Abstract
Background
Public concerns regarding the safety of blood have prompted reconsideration of the use of allogeneic blood (blood from an unrelated donor) transfusion and a range of techniques designed to minimise transfusion requirements.
Objectives
To examine the efficacy of desmopressin acetate (1-deamino-8-D-arginine-vasopressin) in reducing peri-operative blood loss and the need for red blood cell (RBC) transfusion in patients who do not have congenital bleeding disorders.
Search methods
We identified studies by searching CENTRAL (The Cochrane Library 2008, Issue 1), MEDLINE (1950 to 2008), EMBASE (1980 to 2008), the Internet (to May 2008), and bibliographies of published articles.
Selection criteria
Controlled parallel-group trials in which adult patients scheduled for non-urgent surgery were randomised to desmopressin (DDAVP) or to a control group that did not receive DDAVP treatment. Trials were eligible for inclusion if they reported data on the number of patients exposed to allogeneic red cell transfusion or the volume of blood transfused.
Data collection and analysis
Primary outcomes were: the number of patients exposed to allogeneic red blood cell (RBC) transfusion, and the amount of blood transfused. Other outcomes measured were: blood loss, re-operation for bleeding, post-operative complications (thrombosis, myocardial infarction, stroke), mortality, and length of hospital stay. Treatment effects were pooled using a random-effects model.
Main results
Nineteen trials that included a total of 1387 patients reported data on the number of patients exposed to allogeneic RBC transfusion. DDAVP did not significantly reduce the risk of exposure to allogeneic RBC transfusion (relative risk (RR) 0.96, 95% confidence interval (CI) 0.87 to 1.06). However, the use of DDAVP significantly reduced total blood loss (weighted mean difference (WMD) −241.78 ml, 95% CI −387.55 to −96.01 ml). Although DDAVP appeared to reduce the overall volume of allogeneic blood transfused during the peri-operative period the result would not be considered clinically significant (WMD −0.3 units, 95% CI −0.60 to −0.01 units). Risk of re-operation due to bleeding was not reduced (RR 0.69, 95% CI 0.26 to 1.83). DDAVP treatment was not associated with an increased risk of death or myocardial infarction (RR 1.72, 95% CI 0.68 to 4.33; RR 1.38, 95% CI 0.77 to 2.50, respectively).
Authors’ conclusions
There is no convincing evidence that desmopressin (DDAVP) minimises peri-operative allogeneic RBC transfusion in patients who do not have congenital bleeding disorders. Although the data suggest that there is some benefit of using DDAVP as a means of reducing peri-operative blood loss the observed reductions were small and generally not clinically important. Based on the currently available evidence, the use of DDAVP to reduce peri-operative blood loss or allogeneic RBC transfusion cannot be supported.
Medical Subject Headings (MeSH): Blood Loss, Surgical [* prevention & control]; Deamino Arginine Vasopressin [* administration & dosage]; Erythrocyte Transfusion [* utilization]; Hemostatics [* administration & dosage]; Randomized Controlled Trials as Topic; Transplantation, Homologous
MeSH check words: Adult, Humans
BACKGROUND
Public concern regarding the safety of transfused blood has prompted a reconsideration of the role of allogeneic red blood cell transfusion (using whole blood or packed red cells from an unrelated donor). The risks associated with receiving allogeneic blood that has been screened by a competent blood transfusion program are considered minimal, with very low risks of transmission of HIV or hepatitis C (Whyte 1997). However, this only applies where there is a safe, plentiful, and well-regulated supply. The majority of the world’s population does not have access to such a system, and the risks of transfusion in developing countries may be high (McFarland 1997). Concerns of patients and clinicians regarding blood safety have generated enthusiasm for the use of technologies intended to reduce the use of allogeneic blood (Bryson 1998; Carless 2002; Carless 2003; Carless 2006; Henry 2002; Henry 2007). Some of the alternatives to allogeneic blood are expensive and have their own risks (Coyle 1999; Fergusson 1999).
Generally, interventions fall into three groups: (1) techniques for re-infusing a patient’s own blood (pre-operative autologous donation, acute normovolemic hemodilution, cell salvage, platelet-rich plasmapheresis); (2) the administration of drugs to diminish blood loss (aprotinin, tranexamic acid, epsilon aminocaproic acid); and (3) the administration of drugs to promote red blood cell production (recombinant human erythropoietin (rhEPO)).
DDAVP is a synthetic analogue of arginine vasopressin that induces the release of the contents of endothelial cell-associated Weibel-Palade bodies, including von Willibrand factor (Levi 1999). In 1977, Mannucci et al (Mannucci 1977) were the first to trial DDAVP for the prevention of bleeding and avoidance of blood products in mild haemophilia and von Willibrand disease (vWD) patients requiring dental extractions and other surgical procedures (Mannucci 2008). The rationale for its use in patients with hemophilia A or von Willibrand disease is that the intravenous administration of DDAVP (0.3 ug/kg) produces increases in factor VIII and von Willebrand factor, mimicking replacement therapy with blood products, thereby promoting hemostasis (Franchini 2007). Plasma concentrations of factor VIII and von Willibrand factor have been reported to increase three- to fivefold above baseline levels when DDAVP is administered intravenously or subcutaneously (Federici 2008). The use of DDAVP has also been reported to shorten or normalise the bleeding time in selected patients with congenital defects of platelet function (Mannucci 1998). Since there are changes to platelet function associated with cardiopulmonary bypass, DDAVP has been evaluated in post-operative cardiac surgery patients without inherited bleeding disorders to determine whether it decreases blood loss and can minimise exposure to allogeneic transfusion (Cattaneo 2008). Recently there have been claims that the available evidence suggests that DDAVP is efficacious in reducing blood loss and transfusion requirements in those patients undergoing open heart surgery who experience excessive bleeding (Cattaneo 2008). However, the Clinical Practice Guideline (CPG) produced by The Society of Thoracic Surgeons (STS) and The Society of Cardiovascular Anesthesiologists (SCA) does not recommend routine prophylactic use of DDAVP to reduce bleeding or blood transfusion after cardiac operations (Ferraris 2007). Given the conflict in the literature a review of the currently available evidence is warranted.
Why it is important to do this review
This review builds on the systematic reviews published by Fremes et al (Fremes 1994), Cattaneo et al (Cattaneo 1995), Laupacis et al (Laupacis 1997), Levi et al (Levi 1999) and Carless et al (Carless 2004). It examines the evidence on the efficacy of DDAVP in reducing the need for peri-operative allogeneic red blood cell transfusion in adult, elective surgery; and whether there is a greater reduction in allogeneic transfusion demonstrated in identifiable patient subgroups.
OBJECTIVES
To examine the evidence for the efficacy of DDAVP in reducing peri-operative blood loss, allogeneic blood transfusion, and for any effect on clinical outcomes.
METHODS
Criteria for considering studies for this review
Types of studies
Randomised controlled trials with a concurrent control group.
Types of participants
The study participants were adults (over 18 years) without inherited bleeding disorders. The surgery being conducted was elective or non-urgent.
Types of interventions
The intervention considered was desmopressin acetate (1-deamino-8-D-arginine-vasopressin) administered intravenously (IV) as prophylactic therapy during the peri-operative period.
Types of outcome measures
Primary outcomes
Number of patients who were transfused with allogeneic or autologous blood, or both
Volume of allogeneic or autologous blood transfused (expressed as total units of blood)
Blood loss
Secondary outcomes
Re-operation due to bleeding
Mortality
Myocardial infarction
Stroke
Thrombosis
Search methods for identification of studies
This review drew on the literature search that was constructed as part of the International Study of Perioperative Transfusion (ISPOT) (Laupacis 1997). The searches were not restricted by language or publication status.
Electronic searches
We conducted database searches of:
CENTRAL (The Cochrane Library 2008, Issue 1);
MEDLINE (1950 to March 2008);
EMBASE (1980 to March 2008).
The detailed search strategies are recorded in Appendix 1.
Searching other resources
We searched the bibliographies of eligible trials, review articles, and reports for further potentially relevant studies.
In addition to the computer database searches, we searched Google Scholar™ and manufacturer websites to identify any relevant reports or projects in progress that might be relevant to the review.
Data collection and analysis
Selection of studies
The titles and abstracts identified in the searches were screened for relevance and selected for inclusion if they fulfilled the eligibility criteria. We used an article abstraction form to extract information regarding randomisation criteria, study methodology, the presence of a transfusion protocol, the type of surgery, treatment outcomes, and general comments. Two of the authors (PAC, AJM) examined articles for inclusion and exclusion criteria, with disagreements resolved by consensus.
Data extraction and management
We extracted data from studies using a data extraction form and then entered the data into Review Manager (RevMan). Data on the following outcomes were recorded: the number of patients exposed to allogeneic blood; the amount of allogeneic blood transfused (expressed as whole blood or packed red cells); the number of patients experiencing post-operative complications (thrombosis, myocardial infarction, stroke); and mortality. Data were recorded for: blood loss, and the number of patients requiring re-operation for bleeding. Patient demographic data (age and sex), the type of surgery, and the presence or absence of a transfusion protocol were also recorded.
