Abstract
Aberrant remodelling of the extracellular matrix (ECM) in the developing lung may underlie arrested alveolarisation associated with bronchopulmonary dysplasia (BPD). Transglutaminases are regulators of ECM remodelling. Therefore, the expression and activity of transglutaminases were assessed in lungs from human neonates with BPD, and in a rodent model of BPD.
Transglutaminase expression and localisation were assessed by RT-PCR, immunoblot, activity assay, and immunohistochemical analyses of human and mouse lung tissues. Transglutaminase regulation by transforming growth factor (TGF)-β was investigated in lung cells by luciferase-based reporter assay, and RT-PCR. TGF-β signalling was neutralised in vivo in an animal model of BPD, to determine whether TGF-β mediated the hyperoxia-induced changes in transglutaminase expression.
Transglutaminase 2 expression was upregulated in the lungs of preterm infants with BPD, and in the lungs of hyperoxia-exposed mouse pups, where lung development was arrested. Transglutaminase 2 localised to the developing alveolar septa. TGF-β was identified as a regulator of transglutaminase 2 expression in human and mouse lung epithelial cells. In vivo neutralisation of TGF-β signalling partially restored normal lung structure and normalised lung transglutaminase 2 mRNA expression.
Our data point to a role for perturbed transglutaminase 2 activity in the arrested alveolarisation associated with BPD.
Keywords: Bronchopulmonary dysplasia, transglutaminase, extracellular matrix, TGF-β
INTRODUCTION
Premature infants frequently receive oxygen supplementation and mechanical ventilation as early life-support measures, which while effective, also cause bronchopulmonary dysplasia (BPD), a commonly-encountered cause of morbidity and mortality in a neonatal intensive care setting [1]. While BPD is currently diagnosed exclusively by oxygen dependence criteria, the hallmark pathophysiological characteristic of BPD is an arrest of alveolarisation in the developing lung, which has long-term consequences for survivors. The pathophysiological basis of the arrested alveolarisation is not understood, however, perturbations to remodelling and maturation of the extracellular matrix (ECM) are key features of BPD pathology [2]. Malformed collagen and elastin structures have been reported in the lungs of patients with BPD [3], and in animal models of BPD [4, 5]. How these ECM structures are malformed is not understood, however, one line of thinking is that the ECM cross-linking systems operative in affected lungs are deregulated, generating ECM structures that are consequently aberrantly remodelled, or that cannot be remodelled; thereby impeding the development of the immature lung [5, 6]. Supporting this idea, increased expression and activity of lysyl oxidases – key ECM cross-linking enzymes – have been reported in clinical BPD and experimental animal models of BPD [6]. In the present study, we have addressed the expression and activity of a second family of protein cross-linking enzymes – the transglutaminases – in BPD.
Transglutaminases (E.C. 2.3.2.12) are Ca2+-dependent enzymes which catalyse the formation of isopeptide bonds either within or between polypeptide chains [7]. The transglutaminase family currently consists of nine members, including transglutaminases 1 to 7 (encoded by the TGM1–TGM7 genes in humans, and tgm1–tgm7 genes in mice), as well as the related Factor XIIIa and erythrocyte band 4.2 [7, 8]. The predominant transglutaminase catalytic product is a γ-glutamyl-ε-lysine cross-link, formed by a transamidation reaction between a glutamyl residue γ-carboxamide group and a lysyl residue ε-amino group [7]. Transglutaminases are called “nature’s biological glues” since the γ-glutamyl-ε-lysine cross-link is stable to proteolysis and mechanical stress [8]. Substrates of transglutaminases include the ECM proteins collagen II, V, VII, and XI, the collagen III propeptide, and fibronectin [7, 8]. Given that transglutaminases can modify and cross-link many ECM proteins, and that pulmonary ECM remodelling and maturation are perturbed in clinical BPD and in experimental animal models of BPD, transglutaminases appear to be interesting potential players in BPD pathogenesis. This idea is supported by two recent reports implicating transglutaminase 2 in pulmonary fibrosis [9, 10]. For these reasons, we set out to examine the pulmonary expression, localisation, and regulation of transglutaminases in clinical BPD and in an experimental animal model of BPD.
METHODS
Human patient material
The use of human tissues in this study was approved by the Ethik-Kommission [the equivalent of an Institutional Review Board in Germany] of the University of Giessen School of Medicine under approval number 189/09. Tissue harvesting from preterm and term neonates has been described in detail [6]. Clinical data for patient material are provided in table 1.
Table 1.
Clinical characteristics of control patients, and patients with BPD or at risk for the development of BPD.
| Patient number | Birth Weight grams |
Sex (M/F) |
Gestational age weeks |
Chronological age at death days |
Duration FIO2 >0.50 days |
Mechanical ventilation days |
Cause of death/autopsy diagnosis and medication |
|---|---|---|---|---|---|---|---|
| Group 1: Control group age matched to the BPD group (Group 3) for chronological age at death | |||||||
| 1 | 1625 | M | 33 | 3 | 0 | 0 | Congenital heart malformation. Drugs: atropine, prostaglandin A1 |
| 2 | 2350 | M | 35 | <1 | 0 | <1 h | Perinatal asphyxia. Drugs: atropine, adrenaline |
| 3 | 1740 | F | 34 | <1 | 0 | 0 | Intrauterine death (ventriculomegaly) |
| 4 | 1800 | M | 32 | 5 | 0 | 5 | Meningoencephalitis |
| 5 | 1190 | M | 31 | <1 | 0 | 0 | Placental abruption |
| Median | 1740 | 33 | |||||
| Mean ± SE | 1741 ± 186 | 33 ± 0.7 | |||||
| Group 2: Control group matched to the BPD group (Group 3) for birth weight and gestational age at birth | |||||||
| 6 | 954 | M | 26 | <1 | 0 | 0 | Intracranial haemorrhage |
| 7 | 1210 | M | 28 | <1 | 0 | 0 | Hypoxic-ischemic encephalopathy |
| 8 | 826 | M | 29 | <1 | 0 | 0 | Hydrocephalus, Arnold Chiari malformation |
| 9 | 852 | M | 27 | <1 | 0 | 0 | Hypoxic-ischemic encephalopathy |
| 10 | 995 | M | 27 | <1 | 0 | 0 | Placental abruption |
| Median | 954 | 27 | |||||
| Mean ± SE | 967 ± 68 | 27 ± 0.5 | |||||
| Group 3: BPD group, including patients with BPD for at risk for BPD | |||||||
| 11# | 720 | M | 29 | 62 | 13 | 62 | BPD, IRDS, Staphylococcus aureus sepsis. Drugs: surfactant, inotropes, tobramycin, flucloxacillin, cortisone |
| 12 | 825 | F | 27 | 6 | 6 | 6 | BPD, IRDS, bronchopneumonia, intracranial haemorrhage. Drugs: surfactant, inotropes, tobramycin, penicillin |
| 13# | 835 | M | 26 | 65 | 27 | 65 | BPD, cerebral bleeding, ductus arteriosus. Drugs: surfactant, inotropes, dexamethasone, theophylline |
| 14# | 930 | M | 26 | 99 | 98 | 99 | BPD, IRDS, pneumothorax, subependymal haemorrhage. Drugs: surfactant, inotropes, dexamethasone, tobramycin, penicillin, amphotericin |
| 15# | 1250 | F | 28 | 34 | 34 | 34 | BPD, IRDS, Staphylococcus epidermidis sepsis. Drugs: surfactant, furosemide, amoxicillin, erythromycin |
| 16# | 1220 | M | 31 | 35 | 35 | 35 | BPD, IRDS, right ventricular hypertrophy, anaemia, rickets. Drugs: furosemide, amoxicillin, vancomycin |
| Median | 882 | 26 | 49 | 31 | 49 | ||
| Mean ± SE | 963 ± 90 | 28 ± 0.8 | 50 ± 13 | 36 ± 13 | 50 ± 13 | ||
| P value versus Group 1¶ | 0.0032 | 0.0004 | 0.229 (NS) | ||||
| P value versus Group 2¶ | 0.987 (NS) | 0.672 (NS) | <0.0001 | ||||
Patient had clinically-defined BPD.
