Abstract
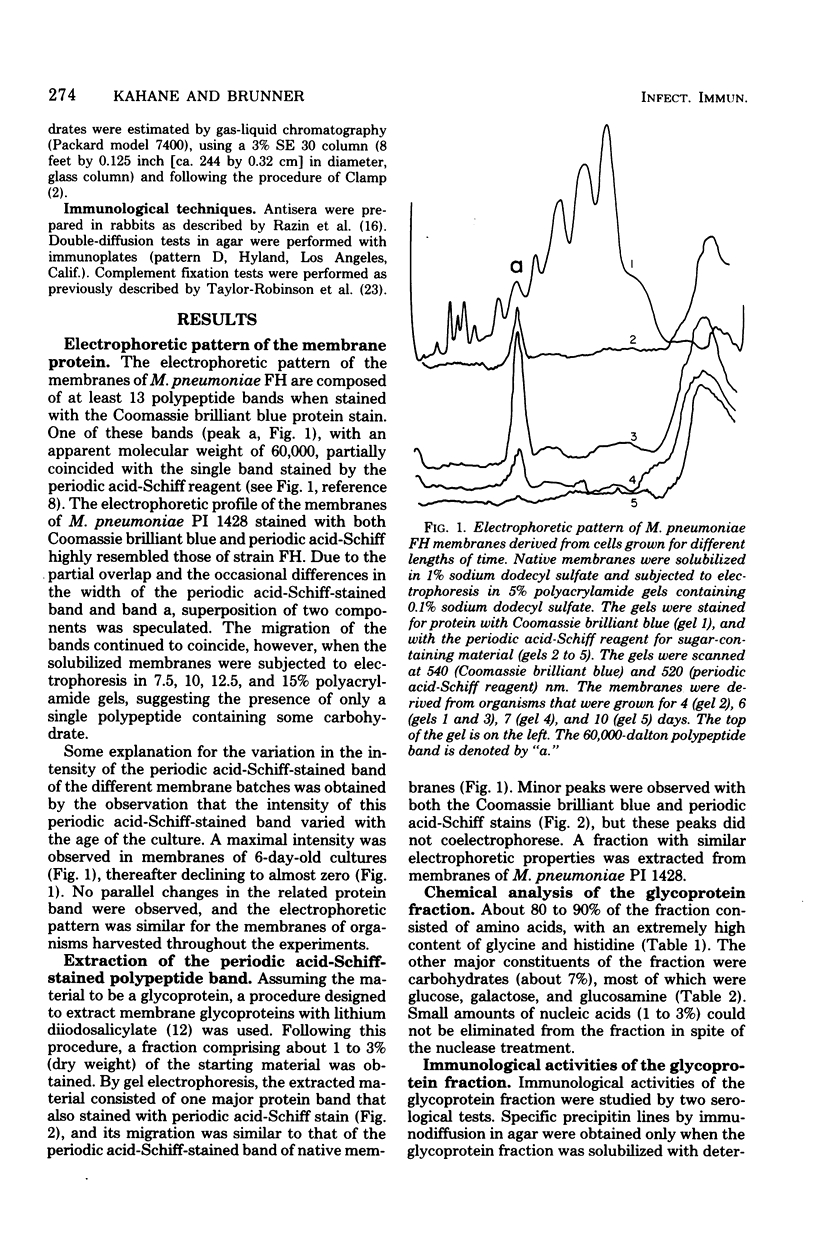
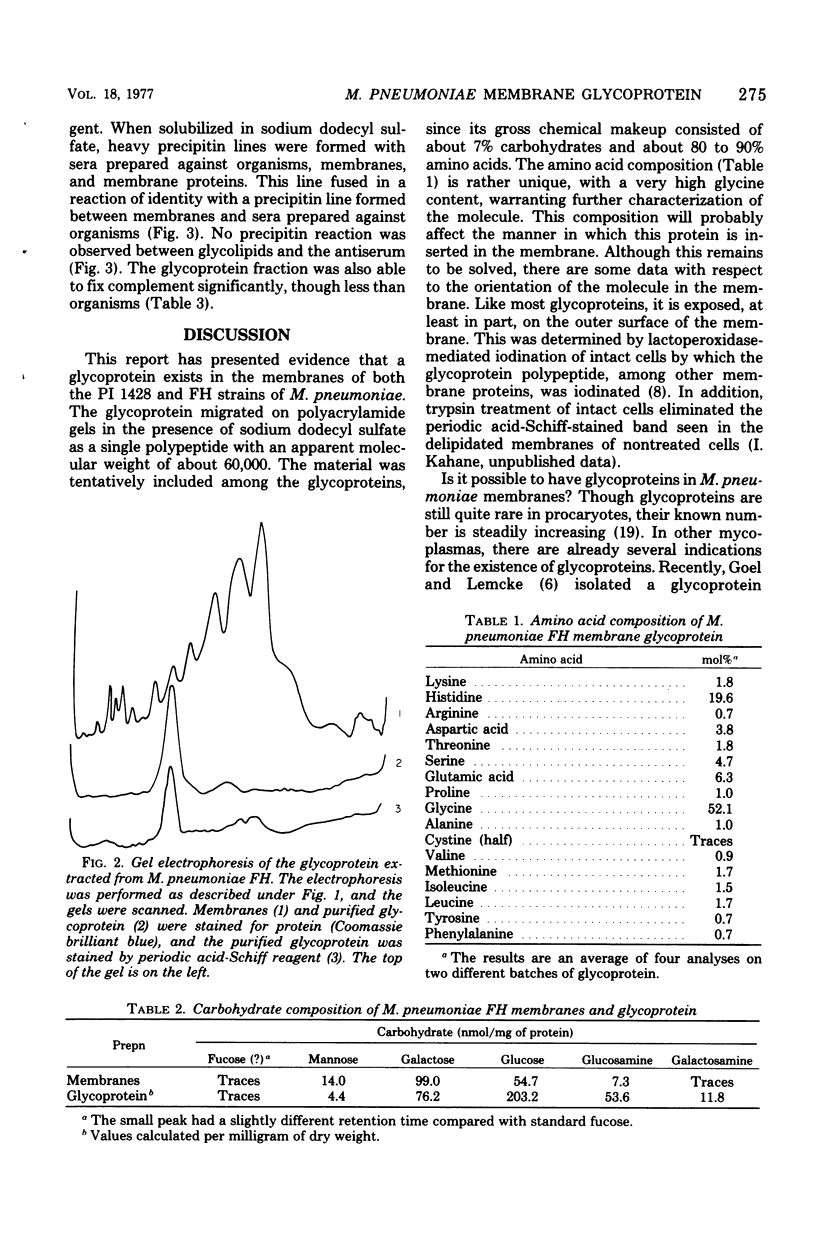
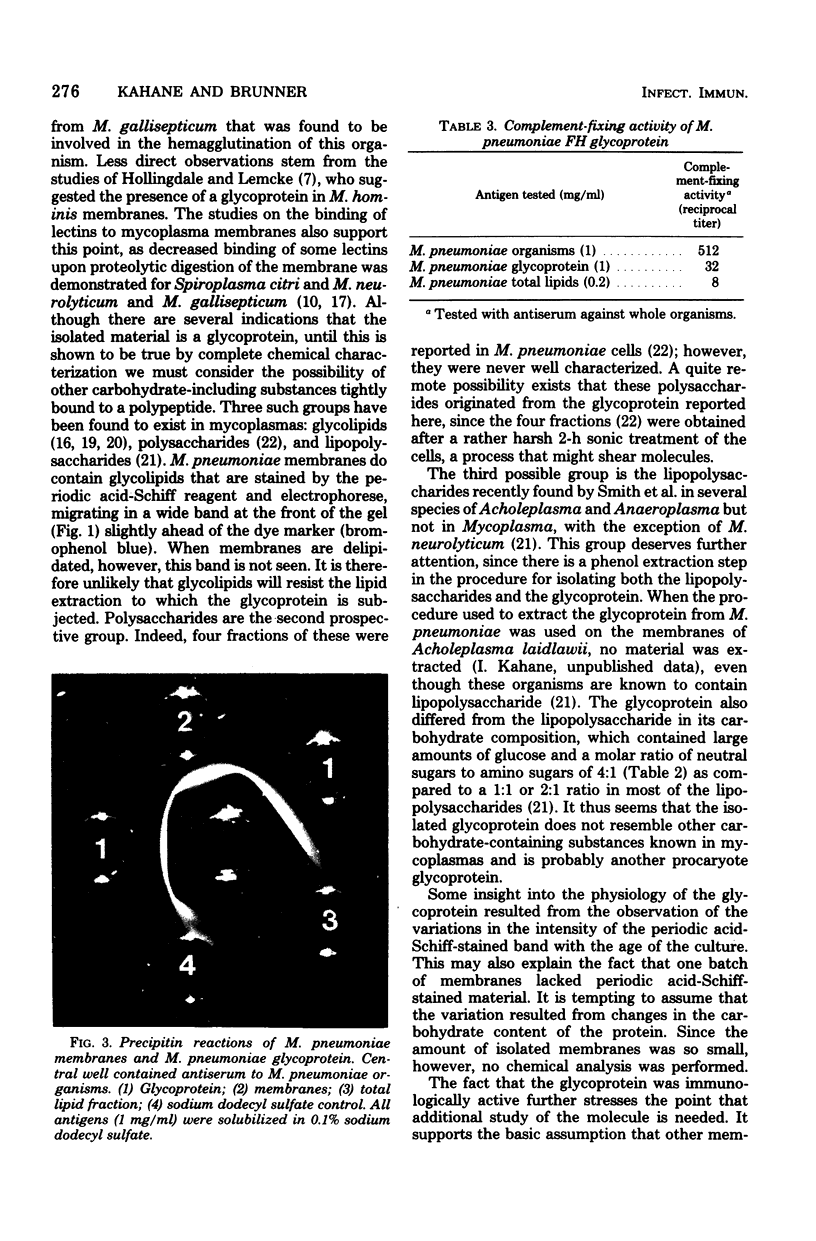
A glycoprotein was detected in Mycoplasma pneumoniae membranes. Its apparent molecular weight was about 60,000, as observed by polyacrylamide gel electrophoresis in the presence of sodium dodecyl sulfate. It corresponded to the single band that was detected on the gels by the carbohydrate stain, periodic acid-Schiff reagent. The intensity of this stained band varied for membranes derived from cells harvested between 4 and 10 days, with maximal intensity found for cells grown for 6 days. The carbohydrate-containing polypeptide was extracted with lithium diiodosalicylate. The extracted fraction consisted of about 80 to 90% amino acids (mainly glycine and histidine) and about 7% carbohydrates (mainly glucose, galactose, and glucosamine). The fraction was immunologically active, as indicated by the complement fixation and precipitin tests with antisera against whole cells, membranes, and membrane proteins.
