Abstract
Tracking thermally induced reactions has always been challenging for electrode materials of electrochemical battery systems. Traditionally, a variety of calorimetric techniques and in situ XRD at elevated temperatures has been used to evaluate the thermal stability of electrode materials. These techniques are capable of providing variations in heat capacity, mass and average bulk composition of materials only. Herein, we report investigation of thermal characteristics of Li0.33Ni0.8Co0.15Al0.05O2 by using in situ soft XAS measurements in combination with XRD. Fluorescence yield and partial electron yield measurements are used simultaneously to obtain element selective surface and bulk information. Fluorescence yield measurements reveal no energy change of the absorption peak and thus no valence state change in the bulk. However, electron yield measurements indicate that NiO-type rock salt structure is formed at the surface at temperatures above 200°C while no evidence for a surface reaction near Co sites in investigated temperature range is found. These results clearly show that in situ soft XAS can give a unique understanding of the role of each element in the structural transformation under thermal abuse offering a useful guidance in developing new battery system with improved safety performance.
Safety is an essential challenge for electrochemical battery technology. Current generation of high energy battery system is prone to thermal runaway reactions. For modern applications of battery system like electric vehicles, price of battery pack is heavily influenced by performance as well as its operating temperature profile. Therefore, it is important to evaluate the type of chemical reactions and structure changes which occur at high temperatures. Further, it is generally accepted that thermal runaway reactions are initiated at the surfaces of electrodes. Consequently, there have been several studies that involve tailoring the material surface composition by controlled synthesis or surface treatments, aimed at improving safety aspects of positive electrodes1,2. However, a fundamental understanding of the surface chemistry and structures when the electrodes are exposed to temperature excursions is still somewhat lacking. Undoubtedly, experiments that can monitor both the surface and bulk structures as a function of temperature can provide key information to help develop safer materials for battery applications. In prior publications, we have reported temperature dependent studies on the bulk structural changes of charged nickel based cathode materials, such as Li1-xNiO2, Li1-xNi0.8Co0.15Al0.05O2, Li1-xNi0.5Mn0.5O2 and Li1-xNi0.33Co0.33Mn0.33O2 with and without the presence of electrolyte using synchrotron based X-ray diffraction3,4,5,6. Recently, Nam et al., Bak et al. and Wu et al. investigated the nickel based cathode materials using XRD, mass spectroscopy and TEM to determine the influence of high temperature on the cathode materials7,8,9. These techniques are useful in studying structural changes during the heating process but are not sufficient in elucidating each element's role on the structural stability, especially for simultaneously probing both the bulk and the surface of the electrodes. The partial electron yield (PEY) measurements in soft XAS give information about surface properties (up to ~5 nm), whereas the fluorescent yield (FY) measurements identify more or less bulk properties (up to ~300 nm) similar to XRD measurements10. Therefore, soft XAS is a very powerful tool to determine structural and valence state changes at elevated temperatures in an element specific manner with sensitivity to surface and the bulk structures. The fact that soft XAS measurements are element-specific and allow discrimination between surface and bulk, provides important complementary information that cannot be obtained by the sole use of XRD and/or TEM.
In this work, in situ temperature-dependent soft XAS measurements (both PEY and FY modes) in conjunction with in situ XRD studies have been applied to understand thermal degradation of the charged electrodes. Specifically, we have monitored the element selective structural changes of the charged cathode material at the surface and in the bulk at the same time during the heating process. The results of this study provide valuable guidance to design new electrode materials with enhanced thermal stability.