By unpaired Student t-test. In the case of chronological age at death, the postmenstrual ages at death, rounded to the nearest full week, were compared.
BPD = bronchopulmonary dysplasia; F = female; IRDS = infant respiratory distress syndrome; M = male; ND = not determined; NS = not significant. Inotropes included dopamine, dobutamine, and adrenaline;
Animal model
Animal experiments performed in Germany were approved by the Regierungspräsidium Gießen [housing the Institutional Animal Care and Use Committee equivalent in Germany] under approval 22/2000. The hyperoxia-based animal model of BPD has been described by our group previously [6, 11], where hyperoxia-exposed pups develop a pronounced arrest of alveolarization, apparent within five days. Briefly, mouse pups were randomised to two groups within 12 hours of birth [post natal day (P) 0.5], and exposed to 21% O2 (normoxia) or 85% O2 (hyperoxia).
Cells and TGF-β stimulation
Human lung epithelial A549 (CCL-185™) and H441 (HTB-174™) cells were obtained commercially from the American Type Culture Collection. Primary mouse lung fibroblasts and alveolar type II cells were isolated from adult C57Bl/6J mouse lungs [6, 11]. Primary human lung microvascular endothelial cells (C-12281), pulmonary artery smooth muscle cells (C-12521)) and lung fibroblasts (C-12360) were purchased from PromoCell. For TGF-β stimulation, cells were exposed to TGF-β1 (2 ng/ml final concentration; R&D Systems) for 18 h. This represents a dose well within the standard range (0.2 – 10 ng/ml) for in vitro TGF-β1 stimulation studies [6, 11].
Analysis of gene and protein expression
Real-time RT-PCR was undertaken exactly as described previously [6, 11, 12], using primers listed in table S1, all of which generated Ct values below 35 (fig. S2), for human (see table 1 for number of subjects per group), mouse (n = 5, per group) or cell culture (n = 3 per group) material. The cDNA was synthesized as described previously [6, 11, 12] from total RNA pools prepared from lung tissue or cultured cell homogenates. Immunoblotting was performed exactly as previously described [6, 11, 12] using: goat anti-Tgm1 (SantaCruz, SC-18127; 1:200); goat anti-Tgm2 (Upstate, 06-471; 1:1000); and rabbit anti-α-tubulin (SantaCruz, SC-5286, 1:2500). Immune complexes were detected (n = 3 per group, per time-point) with donkey anti-goat IgG horseradish peroxidase conjugate (SantaCruz, SC-2020; 1:1000) and goat anti-rabbit IgG horseradish peroxidase conjugate (Pierce, 31460; 1:3000), using chemiluminescence.
Immunohistochemistry
Mouse lungs were pressure-fixed at 20 cm H2O pressure and embedded in paraffin. Paraffin-embedded human and mouse lung tissue was sectioned at 3 µm. Transglutaminases or the γ-Glu-ε-Lys cross-link were detected in 3-µm sections as previously described [6, 11, 12] with goat anti-Tgm1 (SantaCruz, SC-18127; 1:50); goat anti-Tgm2 (Upstate, 06-471; 1:50), or mouse anti-N-γ-Glutamyl-ε-Lysine (Abcam, Ab424; 1:100) primary antibodies. Staining specificity was confirmed by the pre-adsorption of primary antibodies with a 100-fold molar excess of a competing peptide (SantaCruz, SC-50062P) for Tgm1, and H-γ-Glu-ε-Lys-OH (Bachem; G-1970) for the γ-Glu-ε-Lys cross-link. No pre-adsorption agent was available for Tgm2 detection, where isotype-matched non-immune antibodies substituted the specific antibody. Target antigen localisation was visualised with biotinylated rabbit anti-mouse (Invitrogen, 95-6543B) and anti-goat (Invitrogen, A10518), coupled with a Streptavidin-horseradish peroxidase complex colorimetric detection system. Histological sections illustrated are representative of the staining patterns seen in at least three samples per group.
Dual luciferase reporter assay
The dual luciferase reporter assay to assess transglutaminase promoter induction by the TGF-β signalling pathway was performed exactly as described previously [6, 11], using either the 2.2-kb human TGM1 [13] or the 4-kb mouse tgm2 [14] promoter to drive firefly luciferase expression from a pGL3 backbone, in A549 or H441 cells (n = 5, per group). Renilla luciferase activity, driven by the herpes simplex virus thymidine kinase promoter in pRLTK (Promega), yielded low to moderate levels of constitutive expression in co-transfected cells, and was used to normalise firefly luciferase activity in a dual luciferase ratio.
In vivo TGF-β neutralisation experiments
The inhibition of TGF-β signalling in mouse pups in the hyperoxia-based animal model of BPD has shown by our own [6, 15] and other [16, 17] groups to normalise TGF-β signalling, and to partially restore normal alveolar development. Neutralization of TGF-β signalling in mouse pup lungs (n = 6, per group) was undertaken exactly as described previously using pan-TGF-β neutralising IgG (1D11; R&D Systems), and an isotype-matched non-immune IgG (MOPC21; Sigma) [6, 15].
Transglutaminase 2 assay
Transglutaminase 2 activity was assessed with a commercially available kit (Sigma, CS1070) with 100 µg protein (n = 7, per group) over a 60-min period. This assay determines transglutaminases 2 activity by measuring the covalent incorporation of the transglutaminase 2-specific substrate biotin-TVQQEL-OH into poly-l-lysine that has been immobilized on an ELISA plate. The biotin-TVQQEL-OH serves as the acyl donor, where conjugation occurs via the γ-carboxamide group of biotin-TVQQEL-OH. The incorporated biotin-TVQQEL-OH was assessed via biotin recognition of a Streptavidin-peroxidase conjugate, detected using a spectrophotometer at 450 nm with 3,3′,5,5′-tetramethylbenzidine as a substrate.
Statistics
Data from patient samples were compared using one-way analysis of variance (ANOVA) with a Newman-Keuls post hoc test. For cell-culture studies, data was compared between two groups using an unpaired Student’s t-test, while groups containing more than two samples were compared using a one-way ANOVA with a Tukey’s post hoc test. Statistical outliers were determined using Grubbs’ test for outliers.
RESULTS
Transglutaminase expression is altered in the lungs of patients with or at risk for BPD
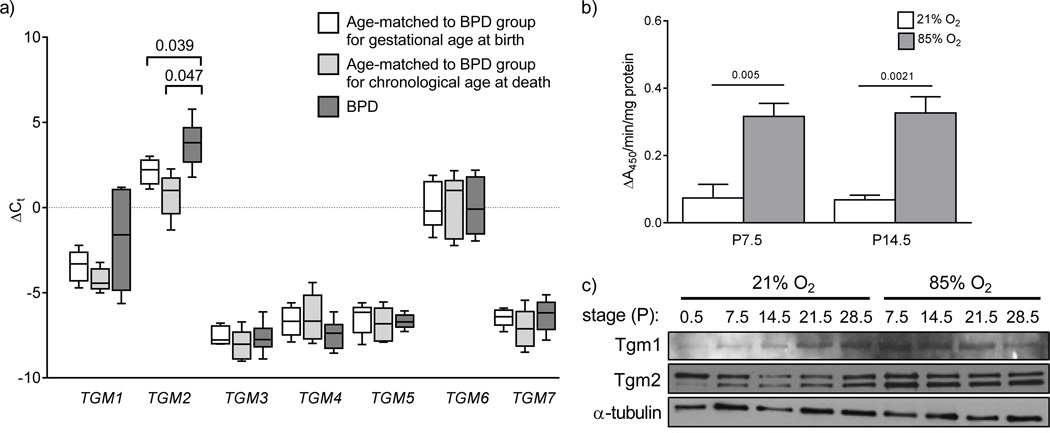
The expression of seven transglutaminases (TGM1 – TGM7) was assessed in the lungs of seven patients with or at risk for BPD. Five patients had clinically-defined BPD under current definition criteria. BPD groups are difficult to control for, since neonates are born prematurely, and prematurely born neonates breathe gaseous air over a period where controls age-matched for gestational age continue to develop in utero with fluid-filled lungs. Therefore, transglutaminase gene expression was assessed in two groups of control patients, one group matched to the BPD group for chronological age at death, while the other control group was matched to the BPD group for gestational age at birth. Only TGM2 expression was impacted in the lungs of neonates with or at risk for BPD, compared to infants age-matched for both gestational age at birth and chronological age at death (fig. 1a). Infants with or at risk for BPD also exhibited an increased range of mRNA expression levels for TGM1, compared to both control groups. However, the expression levels of TGM1 mRNA were not altered compared to either control group, taking p = 0.05 as a threshold value (fig. 1a).
