription factor, which is essential for Tfh development. If the first version is correct, then Tcm-like cells will not form, if the second is correct, then they will.
Figure 1. Model for simultaneous generation of Tem and Tcm cells.
See text for description.
In conclusion, the current evidence indicates that committed Th1, Th2, and perhaps Th17 effector cells survive the contraction phase to form Tem. Other evidence is pointing to B cells as important drivers of the Tem/Tcm decision. However, the big question of why only 10% of the effector cells become memory cells is still unresolved. The answer will likely come from new tools with the power to determine whether CD4+ memory T cells are chance survivors of a stochastic process or descendants of dedicated memory cell precursors.
Acknowledgements
The authors acknowledge Antonio Pagan and Justin Taylor for helpful discussions.
References
- 1.Davis MM. T cell receptor gene diversity and selection. Annu Rev Biochem. 1990;59:475–496. doi: 10.1146/annurev.bi.59.070190.002355. [DOI] [PubMed] [Google Scholar]
- 2.Jenkins MK, et al. In vivo activation of antigen-specific CD4 T cells. Annu Rev Immunol. 2001;19:23–45. doi: 10.1146/annurev.immunol.19.1.23. [DOI] [PubMed] [Google Scholar]
- 3.Swain SL, Weinberg AD, English M. CD4+ T cell subsets. Lymphokine secretion of memory cells and of effector cells that develop from precursors in vitro. J Immunol. 1990;144:1788–1799. [PubMed] [Google Scholar]
- 4.Zhu J, Yamane H, Paul WE. Differentiation of effector CD4 T cell populations. Annu Rev Immunol. 2010;28:445–489. doi: 10.1146/annurev-immunol-030409-101212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Reinhardt RL, Khoruts A, Merica R, Zell T, Jenkins MK. Visualizing the generation of memory CD4 T cells in the whole body. Nature. 2001;410:101–105. doi: 10.1038/35065111. [DOI] [PubMed] [Google Scholar]
- 6.Seder RA, Ahmed R. Similarities and differences in CD4+ and CD8+ effector and memory T cell generation. Nat Immunol. 2003;4:835–842. doi: 10.1038/ni969. [DOI] [PubMed] [Google Scholar]
- 7.Sallusto F, Geginat J, Lanzavecchia A. Central memory and effector memory T cell subsets: function, generation, and maintenance. Annu Rev Immunol. 2004;22:745–763. doi: 10.1146/annurev.immunol.22.012703.104702. [DOI] [PubMed] [Google Scholar]
- 8.Cyster JG. Chemokines, sphingosine-1-phosphate, and cell migration in secondary lymphoid organs. Annu Rev Immunol. 2005;23:127–159. doi: 10.1146/annurev.immunol.23.021704.115628. [DOI] [PubMed] [Google Scholar]
- 9.Lohning M, et al. Long-lived virus-reactive memory T cells generated from purified cytokine-secreting T helper type 1 and type 2 effectors. J Exp Med. 2008;205:53–61. doi: 10.1084/jem.20071855. **Showed that in vitro-derived CD4+ effector cells can become memory cells.
- 10.Harrington LE, Janowski KM, Oliver JR, Zajac AJ, Weaver CT. Memory CD4 T cells emerge from effector T-cell progenitors. Nature. 2008;452:356–360. doi: 10.1038/nature06672. **Showed that Th1 effector cells can become memory cells in vivo.
- 11.Sallusto F, Lenig D, Forster R, Lipp M, Lanzavecchia A. Two subsets of memory T lymphocytes with distinct homing potentials and effector functions. Nature. 1999;401:708–712. doi: 10.1038/44385. **Defined effector and central memory cells.