Articles identified as duplicate publications were combined to obtain one set of data. The report with the greatest number of patients for that study was then used in the analysis.
Assessment of risk of bias in included studies
Articles were assessed for methodologic quality by two of the authors (PAC, AJM) using criteria proposed by Schulz et al (Schulz 1995). These criteria contain four items of assessment: double blinding, allocation concealment, participant exclusion (withdrawal post-randomisation), and methods used to achieve randomisation. In the case of double blinding, allocation concealment, and participant exclusion, three numeric values (that is 0, 1, or 2) were allocated to each of the three scales within each of these items. For example, trials judged to have adequately concealed treatment allocation scored 2; whereas trials judged not to have concealed treatment allocation scored 0. In the case of the method used to achieve randomisation, two numeric values (that is 0, or 1) were allocated to each of the two scales within this item of assessment. Therefore, trials scored 1 if the method used to generate allocation sequences was judged to have been adequate (for example use of a random number table, computer random number generator); whereas inadequate or unreported methods scored 0.
Inter-rater agreement for each item of methodological quality assessment was assessed by comparing the observed or achieved agreement (the proportion of studies for which the two raters assigned the same score) with that expected by chance (the agreement that would be achieved if the raters assigned scores at random). STATA® statistical software was used to calculate agreement and kappa statistics (k). Disagreements were resolved by consensus. The methodological quality of included trials was also assessed, with particular emphasis on allocation concealment ranked as follows:
Grade A - adequate concealment;
Grade B - uncertain;
Grade C - inadequate allocation concealment.
Data synthesis
Dichotomous data (for example number of patients transfused, thrombosis, patients requiring re-operation for bleeding) and continuous data (for example mean units of blood transfused, mean volume of blood loss) were analysed using Review Manager (RevMan). If standard deviations (SD) or standard error of means (SEM) were not reported for continuous data (or could not be calculated from the reported data) the study was not included in the meta-analysis. Outcomes were expressed as pooled relative risks (RR), risk differences (RD), or weighted mean differences (WMD) using a random effects model. The presence of heterogeneity of treatment effect was assessed using the Q statistic, which has an approximate chi2 distribution with degrees of freedom equal to the number of studies minus one (DerSimonian 1986). A P value less than or equal to 0.10 was used to define statistically significant heterogeneity. The I2 statistic was used to quantify inconsistency across trials. The I2 statistic describes the percentage of the variability in effect estimates that is due to heterogeneity rather than sampling error (Higgins 2002; Higgins 2003).
Transfused blood volume expressed in millilitres (ml) was converted to units by dividing by 300.
Subgroup analysis and investigation of heterogeneity
We performed analysis of a priori subgroups to determine whether effect sizes varied according to the factors: type of surgery, use of transfusion protocols, the use of autologous techniques, pre-operative exposure of patients to acetylsalicylic acid (ASA), and the quality of study methods. In practice, these subgroup analyses were frequently constrained by the small number of trials. In the case of blood loss, data were stratified by the time period of assessment (that is intra-operative; post-operative: 0 to 6 hours, 0 to 12 hours, 0 to 16 hours, 0 to 24 hours); and the length of cardiopulmonary bypass (used as a surrogate for surgical complexity). Funnel plots were inspected for evidence of publication bias.
RESULTS
Description of studies
See: Characteristics of included studies.
Twenty-nine trials of DDAVP fulfilled the inclusion criteria. These trials were conducted in the following countries: United States (n = 13), Canada (n = 3), Spain (n = 3), Sweden (n = 3), Germany (n = 1), Turkey (n = 1), Israel (n = 1), China (n = 1), Norway (n = 1), Finland (n = 1), and the United Kingdom (n = 1). Studies were published between 1986 and 2004. Twenty-two of the 29 included trials studied DDAVP treatment in the setting of cardiac surgery. The seven non-cardiac trials were conducted in the following settings: orthognathic surgery (Guyuron 1996), orthopaedic surgery (Ellis 2001; Flordal 1992; Schott 1995), hepatic surgery (Wong 2003), and vascular surgery (Clagett 1995; Lethagen 1991). All of the 29 included trials were small. The median number of patients randomised to each trial arm was only 30 (range 9 to 76 patients).
Of those studies that provided demographic data, the mean age of the patients randomised to DDAVP ranged from 47 to 72 years compared to 53 to 72 years for those patients randomised to control. Of the 29 trials included in the analysis, there was a greater proportion of males enrolled with the ratio of male to female being approximately 2:1 in both the DDAVP and control groups.
Description of dose regimens
In the 22 trials involving cardiac surgery, DDAVP was generally administered at a dose of 0.3 ug/kg (microgram per kilogram of body weight) given intravenously (IV) in 25 to 100 ml of normal saline over a period of 10 to 30 minutes. The DDAVP was given after the cessation of cardiopulmonary bypass (CPB) and after heparin reversal with protamine sulphate.
Five of the 22 cardiac trials administered DDAVP as follows: (1) Sheridan et al (Sheridan 1994) administered 10 ug of DDAVP per metre squared of body surface area diluted in saline and infused intravenously over 20 minutes after the completion of CPB and the reversal of heparin with protamine sulphate; (2) Marquez et al (Marquez 1992) administered 0.3 ug/kg of DDAVP intravenously immediately after the reversal of heparin with protamine sulphate then 0.3 ug/kg of DDAVP intravenously 12 hours later in intensive care. Marquez et al (Marquez 1992) concurrently compared this regimen with a single dose regimen (0.3 ug/kg of DDAVP intravenously after heparin reversal followed by a saline placebo 12 hours later in intensive care); (3) Rocha et al (Rocha 1994) administered 0.3 ug/kg of DDAVP diluted in 50 ml of normal saline intravenously over a period of 20 minutes, on completion of CPB and immediately after the administration of protamine, then administered another 0.3 ug/kg of DDAVP intravenously six hours after surgery. Rocha et al (Rocha 1994) concurrently compared this regimen with a single dose regimen (0.3 ug/kg of DDAVP diluted in 50 ml normal saline infused intravenously over a period of 20 minutes after the administration of protamine); (4) Casas et al (Casas 1995) administered 0.3 ug/kg to 0.4 ug/kg of DDAVP in 50 ml of normal saline solution by infusion over a 20 to 30 minute period 15 minutes after protamine administration; and (5) Despotis et al (Despotis 1999) administered 0.3 ug/kg to 0.4 ug/kg of DDAVP over a 30-minute period, after protamine administration.
The seven non-cardiac trials (Clagett 1995; Ellis 2001; Flordal 1992; Guyuron 1996; Lethagen 1991; Schott 1995; Wong 2003) used various regimens for the administration of DDAVP. Clagett et al (Clagett 1995) administered 20 ug of DDAVP in 50 ml normal saline solution over a 15-minute period, post-heparisation and just before aortic cross-clamp application. Guyuron et al (Guyuron 1996) administered 20 ug of DDAVP in 50 ml of normal saline intravenously over a period of 30 minutes prior to the surgical procedure. Schott et al (Schott 1995) administered 0.3 ug/kg of DDAVP diluted in 50 ml normal saline and infused over 15 minutes, and then administered another dose six hours later (post surgery) in the same way. Ellis et al (Ellis 2001) administered 0.3 ug/kg of DDAVP intravenously over a 30-minute period one half hour before deflating the limb tourniquet. Wong et al (Wong 2003) administered 0.3 ug/kg of DDAVP intravenously over a 20-minute period in 50 ml of normal saline, shortly after the induction of anaesthesia. Flordal et al (Flordal 1992) administered 0.3 ug/kg of DDAVP diluted in 50 ml of saline over 20 to 30 minutes at the start of surgery and repeated the same dose six hours later. Lethagen et al (Lethagen 1991) diluted 4 ug/ml of DDAVP in 10 ml of physiologic saline and injected this over 10 minutes at a dose of 0.3 ug/kg immediately before the start of surgery.
Transfusion triggers or thresholds
Of the 29 trials included in the analysis, 13 (45%) reported the use of a transfusion protocol to guide transfusion practice. There was considerable variation between trials in the transfusion trigger or threshold used. Dilthey et al (Dilthey 1993) and Spyt et al (Spyt 1990) initiated a transfusion of allogeneic red blood cells (RBC) when a patient’s hematocrit fell to less than 30%. Casas et al (Casas 1995) commenced RBC transfusion when the hemoglobin (Hb) level was less than 70 g/L (grams per litre) during cardiopulmonary bypass, or the Hb level fell to less than 80 g/L post CPB. Marquez et al (Marquez 1992) transfused allogeneic RBC when the hemoglobin level fell below 100 g/L. Horrow et al (Horrow 1991) initiated blood transfusion when the hematocrit (Hct) fell below 21% one-hour post-surgery, or the Hct fell below 24% with hemodynamic instability. Mongan et al (Mongan 1992) transfused patients post-operatively when the Hct fell below 24%. Schott et al (Schott 1995) transfused heterologous erythrocyte concentrate (SAGM-ERC) to correct an erythrocyte volume fraction (EVF) so that it was greater than 27%. Ozkisacik et al (Ozkisacik 2001) maintained an on-CPB Hct of between 20% and 25% and transfused patients post-operatively when the Hct fell below 28%. Ellis et al (Ellis 2001) transfused patients with allogeneic blood throughout the post-operative period when the Hct fell below 27%. Wong et al (Wong 2003) transfused patients when the Hct fell below 30%. Kuitunen et al (Kuitunen 1992) maintained the Hct over 20% during CPB, and over 30% during the post-operative period. Lethagen et al (Lethagen 1991) maintained the Hct between 30% and 35%. Pleym et al (Pleym 2004) transfused patients if the Hct fell to less than 20% during CPB, and less than 25% post-operatively.