FIGURE 1.
Lung transglutaminase expression human neonates and mouse pups with normal and aberrant late lung development. a) The mRNA expression levels of all seven classical members (TGM1 – TGM7) of the transglutaminase family were assessed by real-time RT-PCR with the primers listed in table S1, using mRNA pools from patients afflicted with or at risk for bronchopulmonary dysplasia (BPD; dark grey bars), as well as mRNA pools from control patients matched to the BPD group either for gestational age at birth (open bars) or for chronological age at death (light grey bars). Clinical characteristics of the patients are provided in Table 1. The HPRT gene was used as a reference. The bars in the graph represent the range of the data points, while the boxes represent the lower and upper quartiles, and the solid line within the quartile box indicates the median. All data sets were screened by Grubbs’ test for significant outlier, but none were found. The numbers above the brackets indicate the p values (when below 0.05), which were assessed by one-way analysis of variance with the Newman-Keuls post hoc test. b) Transglutaminase 2 activity was assessed by the incorporation of biotin-TVQQEL-OH into immobilised poly-l-lysine, via the γ-carboxamide group of biotin-TVQQEL-OH. Data represent mean (± S.D.) Tgm2 conjugating activity in mouse lung extracts. The numbers above the horizontal lines indicate the p values, which were assessed by unpaired Student’s t-test (n = 7; per group). c) Protein expression of Tgm1 and Tgm2 was assessed in protein extracts from whole-lung homogenates from mouse pups over the course of late lung development, during exposure to 21% O2 or 85% O2 from P0.5, by immunoblot. A single representative series is illustrated, that is representative of at least two other series’, which are quantified by densitometry in fig. S1.
Lung transglutaminase expression is altered in an experimental animal model of BPD
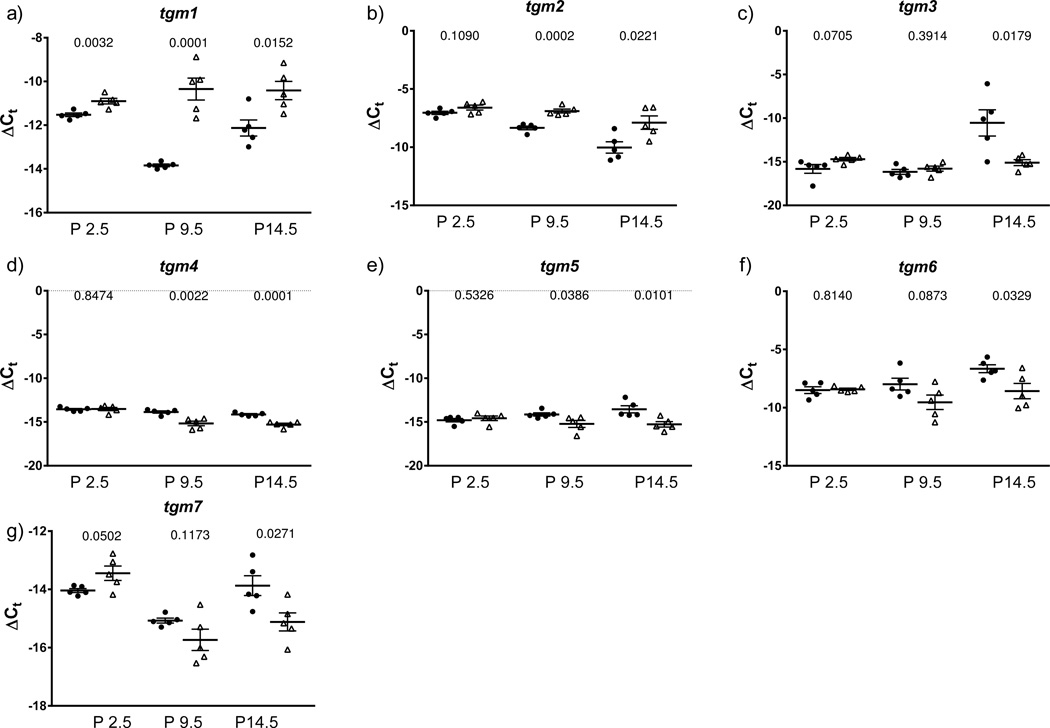
The expression of all seven members of the transglutaminases was assessed in normally developing mouse pups over the first month of post-natal lung development, as well as in the lungs of mouse pups exposed to normobaric hyperoxia (85% O2), where a pronounced arrest of alveolarisation has been noted [6, 11, 17, 18]. In line with the findings made with human BPD patients, transglutaminase 2 activity was also increased in aberrantly-developing lungs from hyperoxia-exposed mouse pups at P7.5 and P14.5 (fig. 1b). Consistent with this observation, immunoblot analysis of Tgm1 and Tgm2 expression in the lungs of mouse pups over the first month of post-natal life, by which time lung development is complete, revealed a pronounced increase in protein abundance of Tgm1 and Tgm2 in hyperoxia-exposed mouse pups at P7.5 and P14.5 (fig. 1c; quantified in fig. S1). Particularly noteworthy was a sustained increase in protein abundance of Tgm2, which was correctly detected as a doublet, in the lungs of hyperoxia-exposed mouse pups at P7.5, P14.5 and P21.5, compared to littermates exposed to 21% O2 (fig. 1c; quantified in fig. S1b). These data indicate that, similar to human patients with or at risk for BPD, the lung expression of Tgm2 is elevated in mouse pups in an experimental animal model of BPD. These observations prompted a screen of transglutaminase gene expression over the first two weeks of life, which is the critical secondary septation period. As early as day P2.5 (after 2 days of exposure to 85% O2), elevated expression of tgm1 was observed (fig. 2). Over the first 14 days of life, hyperoxia-exposed mouse pups exhibited stably elevated tgm1 expression, in contrast to normoxia-exposed pups, where a dynamic expression pattern was evident, reaching a trough at P9.5, perhaps reflecting the normal developmental expression of (and thus changing need for) Tgm1 (fig. 2a). By day 9.5, by which time alveolarisation is well underway, the expression of tgm1 and tgm2 was elevated in the lungs of hyperoxia-exposed mouse pups; while expression of tgm4 and tgm5 was down-regulated (fig. 2).
FIGURE 2.
Expression of transglutaminase genes in the lungs neonatal mouse pups with normal or aberrant late lung development. a) – g) The mRNA expression levels of all seven classical members (tgm1 – tgm7) of the transglutaminase family of protein cross-linking enzymes were assessed by real-time RT-PCR with the primers listed in table 2, using mRNA pools from lung homogenates from mouse pups at post natal day (P) 2.5, 9.5, and 14.5, after exposure to 21% O2 (●) or 85% O2 (△) from P0.5. The 18s rRNA was used as a reference. Data reflect the mean ± S.E. (n = 5). The number above the paired datasets indicate the p values, which compared the 21% O2 and 85% O2 groups, and were assessed by one-way ANOVA with Tukey’s post hoc test.