Results and Discussion
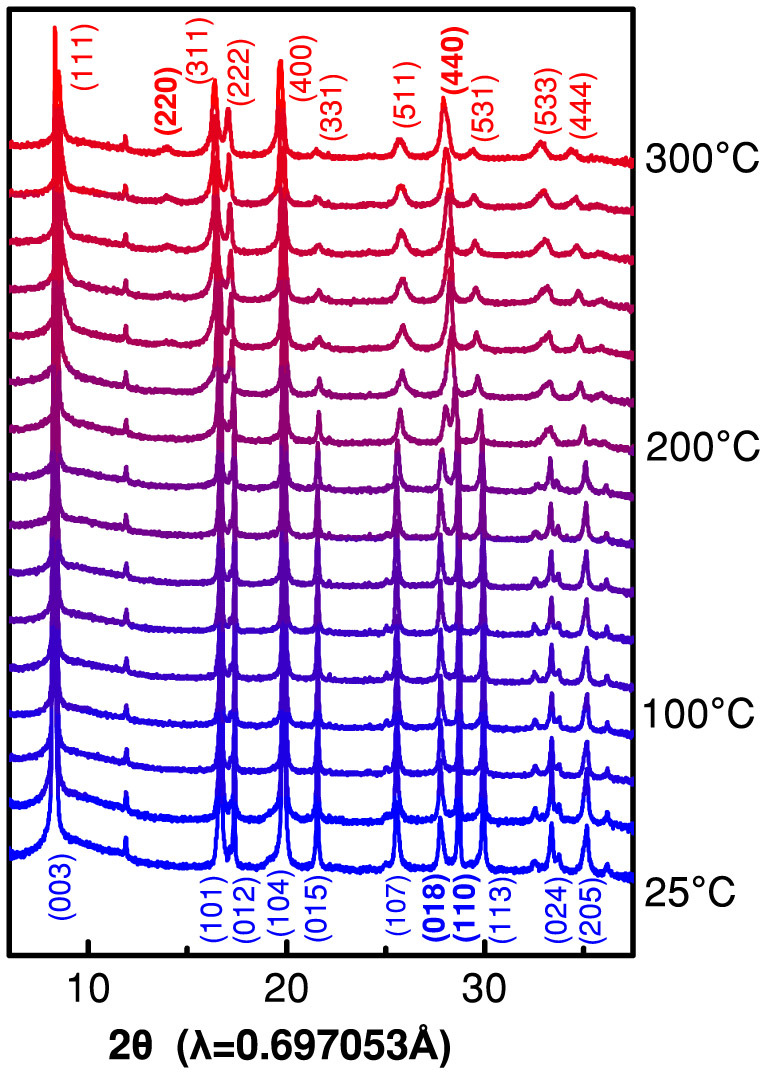
In situ XRD measurements were performed to monitor the bulk structural changes in charged Li0.33Ni0.8Co0.15Al0.05O2 cathode material during the heating process. The XRD patterns, measured with the temperature increased in a stepwise manner are shown in Figure 1. They reveal structure changes beginning at ~200°C. At lower temperature a diffraction pattern typical for layered structure with space group R-3m was obtained. At higher temperature above 200°C the layered structure gradually changed into the Fd-3m type spinel structure. This transformation is indicated by reflection (440) that emerges from the coalescence of reflections (108) and (110) of the layered structure. Moreover, the evolution of peak (220) is another indicator of the cubic spinel phase; this reflection is not expected in the rhombohedral structure4. Transition from a rhombohedral to a cubic unit cell involves cations reordering in the crystal structure, although transition metals might maintain their valence during this rearrangement. A closer look at the XRD patterns indicates continuous increase in intensity of the reflection (220) between 200–300°C, which suggests further rearrangements of cations. Substitution of Li+ at 8a tetrahedral sites by transition metal from 16d octahedral positions decreases the intensity ratio (111)/(220)11,12.
Figure 1. In situ XRD patterns of Li0.33Ni0.8Co0.15Al0.05O2 cathode material when heated from 25°C to 300°C in the absence of electrolyte.
Schematic representation of in situ soft X-ray experimental setup is shown in Figure 2(a) and a photograph of the purpose-built heatable sample holder along with its schematic is presented in Figure 2(b). The soft X-rays enter the vacuum chamber and impinge the sample at an angle of 45°. The FY and PEY detectors were placed 90° out of the incoming X-rays. Inelastic and Compton scattered X-rays are reduced by placing the FY detector normal to the incident beam. The sample was mounted on the heatable sample holder, where a tantalum foil was used as a sample support and a resistance heater. The thermocouple used to register the sample temperature was welded directly on the tantalum foil of the sample support to get precise temperature readings. As alluded to earlier soft XAS experiments13,14,15, it is possible to characterize surface and bulk properties of materials separately by detecting the electron and fluorescence yield simultaneously because of the smaller mean free path length of electrons as compared to the escape depth of photons. In comparison to total electron yield (TEY) mode, more of the Auger electrons and less of the inelastic secondary electrons are collected in PEY detection. This preferential collection renders higher surface sensitivity to the PEY mode. In addition, the measurements are intrinsically element-specific; specifically in the case of Li0.33Ni0.8Co0.15Al0.05O2, the unique electronic structure of nickel, cobalt and oxygen could be explicitly measured and evaluated. The Ni L-edge spectra are based on transitions starting at the P1/2 and P3/2 energy levels, where the strong peaks are due to transitions into 3d orbitals (eg*). In a complex with octahedral coordination, 3d orbitals split into t2g and eg orbitals by crystal field effects. Since the bonding t2g and eg orbitals along with the non-bonding t2g* orbitals are completely occupied, only transitions to the non-bonding eg* orbitals and to empty orbitals above the d-orbitals are possible.