Duration of surgery and cardiopulmonary bypass (CPB)
Seven trials, all involving cardiac surgery, reported the time taken to complete surgery. For these trials the time taken to complete surgery ranged from 133 minutes to 392 minutes (Table 1). The duration of CPB also varied considerably between trials, ranging from around 51 minutes to 168 minutes (Table 2). These were not unexpected findings given the varying degree of surgical complexity across trials.
Risk of bias in included studies
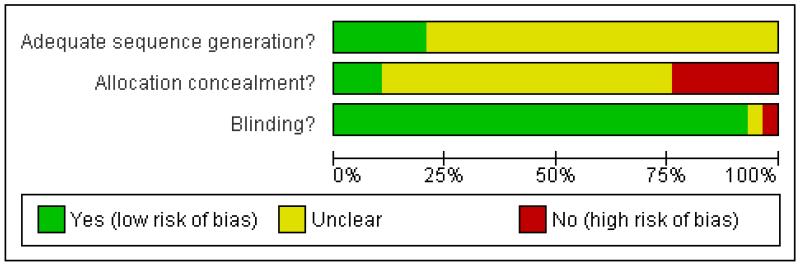
Allocation concealment
Using the Cochrane grading system for allocation concealment, allocation concealment was judged to be adequate (Grade A) in three trials, inadequate (Grade C) in six, and not clearly described (Grade B) in 20. Using the Schulz criteria (Schulz 1995) for the item pertaining to allocation concealment, the consensus scores (out of a possible score of 2) were: 2 in three trials, 1 in six trials, and 0 in 20 trials. For the item pertaining to allocation concealment there was 89.7% agreement between the two raters (k = 0.80).
Blinding
Of the 29 included trials, 26 trials (89.7%) were assessed to be double blind. Two trials (8%) indicated double blinding but the method of blinding was unclear and one trial was assessed not to be double blind. For this item of the Schulz criteria there was 96.6% agreement between the two raters (k = 0.82).
Generation of allocation sequences - randomisation
All of the 29 trials included in the analysis were described as being randomised. However, in 22 trials (75.9%) the method of generating allocation sequences was judged to be either inadequate or not reported. The remaining seven trials were judged to have had an adequate method for generating allocation sequences. These trials used either a series of computer-generated random numbers (Ansell 1992; Ellis 2001; Hedderich 1990; Mongan 1992; Pleym 2004) or a table of random numbers (Despotis 1999; Horrow 1991). For this item of the Schulz criteria, the consensus scores were (out of a possible score of 1): 1 in seven trials, and 0 in 22 trials. There was 96.6% agreement between the two raters (k = 0.91).
Inclusion of all randomised participants
Of the 29 included trials, 11 reported either there were no exclusions or that an intention-to-treat analysis was used. For the 17 trials that reported exclusions, these exclusions were judged unlikely to cause bias. For the remaining trial, exclusions were not explicitly reported. For this item of the Schulz criteria, the consensus scores (out of a possible score of 2) were: 2 in 11 trials, 1 in 17 trials, and 0 in one trial. There was 86.2% agreement between the two raters (k = 0.76).
Aggregate scores
Using the quality assessment instrument based on the Schulz criteria (with a possible score of 7): one trial scored 6, six trials scored 5, 12 trials scored 4, eight trials scored 3, and two trials scored 2. These results represent the consensus scores of the two independent raters (PAC, AJM).
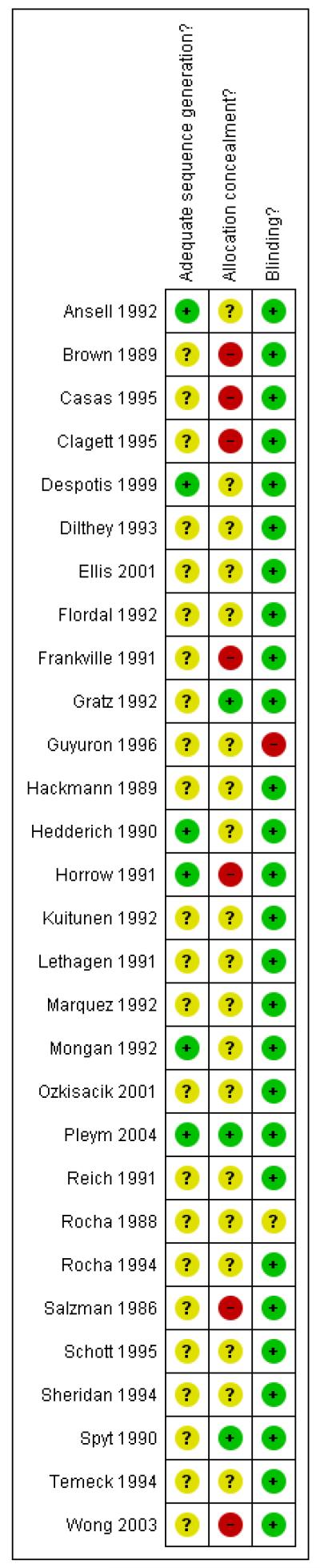
Figure 1 and Figure 2 give a visual representation of the risk of bias for each included study. The risk of bias figures indicate that the general methodological quality of the trials was poor. Although all of the trials included in this review were described as being randomised, the majority of trials failed to report the method used to generate allocation sequences. There was a similar lack of reporting of the methods used to conceal treatment allocation. Given that the majority of trials compared DDAVP with an identical placebo, double blinding was achieved for most of the trials. The methodological quality of the included trials is discussed in more detail in the ‘Effects of interventions’ section of this review.
Figure 1 . Methodological quality graph: review authors’ judgments about each methodological quality item presented as percentages across all included studies.
Figure 2 . Methodological quality summary: review authors’ judgments about each methodological quality item for each included study.
Effects of interventions
Exposure to allogeneic blood transfusion
There were 19 trials of DDAVP that reported data on the number of patients exposed to allogeneic RBC transfusion. These trials included a total of 1387 patients of whom 703 were randomised to DDAVP treatment. The use of DDAVP did not reduce the risk of exposure to allogeneic RBC transfusion compared to control (RR 0.96, 95% CI 0.87 to 1.06). Heterogeneity in treatment effect was not statistically significant (P = 0.19; I2 = 22.3%).
Exposure to allogeneic blood transfusion - type of surgery
Fifteen trials involving cardiac surgery reported data on the number of patients exposed to allogeneic RBC transfusion. These trials included a total of 1196 of whom 610 were randomised to DDAVP. In the context of cardiac surgery DDAVP did reduce the risk of requiring an allogeneic blood transfusion (RR 0.95, 95% CI 0.84 to 1.07). Heterogeneity in treatment effect was not statistically significant (P = 0.11; I2 = 33.2%). For the four non-cardiac trials the risk of requiring an allogeneic blood transfusion in those patients treated with DDAVP was 1.01 (95% CI 0.81 to 1.26). Heterogeneity in treatment effect was not statistically significant (P = 0.59; I2 = 0%).
Exposure to allogeneic blood transfusion - cardiac surgery
Of the 15 trials that involved cardiac surgery, nine involved primary coronary artery bypass graft (CABG) surgery and six involved more complex cardiac surgery (that is CABG and valvular surgery with or without re-do, and combination surgery). The nine cardiac trials that involved primary CABG surgery included a total of 586 patients of whom 299 were randomised to DDAVP treatment. Although there appeared to be a trend toward reduced exposure to allogeneic blood in those patients treated with DDAVP the result was marginal using a random-effects model analysis (RR 0.85, 95% CI 0.73 to 0.99) and not statistically significant using a fixed-effect model analysis (RR 0.89, 95% CI 0.75 to 1.05). Given there was little evidence of statistically significant heterogeneity in the treatment effect (P = 0.43; I2 = 0.1%) the fixed-effect model is the more appropriate method of statistical analysis. The six trials that involved more complex cardiac surgery included a total of 610 patients of whom 311 were randomised to DDAVP treatment. In this subgroup of trials DDAVP appeared even less effective (RR 1.03, 95% CI 0.88 to 1.19). For this subgroup analysis there appeared to be some evidence of heterogeneity in the treatment effect (P = 0.14; I2 = 40%).