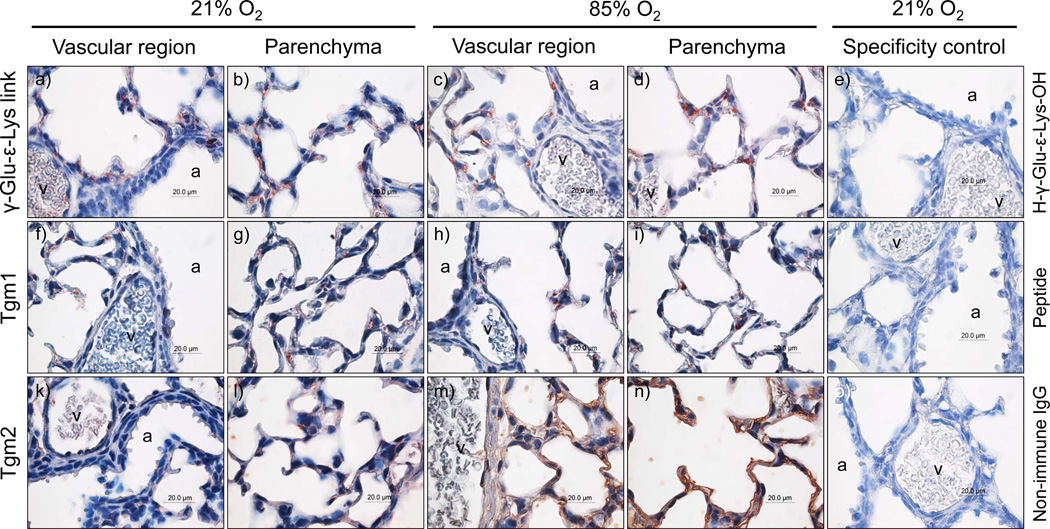
Transglutaminases and transglutaminase activity localise to regions of intense remodelling during alveolarisation
Transglutaminase activity, assessed by the presence of the transglutaminase-generated γ-glutamyl-ε-lysine cross-link, was detected in the developing mouse lungs (fig. 3), where staining was noted in the developing septa and epithelial structures of the parenchyma. A parallel pattern of expression was also noted for both Tgm1 (fig. 3f – 3i) and Tgm2 (fig. 3k – 3n). Furthermore, Tgm2 staining could be seen in the endothelial lining of large vessels, as well as in regions of the vessel wall (fig. 3h). While the authors do not consider the relative intensity of immunohistochemical staining to be quantitative, the apparent increase in staining intensity for Tgm2 in the parenchymal regions of lungs from mouse pups exposed to 85% O2 (fig. 3m – 3n), compared with comparatively less Tgm2 staining in the lungs of 21% O2-exposed littermates (fig. 3k – 3l), was consistent with the Tgm2 immunoblot data (fig. 1c), which revealed an increased lung Tgm2 expression after exposure to hyperoxia.
FIGURE 3.
Localization of transglutaminase expression in the parenchyma and vascular regions of the lungs of mouse pups at post natal day (P) 7.5, after exposure to 21% O2 or 85% O2 from P0.5. The specificity control column of photomicrographs indicates staining of the parenchyma and vascular regions of the lungs of mouse pups at P7.5, after exposure to 21% O2 from P0.5. For specificity control, primary antibodies against the γ-Glu-ε-Lys cross-link and Tgm1 were pre-adsorbed with the H-γ-Glu-ε-Lys-OH dipeptide or the Tgm1 immunogenic peptide, respectively. In the case of Tgm2, primary antibodies were replaced with non-immune isotype-matched control IgG, as no pre-adsorption agent is available for anti-Tgm2 antibodies. The airways (aw) and vessels (v) are indicated.
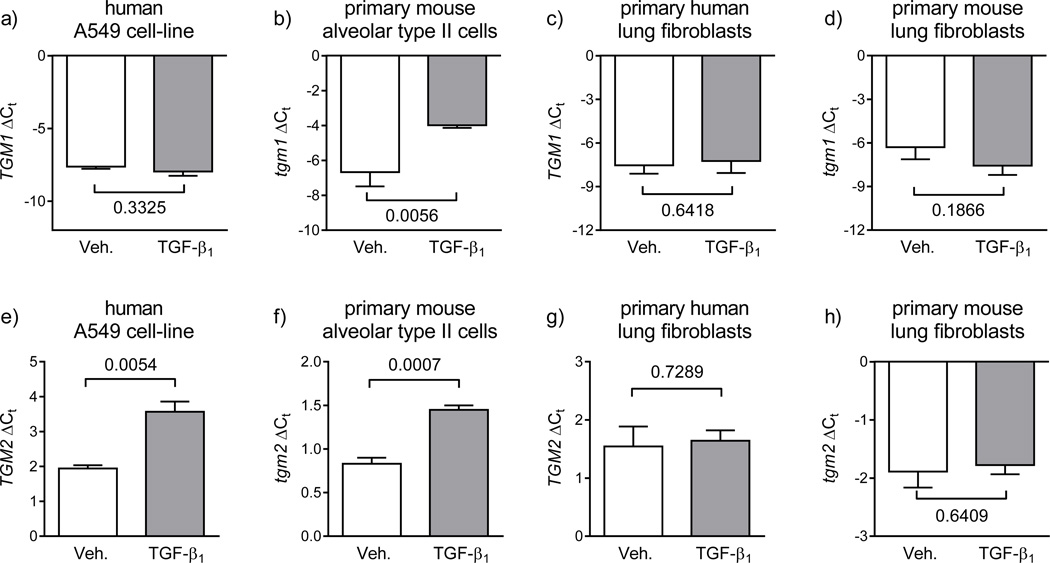
Transglutaminase expression is regulated by TGF-β1
The regulation of transglutaminase expression by TGF-β1 was assessed in the constituent cell types of the developing alveolus. While TGF-β1 did not impact TGM1 expression in the human A549 cell-line (fig. 4a), TGF-β1 did up-regulate mRNA levels of tgm1 in primary mouse alveolar type II cells (fig. 4b), and also upregulated expression of TGM2 in A549 cells (fig. 4e) and tgm2 in primary mouse alveolar type II cells (fig. 4f). TGF-β1 was without effect on TGM1 (fig. 4c) or TGM2 (fig. 4g) expression in primary human lung fibroblasts, or on tgm1 (fig. 4d) or tgm2 (fig. 4h) expression in primary mouse lung fibroblasts. The impact of TGF-β1 was also assessed for all other transglutaminases, where the only notable effects of TGF-β1 were the down-regulation of TGM3 mRNA levels in A549 cells (fig. S3), and a down-regulation of TGM7 mRNA levels in primary human lung microvascular endothelial cells (fig. S4a). Interestingly, the downregulation of TGM3 and TGM7 expression by TGF-β1 correlated with the donwregulation of tgm3 and tgm7 in the mouse model of BPD (fig. 2), suggesting that TGF-β1 may mediate this phenomenon in hyperoxia-exposed mouse pups. There was no impact of TGF-β1 on the expression levels of other tgm genes in primary mouse alveolar type II cells (fig. S5a) or in primary mouse lung fibroblasts (fig. S5b).
FIGURE 4.
Regulation of transglutaminase mRNA levels by TGF-β1. The influence of TGF-β1 on transglutaminase 1 [upper row; a) – d)] and transglutaminase 2 [lower row; e) – h)] gene expression was assessed in the human A549 lung epithelial cell-line [a) and e)], primary mouse alveolar type II cells [b) and f)], primary human lung fibroblasts [c) and g)], and primary mouse lung fibroblasts [d) and h)]. Cells were treated with vehicle alone (Veh., phosphate-buffered saline; open bars) or TGF-β1 (2 ng/ml, in vehicle; grey bars). The HPRT and hrpt genes were employed as reference genes for human and mouse cells, respectively. Primer sequences are provided in table 2. The impact of TGF-β1 on transglutaminase gene expression (TGM1 – TGM7) in primary human lung vascular endothelial cells and primary human pulmonary artery smooth muscle cells, as well as for the remaining transglutaminase genes (tgm3 – tgm7) in primary mouse alveolar type II cells are provided in the online supplement in fig. S1 and fig. S2, respectively. The numbers above the brackets indicate the p values, which were assessed by unpaired Student’s t-test (n = 3; per group), and compare vehicle- versus TGF-β1-treated groups.