Figure 2. Schematic diagram of (a) in situ soft XAS experimental setup and (b) sample heater with heating stage for in situ soft XAS experiment.
W. Y. and S. M. created this figure.
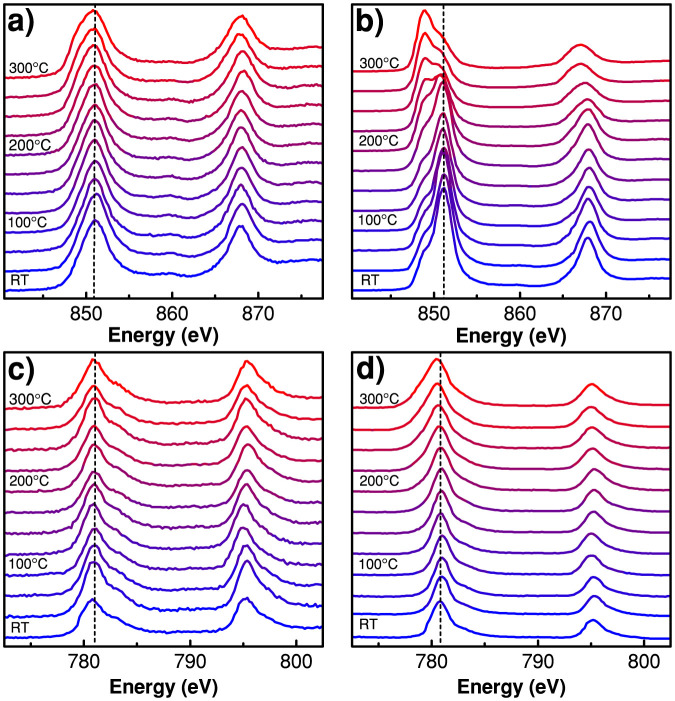
Normalized Ni L-edge spectra of Li0.33Ni0.8Co0.15Al0.05O2 cathode at different temperatures using FY mode are shown in Figure 3(a) and PEY mode spectra are shown in Figure 3(b). Due to spin-orbit interaction of the core hole the absorption spectrum is split into two well separated energy bands namely Ni 2p3/2 (L3 edge) and Ni 2p1/2 (L2 edge). In addition, these bands are expected to split due to 2p-3d interactions and crystal field effects, which should lead to a multiplet structure16. The pattern of these multiplets would offer information about the valence state, the spin state and the symmetry of the Ni coordination. The shape, energy position and other properties like the branching ratio contain information about the valence state and the spin state of the samples. Changes in energy position of the bands can indicate valence state changes during the heating process since energy position shifts about 1 eV per oxidation state change17. Ni L3 and L2 spectra obtained in the bulk sensitive fluorescent yield mode indicate no energy position changes. In fact the change to the Fd-3m structure does not involve a valence state change thus shift in energy position is not expected. However, the energy position of Ni L3 and L2 spectrum moves to lower energy levels in case of surface sensitive electron yield mode, where a rather strong shift takes place at ~200°C indicating the presence of NiO-type rock salt structure on the surface at this temperature.
Figure 3. Normalized XAS spectra of Li0.33Ni0.8Co0.15Al0.05O2 cathode material at different temperatures using (a) Ni L-edge FY mode, (b) Ni L-edge PEY mode, (c) Co L-edge FY mode and (d) Co L-edge PEY mode.
The LiNi0.8Co0.15Al0.05O2 cathode material contained only 15% cobalt species but yet high quality L-edge spectra were obtained. Figure 3(c) and (d) illustrates normalized Co L-edge XAS spectra at different temperatures using FY and PEY mode respectively. In contrast to the Ni L-edge spectra, the electron yield spectra of the Co species do not show energy shifts. There are no visible changes in both the fluorescence yield and the electron yield spectra. It indicates that cobalt ions have superior thermal stability than the nickel ions. Partial substitution of nickel by cobalt in the cathode materials contribute to enhancement of the thermal stability. This finding is consistent with earlier XRD results, wherein a significant thermal stability improvement by Co doping has been seen, as the thermally induced structural changes in Li0.33Ni0.8Co0.15Al0.05O2 cathode occur at much higher temperature compared to LiNiO24,18.