Exposure to allogeneic blood transfusion - cardiac surgery and pre-operative acetylesalicylic acid (ASA) use
Of the 15 trials that involved cardiac surgery and reported data for the number of patients exposed to allogeneic blood transfusion, six reported the use of acetylsalicylic acid (ASA) within seven days of surgery. These six trials included a total of 399 patients of whom 192 were randomised to DDAVP treatment. The use of DDAVP in patients receiving ASA therapy within seven days of surgery did not appear to impact on the rates of exposure to allogeneic blood transfusion (RR 0.89, 95% CI 0.64 to 1.23). Four cardiac trials reported that ASA therapy was ceased seven to 10 days pre-operatively or they excluded patients who had received ASA therapy within seven days of surgery. These four trials included a total of 286 patients of whom 153 were randomised to DDAVP. Although there appeared to be a trend toward a reduction in the risk of exposure to allogeneic blood transfusion in DDAVP patients not receiving ASA within seven days of surgery, the result failed to reach statistical significance (RR 0.79, 95% CI 0.62 to 1.01).
Exposure to allogeneic blood transfusion - transfusion protocol
The presence of a transfusion protocol appeared to have a slight impact on the overall rates of allogeneic RBC transfusion. Of the 19 trials that reported data on the number of patients exposed to allogeneic RBC transfusion 10 reported the use of transfusion protocols. These trials included a total of 736 patients of whom 373 were randomised to DDAVP. For these 10 trials the relative risk of receiving a red cell transfusion was 0.90 (95% CI 0.77 to 1.04) compared to 1.03 (95% CI 0.93 to 1.14) for the nine trials (N = 651 patients) that did not report the use of transfusion protocols to guide transfusion practice.
Exposure to allogeneic blood transfusion - use of autologous techniques (acute normovolemic hemodilution, pre-operative autologous donation, cell savage)
Of the 19 trials that reported data for the number of patients exposed to allogeneic blood transfusion, nine involved the use of techniques that collect, store, and re-infuse a patient’s own blood (that is acute normovolaemic hemodilution (ANH), pre-operative autologous donation (PAD), cell salvage (CS)). Given that these autologous techniques are used to reduce a patient’s risk of exposure to allogeneic blood it was deemed appropriate to examine the effect that these co-interventions had on treatment effect. For the nine trials that reported the use of autologous techniques the relative risk of exposure to allogeneic blood transfusion was 1.00 (95% CI 0.84 to 1.19). Whereas, for those 10 trials that did not use autologous techniques, the relative risk of exposure to allogeneic blood transfusion was 0.91 (95% CI 0.78 to 1.07). Although only marginally different, these results show a trend toward an additive effect of the autologous techniques in reducing exposure to allogeneic blood transfusion.
Exposure to allogeneic blood transfusion - methodological quality
For the five trials that were judged to have used an inappropriate method to conceal treatment allocation (Grade C: Cochrane scale) the relative risk of exposure to allogeneic blood transfusion was 1.11 (95% CI 0.94 to 1.33; I2 = 0%); compared to 0.88 (95% CI 0.75 to 1.03; I2 = 49.8%) for those 11 trials that failed to report the method used to conceal treatment allocation. Only three trials were judged to have adequately concealed treatment allocation. For these three trials the risk of exposure to allogeneic blood transfusion was 0.97 (95% CI 0.75 to 1.24; I2 = 0%).
Volume of blood transfused
Fourteen trials of DDAVP reported data for the volume of allogeneic RBC transfused. Overall there was no evidence of a significant reduction in the volume of red blood cells transfused. The reduced volume with DDAVP was minimal and not clinically significant (WMD −0.3 units, 95% CI −0.60 to −0.01 units). Heterogeneity in treatment effect was statistically significant (P = 0.07; I2 = 38.6%).
Five trials (Ansell 1992; Casas 1995; Dilthey 1993; Gratz 1992; Wong 2003) reported data for the volume of blood transfused, in those patients transfused. These trials included a total of 211 patients of whom 105 were randomised to DDAVP. The use of DDAVP appeared to reduce the volume of blood transfused in those patients receiving a blood transfusion by around half a unit of blood (WMD −0.49 units, 95% CI −0.94 to −0.04). Although this result was statistically significant such a small reduction would not be deemed clinically significant.
Volume of blood transfused - cardiac surgery
Ten trials involving cardiac surgery reported data for the volume of allogeneic blood transfused. These trials included a total of 621 patients of whom 322 were randomised to DDAVP treatment. Although the use of DDAVP reduced the amount of allogeneic blood transfused (WMD −0.39 units, 95% CI −0.77 to −0.01 units) such a small reduction would not be deemed clinically important.
Volume of blood transfused - use of autologous techniques (ANH, PAD, CS)
Although the use of autologous techniques appeared to reduce the volume of blood transfused to a greater degree than when such techniques were not employed (WMD −0.47 units, 95% CI −1.15 to 0.20 units; WMD −0.22 units, 95% CI −0.55 to 0.10 units, respectively) both results failed to reach statistical significance.
Intra-operative blood loss
Seven trials reported intra-operative blood loss data. These trials included a total of 493 patients of whom 243 were randomised to DDAVP treatment. Overall, the use of DDAVP did not statistically significantly reduce intra-operative blood loss (WMD −90.07 ml, 95% CI −199.56 to 19.42 ml). When the three cardiac trials were excluded from the analysis (as DDAVP was administered at the end of CPB and not at the beginning of the operation) a similar non-significant result was observed (WMD −51.99 ml, 95% CI −200.46 to 96.48 ml).
Post-operative blood loss
Eighteen trials reported post-operative blood loss data. These trials included a total of 1201 patients of whom 607 were randomised to DDAVP treatment. Although the use of DDAVP reduced post-operative blood loss, on average by 92.98 ml per patient (WMD −92.98 ml, 95% CI −149.86 to −36.11 ml), such a small reduction would not be deemed clinically significant. Heterogeneity in treatment effect was statistically significant (P = 0.001; I2 = 57.5%). Of the 18 trials that provided data for this outcome, 16 involved cardiac surgery. In the context of cardiac surgery, a similar statistically significant reduction in post-operative blood loss was observed (WMD −96.58, 95% CI −163.04 to −30.12). For this subgroup of trials heterogeneity in treatment effect was statistically significant (P = 0.0006; I2 = 61.8%). Of the 16 cardiac trials that reported post-operative blood loss, 10 trials reported blood loss measured 24-hours post-surgery. Although the use of DDAVP was associated with statistically significant reductions in 24-hour blood loss the reductions were small and not clinically significant (WMD −107.46 ml, 95% CI −207.12 to −7.80 ml). Statistically significant heterogeneity in treatment effect persisted (P = 0.002; I2 = 65.3%). Only three cardiac trials reported post-operative blood loss measured 12 hours after surgery. On average, the use of DDAVP did not significantly reduce blood loss during this time period (WMD −114.05 ml, 95% CI −269.46 to 41.36 ml).
Post-operative blood loss - cardiac surgery and pre-operative ASA use
Of the 16 trials that involved cardiac surgery and reported data for post-operative blood loss, 10 reported the use of acetylsalicylic acid (ASA) within seven days of surgery. These 10 trials included a total of 633 patients of whom 324 were randomised to DDAVP treatment. Although the use of DDAVP was associated with a statistically significant reduction in post-operative blood loss in patients receving ASA therapy within seven days of surgery, the reduction would not be considered clinically significant (WMD −109.57 ml, 95% CI −200.11 to −19.03 ml). Heterogeneity in treatment effect was statistically significant (P = 0.007; I2 = 60%). Three cardiac trials reported that ASA therapy was ceased seven to 10 days pre-operatively or they excluded patients that had received ASA therapy within seven days of surgery. These three trials included a total of 221 patients of whom 110 were randomised to DDAVP. Although there appeared to be a trend toward a reduction in post-operative blood loss in DDAVP patients who did not receive ASA within seven days of surgery the result failed to reach statistical sigificance (WMD −112.69 ml, 95% CI −227.59 to 2.22 ml).
Post-operative blood loss - CPB times in cardiac surgery trials
When post-operative blood loss was stratified by CPB times, trials with the longest bypass times (that is greater than 140 minutes) showed the greatest reductions in blood loss (WMD −344.74 ml, 95% CI −478.50 to −210.97 ml). Interestingly these two trials (Despotis 1999; Salzman 1986) both recorded mean reductions in the amount of allogeneic blood transfused of 1.1 units per patient with DDAVP compared to control. The only other subgroup that showed a statistically significant reduction in post-operative blood loss was for those trials that reported bypass times of between 80 and 100 minutes. For these five trials, the use of DDAVP resulted in an average reduction in post-operative blood loss of around 104 ml per patient (WMD −104.18 ml, 95% CI −184.75 to −23.61 ml). Heterogeneity in treatment effect was statistically significant (P < 0.1) for two of the five subgroups (CPB times less than 80 min, CPB times between 121 and 140 min).