TGF-β signalling drives transglutaminase promoter activation in lung epithelial cells
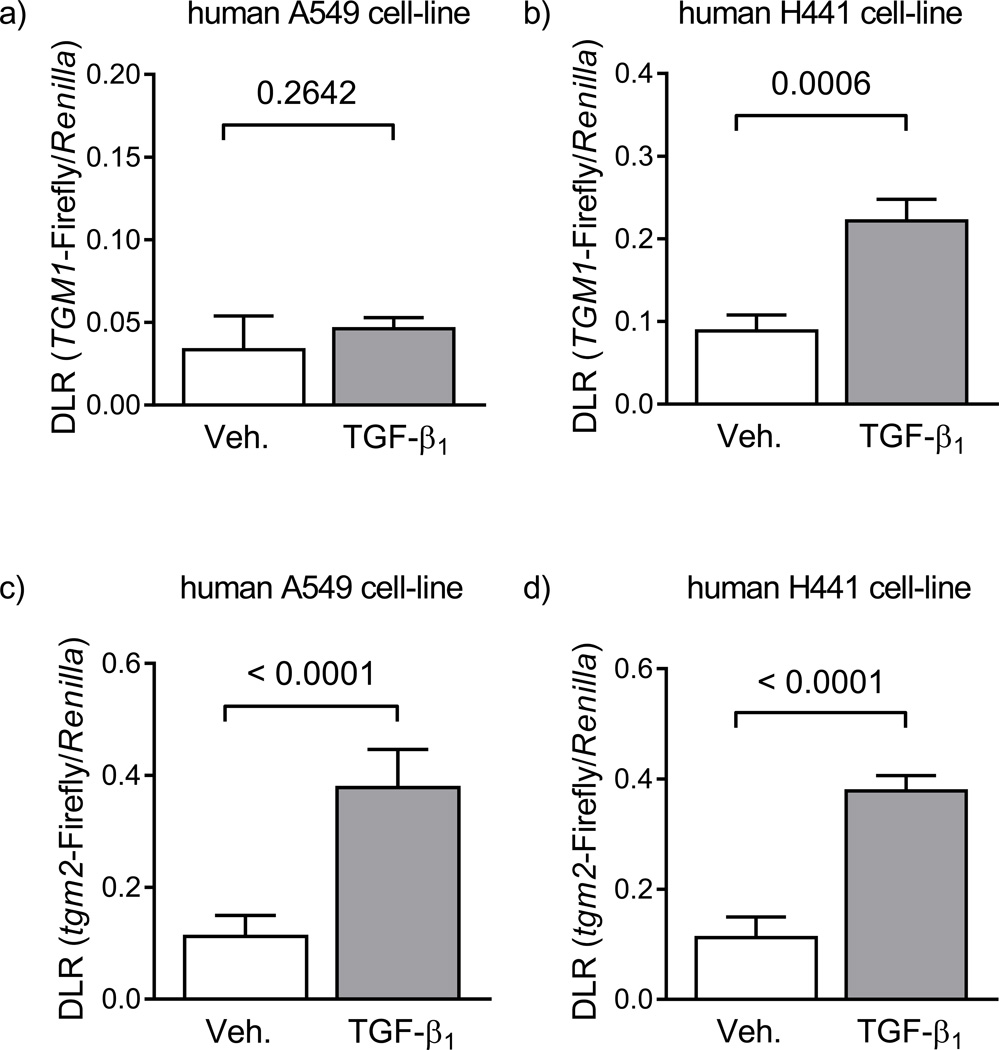
The human TGM1 promoter was not activated by stimulation of A549 cells with TGF-β1 (fig. 5a); however, TGF-β1 stimulation did activate the TGM1 promoter in H441 cells (fig. 5a). Furthermore, the mouse tgm2 promoter was activated by TGF-β1 stimulation of both A549 (fig. 5c) and H441 (fig. 5d) cells. These data indicate that TGF-β1 can be a regulator of transglutaminase expression in the lung epithelium, and are supported by the mRNA expression data in A549 cells and primary mouse alveolar type II cells (fig. 4).
FIGURE 5.
Regulation of transglutaminase promoter activity by TGF-β signalling in human epithelial cell lines. Following transfection with plasmid constructs, the activity of the human TGM1 [upper row; a) – b)] and mouse tgm2 [upper row; c) – d)] promoters was assessed by dual luciferase assay in the human A549 cell-line, as a model of the alveolar epithelium [left-hand column; a) and c)] and in the human H441 cell-line, as a model of the conducting airway epithelium [right-hand column; b) and d)]. Transfected cells were stimulated with vehicle (4 mM HCl, 1 mg/ml bovine serum albumin, in H2O, diluted 1:500 in cell-culture medium; open bars) or TGF-β1 alone (2 ng/ml, in cell-culture medium; grey bars). Data points reflect the dual luciferase ratio (DLR) in which transglutaminase-driven firefly luciferase is normalised to Renilla luciferase expression driven by the herpes simplex virus thymidine kinase promoter, providing low to moderate levels of constitutive expression in co-transfected mammalian cells. The numbers above the brackets reflect the p values, which compare vehicle- versus TGF-β-treated groups, or MOPC21 versus 1D11 control groups, and were assessed by unpaired Student’s t-test (n = 5; per group).
Hyperoxia-induced changes in lung Tgm2 expression are mediated by TGF-β1
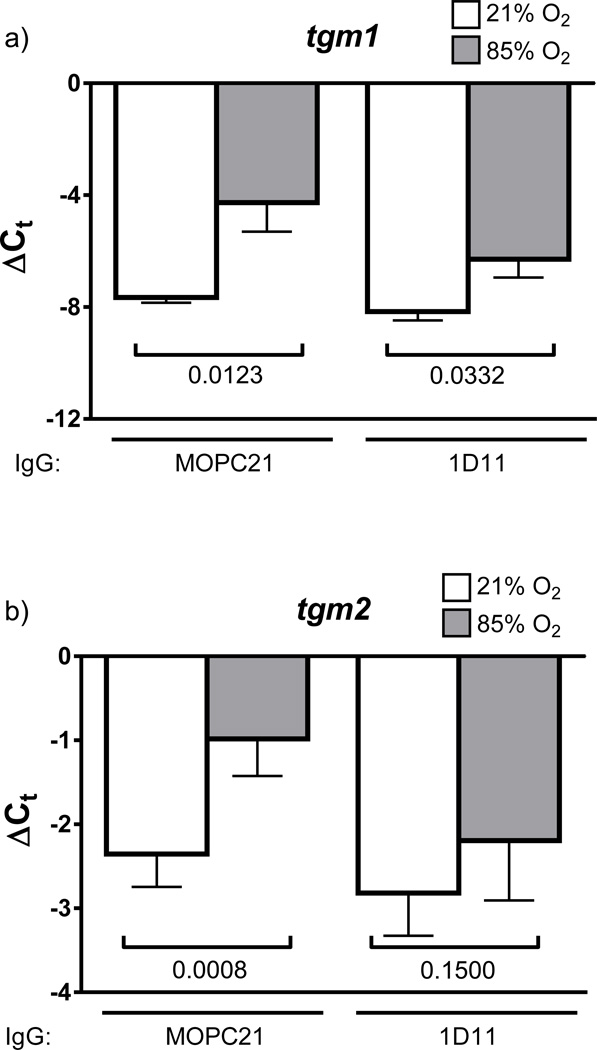
Neutralisation of TGF-β signalling in animal models of arrested lung development both normalises lung TGF-β signalling, and partially restores normal alveolarisation [17–19]. In the present study, it was revealed that in vivo neutralisation of TGF-β signalling has no impact on hyperoxia-induced increases in tgm1 expression (fig. 6a). However, neutralisation of TGF-β signalling with the 1D11 antibody did normalise tgm2 expression in the lungs of hyperoxia-exposed mouse pups. While the authors do not consider the intensity of immunohistochemical staining to be quantitative, the apparent increase in staining intensity for Tgm2 in the developing septa of lungs from mouse pups exposed to 85% O2 (fig. S6c), contrasted with comparatively less Tgm2 staining in the lungs of 21% O2-exposed littermates (fig. S6a), appeared to be diminished when compared with developing septa of lungs from mouse pups exposed to 85% O2 that received a TGF-β-neutralising antibody (fig. S6d, compared with fig. S6c).