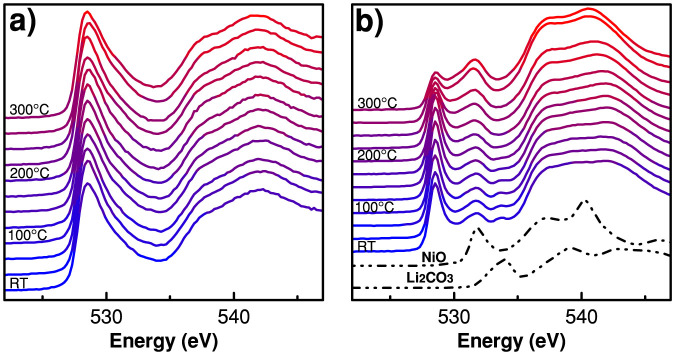
Normalized O K-edge XAS spectra of Li0.33Ni0.8Co0.15Al0.05O2 cathode material at different temperatures, using FY mode are shown in Figure 4(a) and PEY mode spectra are shown in Figure 4(b). The first single intense absorption peak at 528.5 eV corresponds to transition from oxygen 1s orbital to a hybridized state of metal 3d with O 2p orbitals19,20,21. The oxygen K-edge spectra contain information about the unoccupied d-p hybrid orbitals of the metal-oxygen bond. The broad peak above ~535 eV has been associated with transitions to hybridized states of O 2p - Ni 4sp and other empty orbitals in this energy region. Similar to the L-edge spectra, there is no significant change in the fluorescence yield spectra but the surface sensitive electron yield spectra show a remarkable decrease of the peak at 528.5 eV at temperature above 200°C. Also in contrast to the FY data, the PEY data show other distinct differences in spectral evolution. We point, in particular, to features at ~532 eV and ~534 eV in the PEY data. The distinct peak at ~534 eV is disappearing, whereas the peak at ~532 eV is growing with increasing temperature. Similar peaks, at the same energy positions, were observed in one of our previous soft XAS study on Li-ion deintercalation of LiNiO222. The features at ~532 eV and ~534 eV can be attributed to the presence of NiO and Li2CO3, respectively; this is evident from the spectra of the standards shown in Figure 4(b). On heating, features at ~534 eV diminish in intensity; this suggests that carbonate present on the surface is gradually decomposed and disintegrated. Conversely, the intensity of the ~532 eV peak increases with temperature, particularly above 200°C. Concomitantly, the intensity of the 528.5 eV peak diminishes. These observations suggest the formation of reduced divalent nickel, similar to that seen in rock salt NiO. This finding is consistent with the Ni-L edge measurements (vide supra). The presence of NiO-type rock salt structure and its increasing formation at electrode surface with increasing temperature reveal nickel oxides have the tendency to release oxygen at higher temperature leaving the metal in a lower oxidation state. The oxygen K-edge spectra are in complete agreement with the data obtained from the Ni L-edge and point to the initiation of thermal reduction reactions specifically at Ni sites on the surface of the Li0.33Ni0.8Co0.15Al0.05O2 cathode material.
Figure 4. Normalized O K-edge XAS spectra of Li0.33Ni0.8Co0.15Al0.05O2 cathode material at different temperatures using (a) FY mode and (b) PEY mode.
In summary, we successfully demonstrated the capability of in situ soft XAS techniques to investigate thermal behavior of cathode materials. In combination with in situ XRD and fluorescence yield soft XAS, comprehensive and element specific structural information of the bulk was obtained. These investigations clearly show that no valence state change takes place in the bulk even though the layered structure (R-3m) of the Li0.33Ni0.8Co0.15Al0.05O2 cathode material changes to spinel structure (Fd-3m) as indicated by the XRD measurements. The surface sensitive electron yield measurements allow the evaluation of the electronic structure of electrode material at the surface. It reveals that this electrode material loses oxygen at elevated temperatures leading to a lower valence state of Ni and formation of NiO-like rock salt structure. Interestingly, no evidence for a surface reaction near Co sites in the investigated temperature range was found. Therefore, it can be concluded that the Co species are more stable at elevated temperatures than the Ni species in Li0.33Ni0.8Co0.15Al0.05O2. These results indicate that soft XAS can be used as a powerful technique to study the thermal stability of electrode materials. In combination with in situ thermal XRD, soft XAS studies can provide valuable information for understanding the role of each element in the structural transformation under thermal abuse. Undoubtedly, the ability of soft XAS to decipher both the surface and bulk electronic structures with element specificity, makes it an invaluable addition to the arsenal of advanced diagnostic tools that help understand thermal behavior of battery electrodes.