Total blood loss - intra- and post-operative blood loss
Ten trials reported total blood loss data (intra- and post-operative). These trials included a total of 669 patients of whom 331 were randomised to DDAVP. In aggregate, the use of DDAVP significantly reduced the volume of blood lost during the intra- and post-operative periods (WMD −241.78 ml, 95% CI −387.55 to −96.01 ml). Of the 10 trials that provided data for this outcome seven trials involved cardiac surgery. For these seven cardiac trials, DDAVP was associated with a significant reduction in intra- and post-operative blood loss of around 238 mls per patient (WMD −237.92 ml, 95%CI −413.43 to −62.40 ml). Heterogeneity in treatment effect was statistically significant (P = 0.0008; I2 = 74.1%). For the three non-cardiac trials, where DDAVP was administered at the start of surgery, total blood loss was reduced on average by 280 ml per patient (WMD −280.32 ml, 95% CI −500.95 to −59.69 ml). Heterogeneity in treatment effect was not statistically significant (P = 0.47; I2 = 0%).
Adverse events and other outcomes
Re-operation for bleeding
Eleven trials reported data on the rates of re-operation due to bleeding. These trials included a total of 778 patients of whom 383 were randomised to DDAVP. Although the rate of re-operation for bleeding was lower in the DDAVP group (1.83%) than in the control group (3.54%) the difference in absolute terms was not statistically significant (RD −0.02, 95% CI −0.04 to 0.01; I2 = 9.5%).
Mortality
Twelve trials reported mortality data. These trials included a total of 1061 patients of whom 534 were randomised to DDAVP treatment. In aggregate, mortality was slightly higher in DDAVP treated patients (2.43%) than control patients (1.33%). However, the difference in mortality rates was not statistically significant (RD 0.01, 95% CI −0.01 to 0.03; I2 = 0%).
Myocardial infarction
Twelve trials reported data for myocardial infarction (MI). These trials included a total of 876 patients of whom 441 were randomised to DDAVP. The overall rate of MI was 5.25% with 28 DDAVP patients (6.35%) sustaining a MI compared with 18 in the control group (4.14%). Although there appeared to be a trend toward an increased risk of MI with DDAVP treatment, the result failed to reach statistical significance (RR 1.38, 95% CI 0.77 to 2.50; I2 = 0%).
Stroke
Five trials reported data for stroke. These trials included a total of 360 patients of whom 184 were randomised to DDAVP. The overall rate of stroke was 2.7%, with eight DDAVP patients (4.35%) suffering a stroke compared with only two in the control group (1.14%). However, the risk of developing a stroke in those patients treated with DDAVP was not significantly different from that for control patients (RR 2.4, 95% CI 0.68 to 8.43; I2 = 0%).
Any thrombosis
There were nine trials that reported data for thrombosis of any type. These trials included a total of 691 patients of whom 361 were randomised to DDAVP. A thrombotic event occurred in 24 patients (n = 14 (DDAVP) versus n = 10 (control)). The relative risk of developing thrombosis of any type in those patients treated with DDAVP was 1.46 (95% CI 0.64 to 3.35; I2 = 0%) compared to control.
Hypotension
Five trials reported episodes of hypotension requiring urgent treatment (that is fluids, vasoactive drugs) during the infusion of the trial agents. Overall, there were 43 episodes of hypotension during the administration of the trial agents (n = 34 (DDAVP) versus n = 9 (control)). The risk of developing hypotension that required urgent intervention was statistically significant for patients treated with DDAVP (RR 2.81, 95% CI 1.50 to 5.27; I2 = 0%).
DISCUSSION
Evidence of benefit
Desmopressin remains a valuable treatment for patients with mild haemophilia or type I von Willebrand’s disease who have spontaneous bleeding or are scheduled for surgery (Mannucci 1998). Although this review found that the use of DDAVP in elective surgery reduced post-operative and total blood loss in patients without hereditary bleeding disorders, we found no evidence of benefit with DDAVP in terms of reduced red cell transfusion. There appeared to be a trend toward a lower rate of re-operation due to recurrent or continued bleeding in those patients treated with DDAVP. However, this analysis was based on a very small number of events and the difference did not reach statistical significance. Furthermore, this review found no evidence of reduced mortality in patients treated with DDAVP. The results of our meta-analysis are consistent with other published meta-analyses in which DDAVP treatment was shown to have a minor effect on reducing surgical blood loss but no statistically significant effect on reducing transfusion requirements (Cattaneo 1995; Levi 1999).
Adverse events
In individual studies the numbers of adverse events were small. Although we found a small increase in the rates of myocardial infarction, stroke, and any thrombosis in DDAVP treated patients, these trends did not reach statistical significance. The data do not appear to point to unacceptable toxicity. Rather the apparent lack of efficacy is a greater concern. Although only five small trials reported episodes of hypotension during the infusion of the trial agents, in aggregate the results indicate that patients receiving an infusion of DDAVP are 2.8 times as likely (RR 2.81, 95% CI 1.50 to 5.27) to develop hypotension requiring urgent treatment with fluids and vasoactive agents (for example intravenous epinephrine) than those patients who received a placebo control.
Sources of bias
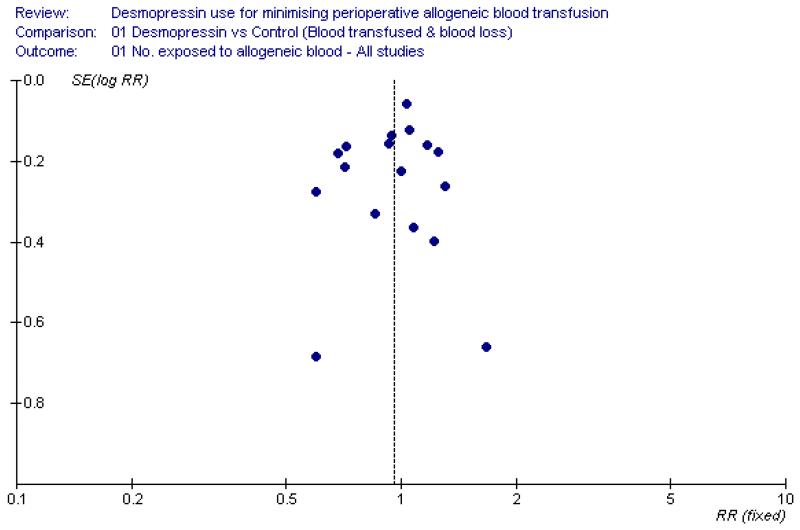
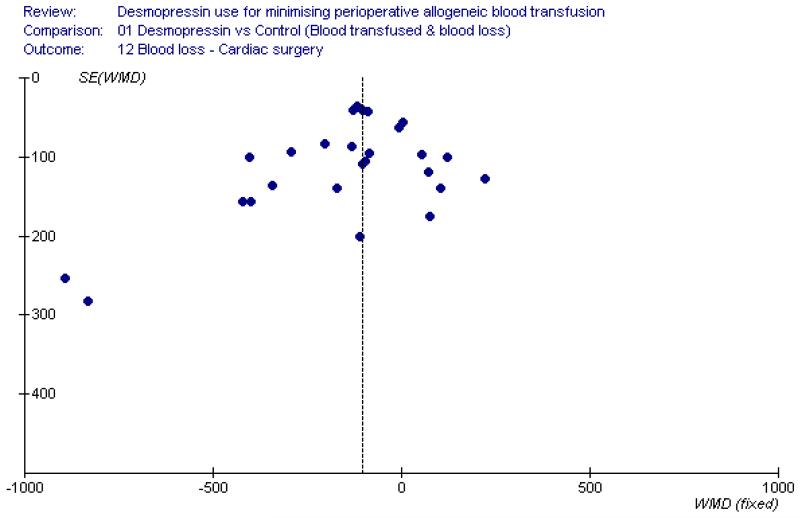
In our review we found a number of small trials. Reliance on small trials raises concerns about the effects of publication bias. However for the primary outcome of this review the results are negative, so it is unlikely that publication bias has had an important effect. Examination of the funnel plot for allogeneic blood transfusion (Figure 3) shows that the results are generally symmetrical around the point estimate (RR 0.96), indicating there is little evidence of publication bias for this outcome. This is also the case with blood loss in the context of cardiac surgery, where the funnel plot appears to be reasonably symmetrical (Figure 4). The primary study outcome of this review is a practise variable involving the decision to transfuse a patient with allogeneic red cells. In the case of the trials reviewed, the decision to transfuse a patient with allogeneic blood required a degree of subjectivity on the part of clinicians and was probably not a source of bias as 93% of the trials involved a placebo and in 97% of trials the outcomes assessor was blind to the allocated treatment.
Figure 3 . Funnel plot assessment for the presence of publication bias.
Figure 4 . Funnel plot assessment for the presence of publication bias.
Clinical significance of the results
It is important to consider the relevance of DDAVP as an adjunct to surgical practices. Although the majority of trials included in this review were conducted in the setting of cardiac surgery (76%) it is likely that these results can be applied to other surgical settings. Blood loss tends to be high during cardiac surgery, so that any beneficial effects of treatment with DDAVP should be readily demonstrated. On the basis of the currently available evidence, there appears to be no clear beneficial effect of using DDAVP to minimise peri-operative transfusion with allogeneic blood. Although there appeared to be evidence that DDAVP reduced post-operative and total blood loss in the context of cardiac surgery, these may not be regarded as clinically meaningful reductions.