Full text
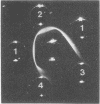
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Brunner H., James W. D., Horswood R. L., Chanock R. M. Measurement of Mycoplasma pneumoniae mycoplasmacidal antibody in human serum. J Immunol. 1972 Jun;108(6):1491–1498. [PubMed] [Google Scholar]
- Clamp J. R. Analysis of glycoproteins. Biochem Soc Symp. 1974;(40):3–16. [PubMed] [Google Scholar]
- Cuatrecasas P., Fuchs S., Anfinsen C. B. Catalytic properties and specificity of the extracellular nuclease of Staphylococcus aureus. J Biol Chem. 1967 Apr 10;242(7):1541–1547. [PubMed] [Google Scholar]
- Freundt E. A. Present status of the medical importance of mycoplasmas. Pathol Microbiol (Basel) 1974;40(3):155–187. doi: 10.1159/000162521. [DOI] [PubMed] [Google Scholar]
- Gabridge M. G., Singer S. E., Esposito R. A. Gradient, polyacrylamide gel electrophoresis of proteins from cytotoxic mycoplasma membranes. Biochem Biophys Res Commun. 1976 May 3;70(1):271–279. doi: 10.1016/0006-291x(76)91138-4. [DOI] [PubMed] [Google Scholar]
- Goel M. C., Lemcke R. M. Dissociation of Mycoplasma gallisepticum membranes with lithium diiodosalicylate and isolation of glycoprotein. Ann Microbiol (Paris) 1975 Oct-Nov;126(3):299–312. [PubMed] [Google Scholar]
- Hollingdale M. R., Lemcke R. M. Membrane antigens of Mycoplasma hominis. J Hyg (Lond) 1972 Mar;70(1):85–98. doi: 10.1017/s0022172400022129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kahane I., Greenstein S., Razin S. Carbohydrate content and enzymic activites in the membrane of Spiroplasma citri. J Gen Microbiol. 1977 Jul;101(1):173–176. doi: 10.1099/00221287-101-1-173. [DOI] [PubMed] [Google Scholar]
- Kahane I., Razin S. Immunological analysis of Mycoplasma membranes. J Bacteriol. 1969 Oct;100(1):187–194. doi: 10.1128/jb.100.1.187-194.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kahane I., Tully J. G. Binding of plant lectins to mycoplasma cells and membranes. J Bacteriol. 1976 Oct;128(1):1–7. doi: 10.1128/jb.128.1.1-7.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Marchesi V. T., Andrews E. P. Glycoproteins: isolation from cellmembranes with lithium diiodosalicylate. Science. 1971 Dec 17;174(4015):1247–1248. doi: 10.1126/science.174.4015.1247. [DOI] [PubMed] [Google Scholar]
- Plackett P., Marmion B. P., Shaw E. J., Lemcke R. M. Immunochemical analysis of Mycoplasma pneumoniae. 3. Separation and chemical identification of serologically active lipids. Aust J Exp Biol Med Sci. 1969 Apr;47(2):171–195. doi: 10.1038/icb.1969.19. [DOI] [PubMed] [Google Scholar]
- Pollack J. D., Somerson N. L., Senterfit L. B. Isolation, Characterization, and Immunogenicity of Mycoplasma pneumoniae Membranes. Infect Immun. 1970 Sep;2(3):326–339. doi: 10.1128/iai.2.3.326-339.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Powell D. A., Hu P. C., Wilson M., Collier A. M., Baseman J. B. Attachment of Mycoplasma pneumoniae to respiratory epithelium. Infect Immun. 1976 Mar;13(3):959–966. doi: 10.1128/iai.13.3.959-966.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Razin S., Prescott B., Caldes G., James W. D., Chanock R. M. Role of Glycolipids and Phosphatidylglycerol in the Serological Activity of Mycoplasma pneumoniae. Infect Immun. 1970 Apr;1(4):408–416. doi: 10.1128/iai.1.4.408-416.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schiefer H. G., Gerhardt U., Brunner H., Krüpe M. Studies with lectins on the surface carbohydrate structures of mycoplasma membranes. J Bacteriol. 1974 Oct;120(1):81–88. doi: 10.1128/jb.120.1.81-88.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith P. F., Langworthy T. A., Mayberry W. R. Distribution and composition of lipopolysaccharides from mycoplasmas. J Bacteriol. 1976 Mar;125(3):916–922. doi: 10.1128/jb.125.3.916-922.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sobeslavsky O., Prescott B., Chanock R. M. Adsorption of Mycoplasma pneumoniae to neuraminic acid receptors of various cells and possible role in virulence. J Bacteriol. 1968 Sep;96(3):695–705. doi: 10.1128/jb.96.3.695-705.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- TAYLOR-ROBINSON D., SOMERSON N. L., TURNER H. C., CHANOCK R. M. SEROLOGICAL RELATIONSHIPS AMONG HUMAN MYCOPLASMAS AS SHOWN BY COMPLEMENT-FIXATION AND GEL DIFFUSION. J Bacteriol. 1963 Jun;85:1261–1273. doi: 10.1128/jb.85.6.1261-1273.1963. [DOI] [PMC free article] [PubMed] [Google Scholar]