Methods
The cathode material in this study was made of Li0.33Ni0.8Co0.15Al0.05O2 (84%) Fuji Chemical, carbon black (4%) Chevron, SFG-6 (4%) Timcal, and PVdF (8%) Kureha. The cathodes, coated on Al foil current collector, were incorporated into 2-electrode test cells. Each of these cells was made of a Li foil anode, a Celgard separator and 1.2 M LiPF6 in a 3:7 EC:EMC solvent as electrolyte. The cell was charged at C/18 rate to a level corresponding to cathode composition of Li0.33Ni0.8Co0.15Al0.05O2 and then transferred to the glove box for disassembly. The charged cathode material was scratched from the current collector and loaded into quartz capillaries of 0.3 mm diameter inside the glove box for the XRD measurements. The capillaries were sealed in a glove box before being mounted on the thermal stage of the diffractometer of beamline X7A at National Synchrotron Light Source (NSLS), Brookhaven National Laboratory (BNL). A monochromatic beam of 0.697053 Å was selected using a channel-cut Ge(111) single crystal. Capillary sample was mounted on the second axis of the diffractometer inside the cryostat. Diffraction data were collected using a position sensitive detector (PSD) stepping in 0.25° intervals over the angular range 5–37.5° in 2θ. Sample temperature was increased up to 300°C and cryostat was rocked by 5° during data collection in order to obtain better powder averaging.
In parallel studies, soft XAS measurements were performed at beamline U7A at the NSLS. The estimated incident X-ray energy resolution was ~0.2 eV with beam size was 1 mm in diameter. The PEY data were recorded using a channel electron multiplier with a high pass filter entrance grid filter set to −150 V to enhance the surface sensitivity while the FY data were recorded using a windowless energy dispersive Si(Li) detector. A linear background fit to the pre-edge region was subtracted from the spectra. The O K-edge spectra were normalized between 585 and 630 eV. To eliminate the effects of incident beam intensity fluctuations and monochromator absorption features, the PEY and FY signals were normalized using the drain current of a gold mesh with 90% transmittance located along the path of the incident X-rays.
Author Contributions
W.Y. conceived, designed, and coordinated the study. W.Y., K.N., D.F., C.J., X.Y. and M.B. performed the experiment and acquired the data. W.Y., O.H., S.M., H.K., W.L., D.K. and M.B. processed the data and wrote the paper; all the authors participated in analysis of the experimental data and discussions of the results as well as preparing the paper.
Acknowledgments
This work was supported by the Human Resources Development program (No. 20124010203270) of the KETEP and the IT R&D program (10041856) of the KEIT funded by the Korea government Ministry of Knowledge Economy. The work at BNL was supported by the U.S. Department of Energy, the Assistant Secretary for Energy Efficiency and Renewable Energy, Office of Vehicle Technologies under Contract Number DEAC02-98CH10886.