In the case of post-operative blood loss in cardiac surgery we observed significant heterogeneity in treatment effect (P = 0.0006; I2 = 61.8%). To investigate this further we stratified post-operative blood loss data by the time period of assessment (that is 0 to 6 hours, 0 to 12 hours, 0 to 16 hours, 0 to 24 hours). Statistically significant heterogeneity was evident in all time periods of assessment, with the exception of one (0 to 16 hours). Only two trials provided data for this particular subgroup. More importantly, we observed considerable differences between trials in the amounts of blood lost during the post-operative period. For the 10 cardiac trials that reported post-operative blood loss data for the 0 to 24-hour period, baseline blood loss volumes ranged from as low as 429 ml to as high as 1386 ml. In patients treated with DDAVP, blood loss volumes ranged from as low as 422 ml to as high as 1214 ml.
It is reasonable to argue that the observed differences in blood loss volumes between trials was due to the different types of surgery performed, and the complexity of the surgery performed. In the case of cardiac surgery, some trials included patients undergoing primary, first-time, or coronary artery bypass graft (CABG) surgery, whilst other trials included patients undergoing more complex cardiac surgery such as re-do CABG, valvular surgery, and combined procedures (for example CAGB and valvular surgery). The duration of surgery also varied considerably between trials, with operative times ranging from 133 minutes to more than 390 minutes (Table 1). Given that the duration of cardiopulmonary bypass (CPB) represents a reasonable surrogate for surgical complexity, with longer CPB times generally associated with an increased risk of greater post-operative blood loss, we stratified post-operative blood loss by CPB times (separated into mutually exclusive categories) to ascertain whether this specific factor may have influenced the results.
Firstly, we tabulated post-operative blood loss volumes for each cardiac trial (where data were available) and the corresponding CPB times (Table 2). The tabulated data did not appear to show any correlation between CPB times and the volume of post-operative blood loss. The results of two trials, in particular, require closer scrutiny. Whilst Salzman et al (Salzman 1986) reported a mean post-operative blood loss of 510 ml (+/− 289) with a mean CPB time of 144 min (+/− 38) in patients treated with DDAVP, Ansell et al (Ansell 1992) reported a mean post-operative blood loss of 1064.8 ml (+/− 647.1) with a mean CPB time of 118.6 min (+/− 39). The latter trial had more than double the average post-opertive blood loss but shorter CPB times of around 25 minutes. Secondly, we performed a meta-analysis of post-operative blood loss stratified by the CPB times to determine whether the use of DDAVP was more or less effective over varying CPB times. Although DDAVP appeared to be most effective in patients undergoing more lengthy CPB (that is greater than 140 minutes in duration) the results were generally mixed, showing no effect in trials with CPB of less than 80 minutes in duration, between 101 and 120 minutes, and between 121 and 140 minutes in duration. Patients receiving DDAVP with CPB times of between 80 and 100 minutes had a statistically significant, but not clinically significant, reduction in post-operative blood loss of around 104 ml (95% CI 23.61 to 184.75 ml). The greatest reductions in post-operative blood loss were observed in patients with CPB times greater than 140 minutes. For this subgroup, post-operative blood loss was reduced by around 345 ml per patient in patients treated with DDAVP compared to control patients (95% 210.97 to 478.50 ml). However, given the sparsity of data these results need to be interpreted with caution.
Interventions other than DDAVP have been shown to be effective in reducing the need for allogeneic red cell transfusion. The anti-fibrinolytic drugs aprotinin, tranexamic acid (TXA), and epsilon aminocaproic acid (EACA) have been studied extensively in cardiac surgery. Results of a meta-analysis for the aforementioned anti-fibrinolytics (Henry 2007) showed that on average aprotinin reduced the need for red blood cell transfusion by a relative 34%; and importantly it reduced the need for re-operation due to recurrent or continued bleeding by a relative 52%. Similar trends were seen with TXA and EACA, although fewer trials have been performed. However, the benefits of aprotinin have been brought into question with the publication of two notable observational studies (Karkouti 2006; Mangano 2006; Mangano 2007) and a large randomised head-to-head comparative trial (Fergusson 2008). The randomised trial conducted by Fergusson et al (Fergusson 2008) in high-risk cardiac surgery examined the comparative efficacy and safety of aprotinin and the lysine analogues (TXA and EACA). The results of this trial showed that there was a strong and consistent negative mortality trend associated with aprotinin compared to the lysine analogues. Fergusson et al (Fergusson 2008) found that at 30 days the rate of death from any cause was 6.0% in the aprotinin group compared with 3.9% in the TXA group (RR 1.55, 95% CI 0.99 to 2.42) and 4.0% in the EACA group (RR 1.52, 95% CI 0.98 to 2.36). The findings of this large, methodologically rigorous randomised trial have raised doubts over the continued use of aprotinin in high-risk cardiac surgery. Whether TXA and EACA represent more cost-effective strategies in the context of high-risk cardiac surgery is yet to be fully elucidated.
A variety of blood-sparing techniques have also been employed in the peri-operative setting. Most involve the re-infusion of autologous blood, either from pre-operative deposit, acute normovolemic hemodilution, or cell salvage. The evidence on the efficacy and safety of these techniques has been thoroughly reviewed (Bryson 1998; Carless 2006; Forgie 1998; Henry 2002; Huet 1999). The literature is generally viewed as being of indifferent quality because of inadequate randomisation and lack of blinding of outcomes assessment. The techniques appear to have a modest blood sparing effect. Significantly, the efficacy of autologous re-infusion techniques appears to be lower when they are used in the context of a rigorous transfusion protocol. This and the growing evidence on the efficacy of transfusion triggers indicates that a more conservative approach to blood transfusion decisions is generally desirable (Carson 1998; Hebert 1995; Hebert 1999). This conservative approach, combined with the use of anti-fibrinolytic drugs, may possibly offer the best approach for managing the transfusion requirements of patients in high-risk settings such as cardiac surgery.
Conclusions
There is no evidence that DDAVP minimises peri-operative allogeneic blood transfusion; nor that its use reduces the need for allogeneic blood or improves health outcomes during or after surgery. Although the data suggest that there is some benefit of using DDAVP as a means of reducing peri-operative blood loss the observed reductions were small and generally not clinically important. However, in the context of cardiac surgery, the use of DDAVP significantly reduced post-operative blood loss in those trials with the longest cardiopulmonary bypass times (that is exceeding 140 minutes). Given the paucity of data this finding needs to be interpreted with caution. Overall, the data appear to rule out a worthwhile benefit of using DDAVP as a means of minimising peri-operative allogeneic blood transfusion in patients who do not have congenital bleeding disorders.
AUTHORS’ CONCLUSIONS
Implications for practice
The use of DDAVP in elective adult surgery for patients who do not suffer from congenital bleeding disorders cannot be justified. The anti-fibrinolytic agents aprotinin, tranexamic acid, and epsilon aminocaproic acid have been extensively researched and their effectiveness in reducing the need for allogeneic blood transfusion is well documented, particularly in the area of cardiac surgery. Given the recent negative findings regarding the use of aprotinin in high-risk cardiac surgery, the lysine analogues tranexamic acid and epsilon aminocaproic acid may offer a safer alternative to aprotinin. In formulating their decision, clinicians need to carefully consider the cost effectiveness of each alternative blood conserving strategy.
Implications for research
Further placebo-controlled clinical trials of DDAVP as an adjunct to surgery in patients who do not have bleeding disorders appears unwarranted.
PLAIN LANGUAGE SUMMARY.
Use of desmopressin to reduce the need for blood transfusions in patients who do not suffer from congenital bleeding disorders
Risks of infection from transfused blood given by an unrelated donor are minimal when blood is screened by a competent transfusion service but concerns still remain. Techniques are available to reduce the need for a transfusion. The review of trials found that there is no convincing evidence that desmopressin reduces the need for blood transfusion in patients who do not have congenital bleeding disorders and are undergoing non-urgent or elective surgery. Other strategies, such as the use of anti-fibrinolytic drugs, may be more effective but are not included in this review.
SOURCES OF SUPPORT
Internal sources
Special purpose grant, Hunter Area Pathology Services, NSW, Australia.
External sources
Australian Health Ministers’ Advisory Committee. National Health and Medical Research Council of Australia, Australia.