- 12.Kearney ER, Pape KA, Loh DY, Jenkins MK. Visualization of peptide-specific T cell immunity and peripheral tolerance induction in vivo. Immunity. 1994;1:327–339. doi: 10.1016/1074-7613(94)90084-1. [DOI] [PubMed] [Google Scholar]
- 13.Pape KA, et al. Use of adoptive transfer of T-cell-antigen-receptor-transgenic T cell for the study of T-cell activation in vivo. Immunological reviews. 1997;156:67–78. doi: 10.1111/j.1600-065x.1997.tb00959.x. [DOI] [PubMed] [Google Scholar]
- 14.Marzo AL, et al. Initial T cell frequency dictates memory CD8+ T cell lineage commitment. Nat Immunol. 2005;6:793–799. doi: 10.1038/ni1227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Foulds KE, Shen H. Clonal competition inhibits the proliferation and differentiation of adoptively transferred TCR transgenic CD4 T cells in response to infection. J Immunol. 2006;176:3037–3043. doi: 10.4049/jimmunol.176.5.3037. [DOI] [PubMed] [Google Scholar]
- 16.Badovinac VP, Haring JS, Harty JT. Initial T cell receptor transgenic cell precursor frequency dictates critical aspects of the CD8(+) T cell response to infection. Immunity. 2007;26:827–841. doi: 10.1016/j.immuni.2007.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Blair DA, Lefrancois L. Increased competition for antigen during priming negatively impacts the generation of memory CD4 T cells. Proc Natl Acad Sci U S A. 2007;104:15045–15050. doi: 10.1073/pnas.0703767104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hataye J, Moon JJ, Khoruts A, Reilly C, Jenkins MK. Naive and memory CD4+ T cell survival controlled by clonal abundance. Science (New York, N.Y. 2006;312:114–116. doi: 10.1126/science.1124228. [DOI] [PubMed] [Google Scholar]
- 19.Moon JJ, et al. Naive CD4(+) T cell frequency varies for different epitopes and predicts repertoire diversity and response magnitude. Immunity. 2007;27:203–213. doi: 10.1016/j.immuni.2007.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pepper M, et al. Different routes of bacterial infection induce long-lived TH1 memory cells and short-lived TH17 cells. Nat Immunol. 2010;11:83–89. doi: 10.1038/ni.1826. **First demonstration of the expansion, contraction , and memory T cell formation by polyclonal pMHCII-specific CD4+ T cells induced by bacterial infection.
- 21.Stephens R, Langhorne J. Effector memory Th1 CD4 T cells are maintained in a mouse model of chronic malaria. PLoS pathogens. 2010;6:e1001208. doi: 10.1371/journal.ppat.1001208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Colpitts SL, Dalton NM, Scott P. IL-7 receptor expression provides the potential for long-term survival of both CD62Lhigh central memory T cells and Th1 effector cells during Leishmania major infection. J Immunol. 2009;182:5702–5711. doi: 10.4049/jimmunol.0803450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Surh CD, Boyman O, Purton JF, Sprent J. Homeostasis of memory T cells. Immunological reviews. 2006;211:154–163. doi: 10.1111/j.0105-2896.2006.00401.x. [DOI] [PubMed] [Google Scholar]
- 24.Homann D, Teyton L, Oldstone MB. Differential regulation of antiviral T-cell immunity results in stable CD8+ but declining CD4+ T-cell memory. Nat Med. 2001;7:913–919. doi: 10.1038/90950. **Provided first evidence that CD4+ memory T cells are less stable than CD8+ memory T cells.
- 25.Lin E, et al. Heterogeneity among viral antigen-specific CD4+ T cells and their de novo recruitment during persistent polyomavirus infection. J Immunol. 2010;185:1692–1700. doi: 10.4049/jimmunol.0904210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Purton JF, et al. Antiviral CD4+ memory T cells are IL-15 dependent. J Exp Med. 2007;204:951–961. doi: 10.1084/jem.20061805. **Revealed an IL-15-dependent mechanism of CD4+ memory T cell survival.