References
- Sun Y.-K. et al. High-energy cathode material for long-life and safe lithium batteries. Nat. Mater. 8, 320–4 (2009). [DOI] [PubMed] [Google Scholar]
- Song J. et al. Co-polyimide-coated polyethylene separators for enhanced thermal stability of lithium ion batteries. Electrochim. Acta 85, 524–530 (2012). [Google Scholar]
- Yoon W.-S., Balasubramanian M., Yang X.-Q., McBreen J. & Hanson J. Time-resolved XRD study on the thermal decomposition of Li1-xNi0.8Co0.15Al0.05O2 cathode materials for Li-ion batteries. Electrochem. Solid-State Lett. 8, A83–A86 (2005). [Google Scholar]
- Yoon W.-S., Hanson J., McBreen J. & Yang X.-Q. A study on the newly observed intermediate structures during the thermal decomposition of nickel-based layered cathode materials using time-resolved XRD. Electrochem. Commun. 8, 859–862 (2006). [Google Scholar]
- Yoon W.-S. et al. Time-resolved XRD study on the thermal decomposition of nickel-based layered cathode materials for Li-ion batteries. J. Power Sources 163, 219–222 (2006). [Google Scholar]
- Nam K.-W., Yoon W.-S. & Yang X.-Q. Structural changes and thermal stability of charged LiNi1/3Co1/3Mn1/3O2 cathode material for Li-ion batteries studied by time-resolved XRD. J. Power Sources 189, 515–518 (2009). [Google Scholar]
- Nam K.-W. et al. Combining in situ synchrotron X-ray diffraction and absorption techniques with transmission electron microscopy to study the origin of thermal instability in overcharged cathode materials for lithium-ion batteries. Adv. Funct. Mater. 23, 1047–1063 (2013). [Google Scholar]
- Bak S. et al. Correlating structural changes and gas evolution during the thermal decomposition of charged LixNi0.8Co0.15Al0.05O2 cathode material. Chem. Mater. 25, 337–351 (2013). [Google Scholar]
- Wu L. et al. Structural origin of overcharge-induced thermal instability of Ni-containing layered-cathodes for high-energy-density lithium batteries. Chem. Mater. 23, 3953–3960 (2011). [Google Scholar]
- Abraham D. P. et al. Microscopy and Spectroscopy of Lithium Nickel Oxide-Based Particles Used in High Power Lithium-Ion Cells. J. Electrochem. Soc. 150, A1450 (2003). [Google Scholar]
- Iturrondobeitia A. et al. Effect of doping LiMn2O4 spinel with a tetravalent species such as Si(IV) versus with a trivalent species such as Ga(III). Electrochemical, magnetic and ESR study. J. Power Sources 216, 482–488 (2012). [Google Scholar]
- Tronel F. et al. New Spinel Cobalt Oxides, Potential Conductive Additives for the Positive Electrode of Ni−MH Batteries. Chem. Mater. 18, 5840–5851 (2006). [Google Scholar]
- Yoon W.-S. et al. Soft X-Ray Absorption Spectroscopic Study of a LiNi0.5Mn0.5O2 Cathode during Charge. J. Electrochem. Soc. 151, A246 (2004). [Google Scholar]
- Yoon W. et al. Investigation of the Charge Compensation Mechanism on the Electrode System by Combination of Soft and Hard X-ray Absorption Spectroscopy. J. Am. Chem. Soc. 127, 17479–17487 (2005). [DOI] [PubMed] [Google Scholar]
- Yoon W.-S., Chung K. Y., McBreen J., Fischer D. a. & Yang, X.-Q. Electronic structural changes of the electrochemically Li-ion deintercalated LiNi0.8Co0.15Al0.05O2 cathode material investigated by X-ray absorption spectroscopy. J. Power Sources 174, 1015–1020 (2007). [Google Scholar]
- Van Elp J. et al. Electronic structure and symmetry in nickel L-edge X-ray absorption spectroscopy: Application to a nickel protein. J. Am. Chem. Soc. 116, 1918–1923 (1994). [Google Scholar]
- Wang H. et al. L-edge X-ray absorption spectroscopy of some Ni enzymes: probe of Ni electronic structure. J. Electron Spectros. Relat. Phenomena 114–116, 855–863 (2001). [Google Scholar]
- Lee K.-K., Yoon W.-S., Kim K.-B., Lee K.-Y. & Hong S.-T. Thermal behavior and the decomposition mechanism of electrochemically delithiated Li1-xNiO2. J. Power Sources 97–98, 321–325 (2001). [Google Scholar]
- Juhin A., de Groot F., Vankó G., Calandra M. & Brouder C. Angular dependence of core hole screening in LiCoO2: A DFT+U calculation of the oxygen and cobalt K-edge X-ray absorption spectra. Phys. Rev. B 81, 115115 (2010). [Google Scholar]
- Yoon W.-S. et al. Oxygen contribution on Li-ion intercalation-deintercalation in LiCoO2 investigated by O K-edge and Co L-edge X-ray absorption spectroscopy. J. Phys. Chem. B 106, 2526–2532 (2002). [Google Scholar]
- Yoon W.-S., Chung K. Y., Nam K.-W. & Kim K.-B. Characterization of LiMn2O4-coated LiCoO2 film electrode prepared by electrostatic spray deposition. J. Power Sources 163, 207–210 (2006). [Google Scholar]
- Yoon W.-S., Chung K. Y., McBreen J., Fischer D. A. & Yang X.-Q. Changes in electronic structure of the electrochemically Li-ion deintercalated LiNiO2 system investigated by soft X-ray absorption spectroscopy. J. Power Sources 163, 234–237 (2006). [Google Scholar]