CHARACTERISTICS OF STUDIES
Characteristics of included studies [ordered by study ID]
| Methods | Randomisation was provided by a series of computer-generated random numbers. All study medication was dispensed by the investigator or hospital pharmacy | |
| Participants | 83 patients undergoing valvular heart operations with or without concomittant coronary artery bypass surgery were randomised to one of two groups
|
|
| Interventions |
NB: Both groups were exposed to pre-operative autologous blood donation (PAD) and postoperative epsilon aminocaproic acid (EACA) |
|
| Outcomes | Number of patients transfused allogeneic blood (n) Amount of allogeneic and autologus blood transfused (units) Fresh frozen plasma (FFP) transfused (n/units) Platelets transfused (n/units) Blood loss (mls) Mortality (n) Myocardial infarction (n) Re-operation for bleeding (n) Bowel infarction (n) Brain stem infarction (n) Femoral artery embolism (n) Cardiac arrest (n) Retinal artery embolism (n) |
|
| Notes | Quality assessment score (Schulz criteria): 4/7 Transfusion protocol not specified. |
|
| Risk of bias | ||
| Item | Authors’ judgement | Description |
| Adequate sequence generation? | Yes | Randomisation was provided by a series of computer-generated random numbers |
| Allocation concealment? | Unclear | B - Unclear. |
| Blinding? All outcomes |
Yes | Double blind. |
| Methods | Methods of randomisation and allocation concealment were not described | |
| Participants | 20 patients scheduled to undergo elective CABG surgery were randomised in double-blind fashion to one of two groups
|
|
| Interventions |
NB: Both groups were exposed to cell salvage. |
|
| Outcomes | Number of patients transfused allogeneic blood (n) Amount of allogeneic blood transfused (units) Blood loss (mls) Thrombosis (n) Re-operation for bleeding (n) Myocardial infarction (n) Hypotension (n) |
|
| Notes | Quality assessment score (Schulz criteria): 3/7 Transfusion protocol not described. |
|
| Risk of bias | ||
| Item | Authors’ judgement | Description |
| Adequate sequence generation? | Unclear | Method of randomisation was not described. |
| Allocation concealment? | No | C - Inadequate. |
| Blinding? All outcomes |
Yes | Double blind. |
| Methods | Allocation concealment was by means of sealed envelopes. The pharmacist prepared the encoded infusions. All study infusions were identical in outward appearance | |
| Participants | 149 patients scheduled for elective coronary artery bypass graft surgery, heart valve replacement or annuloplasty, combined valve replacement and coronary artery bypass grafting, or closure of atrial septal defects were randomly assigned to one of three groups
NB: Three patients requiring re-operation due to bleeding related to the operation (one in the aprotinin group and two in the DDAVP group) were excluded from the final analysis |
|
| Interventions |
|
|
| Outcomes | Number of patients transfused allogeneic blood (n) Amount of allogeneic blood transfused (units) Number of patients transfused fresh frozen plasma (FFP) (n) Number of patients transfused platelets (n) Blood loss (ml) Mortality (n) Re-operation for bleeding (n) Femoral embolism (n) Stroke (n) |
|
| Notes | Quality assessment score (Schulz criteria): 4/7 Transfusion protocol used. |
|
| Risk of bias | ||
| Item | Authors’ judgement | Description |
| Adequate sequence generation? | Unclear | Method of randomisation was not described. |
| Allocation concealment? | No | C - Inadequate. Allocation concealment was by means of sealed envelopes |
| Blinding? All outcomes |
Yes | Double blind. |
| Methods | Randomisation was carried out in blocks of 10 and was stratified for repair of abdominal aortic aneurysm (AAA) or aorto-femoral bypass for occlusive disease. Patients were randomised by drawing a sealed envelope that contained a prescription for either desmopressin (DDAVP) or placebo. The method used to generate allocation sequences was not described. The only person to have knowledge of treatment assignment was the pharmacist, who kept records and prepared the DDAVP or placebo in identical-appearing plastic bags of 50 ml normal saline solution | |
| Participants | 91 male patients undergoing elective aortic surgical operations were randomly assigned to one of two groups
|
|
| Interventions |
NB: Both groups were exposed to autotransfusion and cell salvage |
|
| Outcomes | Number of patients transfused allogeneic blood (n) Amount of allogeneic blood transfused (units) Blood loss (mls) Mortality (n) Myocardial infarction (n) Deep vein thrombosis (n) Pulmonary embolus (n) Acute limb ischemia (n) Bowel infarction (n) |
|
| Notes | Quality assessment score (Schulz criteria): 5/7 Transfusion protocol not described. |
|
| Risk of bias | ||
| Item | Authors’ judgement | Description |
| Adequate sequence generation? | Unclear | Method of randomisation was not described. |
| Allocation concealment? | No | C - Inadequate. Allocation concealment was by means of sealed envelopes |
| Blinding? All outcomes |
Yes | Double blind. |
| Methods | Randomisation was based on a computer-generated random-number table and done according to a sequential allocation schedule that was generated by an investigator not involved in treatment assignment. Desmopressin and placebo were administered intravenously as colourless fluids from unlabelled syringes, aspirated in a separate room and transported to the operating room by one of the investigators (not masked to treatment assignment) | |
| Participants | 101 patients undergoing elective cardiac surgery in whom clot ratios were abnormal (i.e. hemoSTA-TUS-derived clot ratios were <60% of maximum in channel 5) were randomly assigned to one of two groups
|
|
| Interventions |
NB: A proportion of DDAVP and placebo patients received epsilon-aminocaproic acid (EACA) 50% and 61% respectively. EACA was given at the discretion of the managing physician. EACA was administered as follows: 5 g loading dose, 5 g in the CPB circuit, and 1g/hr infusion |
|
| Outcomes | Amount of allogeneic blood transfused (units) Fresh frozen plasma transfused (units) Platelets transfused (units) Blood loss (mls) Mortality (n) Myocardial infarction (n) Re-operation for bleeding (n) Any thrombosis (n) |
|
| Notes | Quality assessment score (Schulz criteria): 5/7 Transfusion of RBC was at the discretion of the managing physicians |
|
| Risk of bias | ||
| Item | Authors’ judgement | Description |
| Adequate sequence generation? | Yes | Randomisation was based on a computer-generated random-number table |
| Allocation concealment? | Unclear | B - Unclear |
| Blinding? All outcomes |
Yes | Double blind. |
| Methods | Methods of randomisation and allocation concealment were not described | |
| Participants | 40 consecutive male patients undergoing elective primary myocardial revascularisation were randomly assigned to one of two groups
NB: Gender data not reported. One patient was excluded from the desmopressin group due to excessive post-operative bleeding requiring re-exploration - surgical cause identified |
|
| Interventions |
NB: Both groups were exposed to cell salvage. |
|
| Outcomes | Number of patients transfused allogeneic blood (n)Amount of allogeneic blood transfused (units) Number of patients transfused platelets (n) Blood loss (ml) Re-operation for bleeding (n) |
|
| Notes | Quality assessment score (Schulz criteria): 3/7 Transfusion protocol used. |
|
| Risk of bias | ||
| Item | Authors’ judgement | Description |
| Adequate sequence generation? | Unclear | Method of randomisation was not described. |
| Allocation concealment? | Unclear | B - Unclear |
| Blinding? All outcomes |
Yes | Double blind. |
| Methods | A computer-generated randomisation table was used to allocate patients to one of three treatment groups. The method used to conceal treatment allocation was not described | |
| Participants | 30 patients undergoing elective total knee replacement were randomly allocated to one of three groups
|
|
| Interventions |
|
|
| Outcomes | Number of patients transfused allogeneic blood (n) Amount of allogeneic blood transfused (units) Hospital length of stay (days) Hematological data |
|
| Notes | Quality assessment score (Schulz criteria): 3/7 Transfusion protocol used. |
|
| Risk of bias | ||
| Item | Authors’ judgement | Description |
| Adequate sequence generation? | Unclear | A computer-generated randomisation table was used to allocate patients |
| Allocation concealment? | Unclear | B - Unclear |
| Blinding? All outcomes |
Yes | Double blind. |
| Methods | Method of randomisation and allocation concealment was not described | |
| Participants | 50 patients scheduled for total hip replacement were randomly assigned to one of two groups
|
|
| Interventions |
|
|
| Outcomes | Amount of allogeneic blood transfused (units) Blood loss (ml) - intra- and post-operative |
|
| Notes | Quality assessment score (Schulz criteria): 4/7 Transfusion protocol not used. |
|
| Risk of bias | ||
| Item | Authors’ judgement | Description |
| Adequate sequence generation? | Unclear | Method of randomisation was not described. |
| Allocation concealment? | Unclear | B - Unclear |
| Blinding? All outcomes |
Yes | Double blind. |
| Methods | Patients were randomly assigned to study drug or placebo by a system of sealed envelopes. The placebo solution was identical in appearance to the study drug solution. The method used to generate allocation sequences was not described | |
| Participants | 40 patients undergoing elective primary coronary artery bypass graft surgery were randomly assigned to one of two groups
|
|
| Interventions |
NB: Both groups were exposed to cell salvage. |
|
| Outcomes | Number of patients transfused allogeneic blood (n) Amount of allogeneic blood transfused (units)xbr Fresh frozen plasma (units) Platelets (units) Blood loss (ml) Re-operation for bleeding (n) Hypotension (n) |