FIGURE 6.
Impact of in vivo neutralization of TGF-β signalling on hyperoxia-induced transglutaminase gene expression. Gene expression of mRNA encoding a) tgm1 and b) tgm2 was assessed in the lungs of mouse pups at post natal day 10.5, which inspired 21% O2 (open bars) or 85% O2 (grey bars) from P0.5, and were treated with a pan TGF-β1,2,3-neutralizing IgG (1D11) or with an isotype-matched control (MOPC21) IgG. The mRNA levels were assessed by real-time RT-PCR in mRNA pools from whole-lung homogenates, using the primers in table 1, with the 18s rRNA serving as a reference. The numbers above the brackets indicate the p values, which were assessed by unpaired Student’s t-test, and were used to compare the 21% O2 and 85% O2 groups, or to compare the MOPC21-treated and 1D11-treated normoxia groups (n = 6; per group).
Transglutaminase 2 is widely expressed in the lungs of human neonates
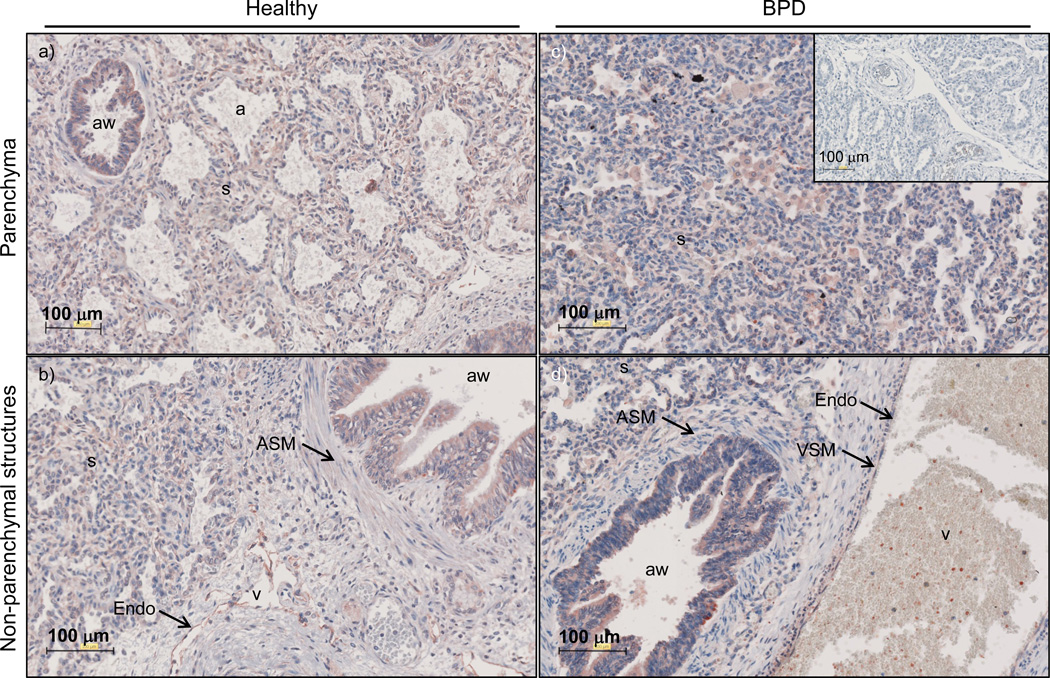
Given the increased mRNA expression of TGM2 in the lungs of human patients with BPD, lung tissues from normally developing human lungs as well as lung tissues from BPD patients were examined for TGM2 localisation by immunohistochemistry (fig. 7). TGM2 immunoreactivity was observed in the alveolar septa, airway epithelium, and airway and vascular smooth muscle walls, as well as the endothelium.
FIGURE 7.
Transglutaminase 2 expression in the lung tissues of human neonates with normally or aberrantly developing lungs. The tissue localisation of TGM2 protein was assessed in the lung tissues of human neonates with normal lung development [in this case patient 2 in table 1; a) and b)], and in the lung tissue of a patient with BPD [in this case patient 15 in table 1; c) and d)]. As a specificity control, primary antibodies were replaced with non-immune isotype-matched control IgG, as no pre-adsorption agent is available for anti-TGM2 antibodies [c) inset]. The airways (aw), airway smooth muscle (ASM), alveolar airspaces (a), endothelium (Endo), vascular smooth muscle (VSM), alveolar septa (s), and vessels (v) are indicated.
DISCUSSION
Although transglutaminases have been widely studied in neurodegenerative and autoimmune diseases, and genetic diseases including lamellar ichthyosis and factor XIII deficiency [7], transglutaminases have received scant attention in the lung. Two recent reports have implicated Tgm2 in pulmonary fibrosis [9, 10], while one earlier study detected Tgm2 in the normally developing rat lung, where externalisation of Tgm2 from intracellular to extracellular pools was thought to delay basement membrane remodelling, and to stabilise extracellular components such as microfibrils [20]. Our data reveal pronounced deregulation of transglutaminase 2 in clinical BPD and in an experimental animal model of BPD. The localisation studies reported here place transglutaminase 2 in the parenchyma of the developing mouse lung, a region of intense remodelling during alveolarisation. TGF-β was identified as a regulator of epithelial tgm2 expression, and as the factor that mediated hyperoxia-induced changes in tgm2 expression, in an experimental animal model of BPD. While the bulk of these data point to perturbed TGM2 expression in clinical BPD, altered Tgm1 expression was also noted in the mouse BPD model. This is interesting, since Tgm1 has been reported to be expressed in the normal respiratory epithelium, as well as in bronchial pre-invasive lesions and lung cancer [21], and increased Tgm1 expression is associated with squamous metaplasia of the respiratory epithelium [22] which is in line with the squamous metaplasia noted in BPD.
Abnormally elevated transglutaminase 1 and 2 expression could promote aberrant late lung development. It has been proposed that ECM cross-linking enzymes, such at the lysyl oxidases, are abnormally active in clinical BPD and an experimental animal model of BPD [6]. The same appears to be true for transglutaminases, which may then drive excessive cross-linking (and hence excessive stabilisation) of the lung ECM, rendering the ECM resistant to proteolysis, thus “locking” the lung structure, and preventing the remodelling processes that normally facilitate alveolarisation. Transglutaminases are particularly active in basement membrane remodelling, and the final phase of late lung development is achieved by fusion of epithelial and endothelial basement membranes [20]. Improperly cross-linked basement membrane collagens and other structural molecules may hinder the prerequisite remodelling of basement membranes to promote basement membrane fusion.
In addition to stabilising the ECM, transglutaminases have other notable functions that may be impacted in BPD. The epithelial lining of the lungs of infants with BPD is damaged by mechanical stretch and strain, and TGM2 is known to promote membrane resealing after mechanical damage to human lung A549 cells [23]. Along these lines, Tgm1 has also been shown to be critical for the structural integrity of mouse epithelial cells, by stabilising cadherin-based adherens junctions [24]. Thus, perturbed transglutaminase 1 and 2 expression may impact epithelial cell structural integrity and membrane repair, which are important processes in a premature, inflamed lung that is subjected to the stresses associated with mechanical ventilation and oxygen toxicity, which form part of the medical management of patients with BPD.