- 27.Turtle CJ, Swanson HM, Fujii N, Estey EH, Riddell SR. A distinct subset of self-renewing human memory CD8+ T cells survives cytotoxic chemotherapy. Immunity. 2009;31:834–844. doi: 10.1016/j.immuni.2009.09.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Stetson DB, Mohrs M, Mallet-Designe V, Teyton L, Locksley RM. Rapid expansion and IL-4 expression by Leishmania-specific naive helper T cells in vivo. Immunity. 2002;17:191–200. doi: 10.1016/s1074-7613(02)00363-1. [DOI] [PubMed] [Google Scholar]
- 29.Mohrs M, Shinkai K, Mohrs K, Locksley RM. Analysis of type 2 immunity in vivo with a bicistronic IL-4 reporter. Immunity. 2001;15:303–311. doi: 10.1016/s1074-7613(01)00186-8. [DOI] [PubMed] [Google Scholar]
- 30.Zaph C, et al. Persistence and function of central and effector memory CD4+ T cells following infection with a gastrointestinal helminth. J Immunol. 2006;177:511–518. doi: 10.4049/jimmunol.177.1.511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hendriks J, Xiao Y, Borst J. CD27 promotes survival of activated T cells and complements CD28 in generation and establishment of the effector T cell pool. J Exp Med. 2003;198:1369–1380. doi: 10.1084/jem.20030916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hirota K, et al. Fate mapping of IL-17-producing T cells in inflammatory responses. Nat Immunol. 2011;12:255–263. doi: 10.1038/ni.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Shi G, et al. Phenotype switching by inflammation-inducing polarized Th17 cells, but not by Th1 cells. J Immunol. 2008;181:7205–7213. doi: 10.4049/jimmunol.181.10.7205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bending D, et al. Highly purified Th17 cells from BDC2.5NOD mice convert into Th1-like cells in NOD/SCID recipient mice. The Journal of Clinical Investigation. 2009 doi: 10.1172/JCI37865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Martin-Orozco N, Chung Y, Chang SH, Wang YH, Dong C. Th17 cells promote pancreatic inflammation but only induce diabetes efficiently in lymphopenic hosts after conversion into Th1 cells. European Journal of Immunology. 2009;39:216–224. doi: 10.1002/eji.200838475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lee YK, et al. Late developmental plasticity in the T helper 17 lineage. Immunity. 2009;30:92–107. doi: 10.1016/j.immuni.2008.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Abromson-Leeman S, Bronson RT, Dorf ME. Encephalitogenic T cells that stably express both T-bet and ROR gamma t consistently produce IFNgamma but have a spectrum of IL-17 profiles. Journal of Neuroimmunology. 2009;215:10–24. doi: 10.1016/j.jneuroim.2009.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Acosta-Rodriguez EV, et al. Surface phenotype and antigenic specificity of human interleukin 17-producing T helper memory cells. Nat Immunol. 2007;8:639–646. doi: 10.1038/ni1467. [DOI] [PubMed] [Google Scholar]
- 39.Annunziato F, et al. Phenotypic and functional features of human Th17 cells. J Exp Med. 2007;204:1849–1861. doi: 10.1084/jem.20070663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ivanov II, et al. The orphan nuclear receptor RORgammat directs the differentiation program of proinflammatory IL-17+ T helper cells. Cell. 2006;126:1121–1133. doi: 10.1016/j.cell.2006.07.035. [DOI] [PubMed] [Google Scholar]
- 41.Lazarevic V, et al. T-bet represses T(H)17 differentiation by preventing Runx1-mediated activation of the gene encoding RORgammat. Nat Immunol. 2011;12:96–104. doi: 10.1038/ni.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ertelt JM, et al. Selective priming and expansion of antigen-specific Foxp3− CD4+ T cells during Listeria monocytogenes infection. J Immunol. 2009;182:3032–3038. doi: 10.4049/jimmunol.0803402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Koch MA, et al. The transcription factor T-bet controls regulatory T cell homeostasis and function during type 1 inflammation. Nat Immunol. 2009;10:595–602. doi: 10.1038/ni.1731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Zhou X, et al. Instability of the transcription factor Foxp3 leads to the generation of pathogenic memory T cells in vivo. Nat Immunol. 2009;10:1000–1007. doi: 10.1038/ni.1774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.King C, Tangye SG, Mackay CR. T follicular helper (TFH) cells in normal and dysregulated immune responses. Annu Rev Immunol. 2008;26:741–766. doi: 10.1146/annurev.immunol.26.021607.090344. [DOI] [PubMed] [Google Scholar]