TGF-β was identified in this study as a regulator of tgm2 expression in the mouse lung epithelium, and as the mediator of hyperoxia-driven lung tgm2 expression in the mouse BPD model. These data add to an emerging body of evidence that highlight TGF-β as a mediator of both experimental [12, 17, 18, 25] and clinical [26] BPD, where it has been solidly established by our own and other groups that in vivo neutralisation of TGF-β in hypoxia or hyperoxia models of BPD partially restores normal lung architecture. Our findings here demonstrate that in vivo TGF-β neutralisation also restored normal tgm2 expression in the lungs of hyperoxia-treated mouse pups makes a strong case for Tgm2 as a pathogenic ECM remodelling factor in this rodent BPD model. This idea is further strengthened by our findings that TGM2 expression is elevated in human neonates with or at risk for BPD.
TGF-β1 did not drive transglutaminase expression in lung fibroblasts, which contrasts with observations made in dermal fibroblasts [27]. However, Olsen et al. [10] also noted that TGF-β1 did not drive TGM2 expression in human lung fibroblasts, although TGF-β1 did rapidly promote the externalisation and secretion of TGM2 by human lung fibroblasts. Thus, in the context of BPD, it is likely that TGF-β drives increased gene expression of transglutaminase II in epithelial cells, and also, increased secretion (and hence, extracellular activity) of fibroblast-derived transglutaminase 2. In addition to ECM cross-linking functions, intracellular Tgm2 has also been accredited with acting (i) as a rheostat, and regulating both apoptosis and autophagy in mouse fibroblasts [28], and (ii) in regulating adhesion [29] and migration [10] of mouse fibroblasts. Thus, perturbations to transglutaminase 2 expression may influence the spatio-temporal organisation of ECM deposition by fibroblasts in the developing alveolar septa. Interestingly, transglutaminase 2 is also known to activate latent TGF-β to active TGF-β [30], suggesting a positive feedback loop, where increased TGF-β activity may drive increased Tgm2 expression and activity, which in turn, then escalates TGF-β activity in the lung, leading to a vicious circle where Tgm2 and TGF-β drive each other’s expression.
BPD is a complicated disease, and it should be noted that the animal model employed only mimics the arrested septation component of the alveolar simplification seen in patients with BPD. Furthermore, the mouse model employed in the current study replicates the response of alveolar development to oxygen-induced lung injury, whereas the humans that develop BPD might also have pathologic contributions to lung disease due to other factors (such as inflammation and volutrauma) in addition to hyperemia. This may be regarded as a limitation of the model. However, it is also notable that term mouse pups are born in the same stage of lung development as are preterm infants that develop BPD, and as such, the mouse model is more suitable for BPD studies than is generally appreciated. Additionally, alveolar simplification due to arrested secondary septation is the key histopathological characteristic of “new” BPD, and this is modelled very well in the mouse pup model. Other limitations of this study include the use of cell derived from adult (not neonatal) humans and mice, which may impact the extrapolation of the in vitro results to the situation in the neonate. It should also be kept in mind that both control patient groups did not survive post-natally, and as such, no control group that was age-matched for post-natal age has been included. Thus, the impact of post-natal life alone on transglutaminase expression has not been estimated in this study.
In spite of these limitations, our data do make a strong case for a pathogenic role for a second family of protein cross-linking enzymes – the transglutaminases – in the arrested lung development associated with clinical BPD and experimental animal models of BPD. Follow-up studies with transglutaminase inhibitors, currently being initiated by the investigators’ laboratories, will assess whether transglutaminases play a causal role, or represent therapeutic targets, in experimental animal models of BPD.
Supplementary Material
take-home message.
Transglutaminase 2 may be a novel pathogenic factor in arrested lung development associated with bronchopulmonary dysplasia.
ACKNOWLEDGEMENTS
The authors thank Richard Bland, Tom Mariani, Bob Mecham, Richard Pierce, Irwin Reiss, Dick Tibboel, and David Warburton for constructive discussions and reagents. The pGL3-TGM1 and pGL3-tgm2 promoter constructs were kind gifts from Bob Rice and László Nagy, respectively.
SUPPORT STATEMENT
This study was supported by the German Research Foundation through grant Mo 1789/1 (to R.E.M.) and Excellence Cluster 147 “Cardio-Pulmonary System” (to S.H., K.M., I.V., W.S., and R.E.M.) as well as the Federal Ministry of Higher Education, Research and the Arts of the State of Hessen LOEWE Program (to S.H., K.M., I.V., W.S., and R.E.M.), and the German Center for Lung Research (Deutsches Zentrum für Lungenforschung; all German authors), and U.S. National Heart, Lung, and Blood Institute grant HL-094608 (to J.D.R).
Footnotes
STATEMENT OF INTEREST
No conflicts of interest, financial or otherwise, are declared by the authors.
REFERENCES
- 1.Jobe AH, Bancalari E. Bronchopulmonary dysplasia. Am J Respir Crit Care Med. 2001;163(7):1723–1729. doi: 10.1164/ajrccm.163.7.2011060. [DOI] [PubMed] [Google Scholar]
- 2.Madurga A, Mizikova I, Ruiz-Camp J, Morty RE. Recent advances in late lung development and the pathogenesis of bronchopulmonary dysplasia. Am J Physiol Lung Cell Mol Physiol. 2013 doi: 10.1152/ajplung.00267.2013. in press. [DOI] [PubMed] [Google Scholar]
- 3.Thibeault DW, Mabry SM, Ekekezie II, Zhang X, Truog WE. Collagen scaffolding during development and its deformation with chronic lung disease. Pediatrics. 2003;111(4 Pt 1):766–776. doi: 10.1542/peds.111.4.766. [DOI] [PubMed] [Google Scholar]
- 4.Albertine KH, Jones GP, Starcher BC, Bohnsack JF, Davis PL, Cho SC, Carlton DP, Bland RD. Chronic lung injury in preterm lambs. Disordered respiratory tract development. Am J Respir Crit Care Med. 1999;159(3):945–958. doi: 10.1164/ajrccm.159.3.9804027. [DOI] [PubMed] [Google Scholar]
- 5.Bourbon J, Boucherat O, Chailley-Heu B, Delacourt C. Control mechanisms of lung alveolar development and their disorders in bronchopulmonary dysplasia. Pediatr Res. 2005;57(5 Pt 2):38R–46R. doi: 10.1203/01.PDR.0000159630.35883.BE. [DOI] [PubMed] [Google Scholar]
- 6.Kumarasamy A, Schmitt I, Nave AH, Reiss I, van der Horst I, Dony E, Roberts JD, Jr, de Krijger RR, Tibboel D, Seeger W, Schermuly RT, Eickelberg O, Morty RE. Lysyl oxidase activity is dysregulated during impaired alveolarization of mouse and human lungs. Am J Respir Crit Care Med. 2009;180(12):1239–1252. doi: 10.1164/rccm.200902-0215OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lorand L, Graham RM. Transglutaminases: crosslinking enzymes with pleiotropic functions. Nat Rev Mol Cell Biol. 2003;4(2):140–156. doi: 10.1038/nrm1014. [DOI] [PubMed] [Google Scholar]
- 8.Griffin M, Casadio R, Bergamini CM. Transglutaminases: nature's biological glues. Biochem J. 2002;368(Pt 2):377–396. doi: 10.1042/BJ20021234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Oh K, Park HB, Byoun OJ, Shin DM, Jeong EM, Kim YW, Kim YS, Melino G, Kim IG, Lee DS. Epithelial transglutaminase 2 is needed for T cell interleukin-17 production and subsequent pulmonary inflammation and fibrosis in bleomycin-treated mice. J Exp Med. 2011;208(8):1707–1719. doi: 10.1084/jem.20101457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Olsen KC, Sapinoro RE, Kottmann RM, Kulkarni AA, Iismaa SE, Johnson GV, Thatcher TH, Phipps RP, Sime PJ. Transglutaminase 2 and its role in pulmonary fibrosis. Am J Respir Crit Care Med. 2011;184(6):699–707. doi: 10.1164/rccm.201101-0013OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Alejandre-Alcázar MA, Kwapiszewska G, Reiss I, Amarie OV, Marsh LM, Sevilla-Pérez J, Wygrecka M, Eul B, Köbrich S, Hesse M, Schermuly RT, Seeger W, Eickelberg O, Morty RE. Hyperoxia modulates TGF-beta/BMP signaling in a mouse model of bronchopulmonary dysplasia. Am J Physiol Lung Cell Mol Physiol. 2007;292(2):L537–L549. doi: 10.1152/ajplung.00050.2006. [DOI] [PubMed] [Google Scholar]
- 12.Alejandre-Alcázar MA, Michiels-Corsten M, Vicencio AG, Reiss I, Ryu J, de Krijger RR, Haddad GG, Tibboel D, Seeger W, Eickelberg O, Morty RE. TGF-beta signaling is dynamically regulated during the alveolarization of rodent and human lungs. Dev Dyn. 2008;237(1):259–269. doi: 10.1002/dvdy.21403. [DOI] [PubMed] [Google Scholar]
- 13.Jessen BA, Qin Q, Rice RH. Functional AP1 and CRE response elements in the human keratinocyte transglutaminase promoter mediating Whn suppression. Gene. 2000;254(1–2):77–85. doi: 10.1016/s0378-1119(00)00291-2. [DOI] [PubMed] [Google Scholar]
- 14.Nagy L, Saydak M, Shipley N, Lu S, Basilion JP, Yan ZH, Syka P, Chandraratna RA, Stein JP, Heyman RA, Davies PJ. Identification and characterization of a versatile retinoid response element (retinoic acid receptor response element-retinoid X receptor response element) in the mouse tissue transglutaminase gene promoter. J Biol Chem. 1996;271(8):4355–4365. doi: 10.1074/jbc.271.8.4355. [DOI] [PubMed] [Google Scholar]
- 15.Nakanishi H, Sugiura T, Streisand JB, Lonning SM, Roberts JD., Jr TGF-beta-neutralizing antibodies improve pulmonary alveologenesis and vasculogenesis in the injured newborn lung. Am J Physiol Lung Cell Mol Physiol. 2007;293(1):L151–L161. doi: 10.1152/ajplung.00389.2006. [DOI] [PubMed] [Google Scholar]
- 16.Hilgendorff A, Parai K, Ertsey R, Juliana Rey-Parra G, Thebaud B, Tamosiuniene R, Jain N, Navarro EF, Starcher BC, Nicolls MR, Rabinovitch M, Bland RD. Neonatal mice genetically modified to express the elastase inhibitor elafin are protected against the adverse effects of mechanical ventilation on lung growth. Am J Physiol Lung Cell Mol Physiol. 2012;303(3):L215–L227. doi: 10.1152/ajplung.00405.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sakurai R, Li Y, Torday JS, Rehan VK. Curcumin augments lung maturation, preventing neonatal lung injury by inhibiting TGF-beta signaling. Am J Physiol Lung Cell Mol Physiol. 2011;301(5):L721–L730. doi: 10.1152/ajplung.00076.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Nicola T, Ambalavanan N, Zhang W, James ML, Rehan V, Halloran B, Olave N, Bulger A, Oparil S, Chen YF. Hypoxia-induced inhibition of lung development is attenuated by the peroxisome proliferator-activated receptor-gamma agonist rosiglitazone. Am J Physiol Lung Cell Mol Physiol. 2011;301(1):L125–L134. doi: 10.1152/ajplung.00074.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Olave N, Nicola T, Zhang W, Bulger A, James M, Oparil S, Chen YF, Ambalavanan N. Transforming growth factor-beta regulates endothelin-1 signaling in the newborn mouse lung during hypoxia exposure. Am J Physiol Lung Cell Mol Physiol. 2012;302(9):L857–L865. doi: 10.1152/ajplung.00258.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Schittny JC, Paulsson M, Vallan C, Burri PH, Kedei N, Aeschlimann D. Protein cross-linking mediated by tissue transglutaminase correlates with the maturation of extracellular matrices during lung development. Am J Respir Cell Mol Biol. 1997;17(3):334–343. doi: 10.1165/ajrcmb.17.3.2737. [DOI] [PubMed] [Google Scholar]
- 21.Martinet N, Bonnard L, Regnault V, Picard E, Burke L, Siat J, Grosdidier G, Martinet Y, Vignaud JM. In vivo transglutaminase type 1 expression in normal lung, preinvasive bronchial lesions, and lung cancer. Am J Respir Cell Mol Biol. 2003;28(4):428–435. doi: 10.1165/rcmb.2002-0114OC. [DOI] [PubMed] [Google Scholar]
- 22.Vollberg TM, George MD, Nervi C, Jetten AM. Regulation of type I and type II transglutaminase in normal human bronchial epithelial and lung carcinoma cells. Am J Respir Cell Mol Biol. 1992;7(1):10–18. doi: 10.1165/ajrcmb/7.1.10. [DOI] [PubMed] [Google Scholar]
- 23.Kawai Y, Wada F, Sugimura Y, Maki M, Hitomi K. Transglutaminase 2 activity promotes membrane resealing after mechanical damage in the lung cancer cell line A549. Cell Biol Int. 2008;32(8):928–934. doi: 10.1016/j.cellbi.2008.04.009. [DOI] [PubMed] [Google Scholar]
- 24.Hiiragi T, Sasaki H, Nagafuchi A, Sabe H, Shen SC, Matsuki M, Yamanishi K, Tsukita S. Transglutaminase type 1 and its cross-linking activity are concentrated at adherens junctions in simple epithelial cells. J Biol Chem. 1999;274(48):34148–34154. doi: 10.1074/jbc.274.48.34148. [DOI] [PubMed] [Google Scholar]
- 25.Tarantal AF, Chen H, Shi TT, Lu CH, Fang AB, Buckley S, Kolb M, Gauldie J, Warburton D, Shi W. Overexpression of transforming growth factor-beta1 in fetal monkey lung results in prenatal pulmonary fibrosis. Eur Respir J. 2010;36(4):907–914. doi: 10.1183/09031936.00011810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Been JV, Debeer A, van Iwaarden JF, Kloosterboer N, Passos VL, Naulaers G, Zimmermann LJ. Early alterations of growth factor patterns in bronchoalveolar lavage fluid from preterm infants developing bronchopulmonary dysplasia. Pediatr Res. 2010;67(1):83–89. doi: 10.1203/PDR.0b013e3181c13276. [DOI] [PubMed] [Google Scholar]
- 27.Quan G, Choi JY, Lee DS, Lee SC. TGF-beta1 up-regulates transglutaminase two and fibronectin in dermal fibroblasts: a possible mechanism for the stabilization of tissue inflammation. Arch Dermatol Res. 2005;297(2):84–90. doi: 10.1007/s00403-005-0582-8. [DOI] [PubMed] [Google Scholar]
- 28.Rossin F, D'Eletto M, Macdonald D, Farrace MG, Piacentini M. TG2 transamidating activity acts as a reostat controlling the interplay between apoptosis and autophagy. Amino Acids. 2012;42(5):1793–1802. doi: 10.1007/s00726-011-0899-x. [DOI] [PubMed] [Google Scholar]
- 29.Gentile V, Thomazy V, Piacentini M, Fesus L, Davies PJ. Expression of tissue transglutaminase in Balb-C 3T3 fibroblasts: effects on cellular morphology and adhesion. J Cell Biol. 1992;119(2):463–474. doi: 10.1083/jcb.119.2.463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kojima S, Nara K, Rifkin DB. Requirement for transglutaminase in the activation of latent transforming growth factor-beta in bovine endothelial cells. J Cell Biol. 1993;121(2):439–448. doi: 10.1083/jcb.121.2.439. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.