- 46.Fazilleau N, Mark L, McHeyzer-Williams LJ, McHeyzer-Williams MG. Follicular helper T cells: lineage and location. Immunity. 2009;30:324–335. doi: 10.1016/j.immuni.2009.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Johnston RJ, et al. Bcl6 and Blimp-1 are reciprocal and antagonistic regulators of T follicular helper cell differentiation. Science. 2009;325:1006–1010. doi: 10.1126/science.1175870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Rasheed AU, Rahn HP, Sallusto F, Lipp M, Muller G. Follicular B helper T cell activity is confined to CXCR5(hi)ICOS(hi) CD4 T cells and is independent of CD57 expression. European Journal of Immunology. 2006;36:1892–1903. doi: 10.1002/eji.200636136. [DOI] [PubMed] [Google Scholar]
- 49.Morita R, et al. Human blood CXCR5(+)CD4(+) T cells are counterparts of T follicular cells and contain specific subsets that differentially support antibody secretion. Immunity. 2011;34:108–121. doi: 10.1016/j.immuni.2010.12.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Breitfeld D, et al. Follicular B helper T cells express CXC chemokine receptor 5, localize to B cell follicles, and support immunoglobulin production. J Exp Med. 2000;192:1545–1552. doi: 10.1084/jem.192.11.1545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Wang X, Mosmann T. In vivo priming of CD4 T cells that produce interleukin (IL)-2 but not IL-4 or interferon (IFN)-gamma, and can subsequently differentiate into IL-4- or IFN-gamma-secreting cells. J Exp Med. 2001;194:1069–1080. doi: 10.1084/jem.194.8.1069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Catron DM, Rusch LK, Hataye J, Itano AA, Jenkins MK. CD4+ T cells that enter the draining lymph nodes after antigen injection participate in the primary response and become central-memory cells. J Exp Med. 2006;203:1045–1054. doi: 10.1084/jem.20051954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Araki K, et al. mTOR regulates memory CD8 T-cell differentiation. Nature. 2009;460:108–112. doi: 10.1038/nature08155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Pearce EL, et al. Enhancing CD8 T-cell memory by modulating fatty acid metabolism. Nature. 2009;460:103–107. doi: 10.1038/nature08097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Rutishauser RL, et al. Transcriptional repressor Blimp-1 promotes CD8(+) T cell terminal differentiation and represses the acquisition of central memory T cell properties. Immunity. 2009;31:296–308. doi: 10.1016/j.immuni.2009.05.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Gudmundsdottir H, Wells AD, Turka LA. Dynamics and requirements of T cell clonal expansion in vivo at the single-cell level: effector function is linked to proliferative capacity. J Immunol. 1999;162:5212–5223. [PubMed] [Google Scholar]
- 57.Caserta S, Kleczkowska J, Mondino A, Zamoyska R. Reduced functional avidity promotes central and effector memory CD4 T cell responses to tumor-associated antigens. J Immunol. 2010;185:6545–6554. doi: 10.4049/jimmunol.1001867. [DOI] [PubMed] [Google Scholar]
- 58.Whitmire JK, et al. Requirement of B cells for generating CD4+ T cell memory. J Immunol. 2009;182:1868–1876. doi: 10.4049/jimmunol.0802501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Dong C, et al. ICOS co-stimulatory receptor is essential for T-cell activation and function. Nature. 2001;409:97–101. doi: 10.1038/35051100. [DOI] [PubMed] [Google Scholar]
- 60.McAdam AJ, et al. Mouse inducible costimulatory molecule (ICOS) expression is enhanced by CD28 costimulation and regulates differentiation of CD4+ T cells. J Immunol. 2000;165:5035–5040. doi: 10.4049/jimmunol.165.9.5035. [DOI] [PubMed] [Google Scholar]
- 61.Kadkhoda K, et al. Th1 cytokine responses fail to effectively control Chlamydia lung infection in ICOS ligand knockout mice. J Immunol. 2010;184:3780–3788. doi: 10.4049/jimmunol.0901384. [DOI] [PubMed] [Google Scholar]
- 62.Grimbacher B, et al. Homozygous loss of ICOS is associated with adult-onset common variable immunodeficiency. Nat Immunol. 2003;4:261–268. doi: 10.1038/ni902. [DOI] [PubMed] [Google Scholar]
- 63.Bossaller L, et al. ICOS deficiency is associated with a severe reduction of CXCR5+CD4 germinal center Th cells. J Immunol. 2006;177:4927–4932. doi: 10.4049/jimmunol.177.7.4927. [DOI] [PubMed] [Google Scholar]
- 64.Nurieva RI, et al. Generation of T follicular helper cells is mediated by interleukin-21 but independent of T helper 1, 2, or 17 cell lineages. Immunity. 2008;29:138–149. doi: 10.1016/j.immuni.2008.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]