|
| Notes | Quality assessment score (Schulz criteria): 5/7 Transfusion protocol not specified. |
|
| Risk of bias | ||
| Item | Authors’ judgement | Description |
| Adequate sequence generation? | Unclear | Method of randomisation was not described. |
| Allocation concealment? | No | C - Inadequate. Allocation concealment was by means of sealed envelopes |
| Blinding? All outcomes |
Yes | Double blind. |
| Methods | Patients were assigned to desmopressin or placebo according to a randomisation schedule. The method used to generate allocation sequences was not described. Study solutions were prepared by a research pharmacist | |
| Participants | 65 patients pre-treated with aspirin within 7 days before scheduled elective coronary artery bypass graft surgery were randomised to one of two groups
NB: Six patients were excluded from the analysis, three from the DDAVP group and three from the placebo group (two died intra-operatively, one inadvertently received DDAVP, and three required the insertion of an intra-aortic ballon pump) |
|
| Interventions |
NB: Both groups were exposed to cell salvage. |
|
| Outcomes | Number of patients transfused allogeneic blood (n) Amount of allogeneic blood transfused (units) Fresh frozen plasma (units) Platelets (units) Blood loss (ml) Mortality (n) Non-fatal myocardial infarction (n) Arrhythmia (n) Encephalopathy (n) |
|
| Notes | Quality assessment score (Schulz criteria): 5/7 Transfusion protocol not specified. |
|
| Risk of bias | ||
| Item | Authors’ judgement | Description |
| Adequate sequence generation? | Unclear | Method of randomisation was not described. |
| Allocation concealment? | Yes | A - Adequate |
| Blinding? All outcomes |
Yes | Double blind. |
| Methods | Methods of randomisation and allocation concealment were not described | |
| Participants | 20 patients undergoing bimaxillary osteotomy were randomly assigned to one of two groups
NB: Age of patients was not reported. |
|
| Interventions |
NB: Both groups were exposed to pre-operative autologous blood donation (PAD) |
|
| Outcomes | Number of patients transfused allogeneic blood (n) Amount of allogeneic and autologous blood transfused (units) Blood loss (ml) Hypotension (n) |
|
| Notes | Quality assessment score (Schulz criteria): 2/7 Transfusion protocol not specified. |
|
| Risk of bias | ||
| Item | Authors’ judgement | Description |
| Adequate sequence generation? | Unclear | Method of randomisation was not described. |
| Allocation concealment? | Unclear | B - Unclear |
| Blinding? All outcomes |
No | Studied an untreated control group. |
| Methods | Methods of randomisation and allocation concealment were not described | |
| Participants | 150 consecutive patients undergoing elective cardiac surgery involving cardiopulmonary bypass (CPB) were randomly assigned to one of two groups
NB: Data for gender was not reported. |
|
| Interventions |
NB: Both groups were exposed to cell salvage. |
|
| Outcomes | Number of patients transfused allogeneic blood (n) Amount of allogeneic blood transfused (units) Number of patients transfused FFP and Hetastarch (n) Amount of FFP and Hetastarch transfused (ml) Number of patients receiving autotransfusion (n) Amount of blood autotransfused (ml) Blood loss (ml) Hematological data |
|
| Notes | Quality assessment score (Schulz criteria): 3/7 Transfusion protocol not specified. |
|
| Risk of bias | ||
| Item | Authors’ judgement | Description |
| Adequate sequence generation? | Unclear | Method of randomisation was not described. |
| Allocation concealment? | Unclear | B - Unclear |
| Blinding? All outcomes |
Yes | Double blind. |
| Methods | Patients were assigned to either treatment or placebo groups using a computer-generated random-number table. The method used to conceal treatment allocation was not described | |
| Participants | 62 patients undergoing elective coronary artery bypass grafting were randomised to one of two groups
|
|
| Interventions |
|
|
| Outcomes | Number of patients transfused allogeneic blood transfusion (n) Amount of allogeneic blood transfused (units) Blood loss (ml) Mortality (n) Myocardial infarction (n) Re-operation for bleeding (n) |
|
| Notes | Quality assessment score (Schulz criteria): 4/7 Transfusion protocol not used. |
|
| Risk of bias | ||
| Item | Authors’ judgement | Description |
| Adequate sequence generation? | Yes | Patients were assigned to either treatment or placebo groups using a computer-generated random-number table |
| Allocation concealment? | Unclear | B - Unclear |
| Blinding? All outcomes |
Yes | Double blind. |
| Methods | A table of random numbers determined patient allocation. Coded infusion bags and sealed envelopes were prepared by a pharmacist not involved in the study | |
| Participants | 163 patients undergoing elective coronary revascularisation, valve replacement, both procedures, or repair of atrial septal defects were randomly assigned to one of four groups
NB: Data for gender not reported. Four patients were excluded from the final analysis (one from the placebo group, one from the TXA group, one from the DDAVP group, and one from the TXA + DDAVP group) |
|
| Interventions |
NB: Both groups were exposed to cell salvage, if available. One patient in the placebo group received pre-operative autologous blood (PAD) |
|
| Outcomes | Number of patients transfused allogeneic blood (n) Number of patients transfused fresh frozen plasma (n) Number of patients transfused platelets (n) Blood loss (ml) Myocardial infarction (n) Stroke (n) Deep vein thrombosis (n) Re-operation for bleeding (n) Ventricular dysfunction (n) Pulmonary dysfunction (n) Cardiac arrhythmia (n) |
|
| Notes | Quality assessment score (Schulz criteria): 4/7 Transfusion protocol used. |
|
| Risk of bias | ||
| Item | Authors’ judgement | Description |
| Adequate sequence generation? | Yes | Randomisation was by means of a table of random numbers. |
| Allocation concealment? | No | C - Inadequate |
| Blinding? All outcomes |
Yes | Single blinding. |
| Methods | Methods of randomisation and allocation concealment were not described | |
| Participants | 33 patients scheduled to undergo elective primary coronary artery bypass grafting were randomly allocated to one of two groups
NB: Three patients were excluded from the final analysis (one from the placebo group and two from the DDAVP group) |
|
| Interventions |
NB: Both groups were exposed to acute normovolemic hemodilution (ANH). After induction of anaesthesia, one unit of whole blood was withdrawn from the patient and replaced with 1000 ml of Ringer’s solution. This autologous blood unit was given back to the patient after the available residual oxygenator perfusate had been returned |
|
| Outcomes | Amount of allogeneic blood transfused (units) Blood loss (ml) Mortality (n) Haemoglobin loss Cardiac failure (n) Crystalloid infusions (ml) |
|
| Notes | Quality assessment score (Schulz criteria): 3/7 Transfusion protocol used. |
|
| Risk of bias | ||
| Item | Authors’ judgement | Description |
| Adequate sequence generation? | Unclear | Method of randomisation was not described. |
| Allocation concealment? | Unclear | B - Unclear |
| Blinding? All outcomes |
Yes | Double blind. |
| Methods | Methods of randomisation and allocation concealment were not described | |
| Participants | 50 patients scheduled for aorto-iliac graft surgery were randomly allocated to one of two groups
NB: Age data were not reported. |
|
| Interventions |
NB: Both groups received 500 ml of dextran 40 (Rheomacrodex) as thromboprophylaxis |
|
| Outcomes | Amount of allogeneic blood transfused (units) Blood loss (ml) Myocardial infarction (n) |
|
| Notes | Quality assessment score (Schulz criteria): 3/7 Transfusion protocol used. |
|
| Risk of bias | ||
| Item | Authors’ judgement | Description |
| Adequate sequence generation? | Unclear | Method of randomisation was not described. |
| Allocation concealment? | Unclear | B - Unclear |
| Blinding? All outcomes |
Yes | Double blind. |
| Methods | Methods of randomisation and allocation concealment were not described | |
| Participants | 70 patients undergoing elective coronary artery bypass graft (CABG) surgery were randomised to one of three groups
NB: Data for gender was not reported. Two patients were excluded prior to randomisation for haemodynamic instability and three patients were excluded after randomisation for mediastinal exploration secondary to surgical bleeding |
|
| Interventions |
|
|
| Outcomes | Number of patients transfused allogeneic blood (n) Amount of allogeneic blood transfused (ml) Number of patients transfused fresh frozen plasma (n) Number of patients transfused platelets (n) Blood loss (ml) Myocardial infarction (n) Deep venous thrombosis (n) Stroke (n) |
|
| Notes | Quality assessment score (Schulz criteria): 3/7 Transfusion protocol used. |
|
| Risk of bias | ||
| Item | Authors’ judgement | Description |
| Adequate sequence generation? | Unclear | Method of randomisation was not described. |
| Allocation concealment? | Unclear | B - Unclear |
| Blinding? All outcomes |
Yes | Double blind. |
| Methods | A computer-generated random-number table was used to assign patients to treatment or control groups. Method used to conceal treatment allocation was not described | |
| Participants | 115 patients undergoing elective coronary artery bypass graft surgery were randomised after cardiopulmonary bypass (CPB) to one of four groups
MA = Mean amplitude of the thromboelastogram (TEG) NB: Patients were divided into groups based upon TEG analysis. Groups 1 and 2 consisted of patients with normal TEG parameters prior to and after separation from CPB (MA>50mm). Groups 3 and 4 consisted ofpatients with normal TEG parameters prior to CPB who demonstrated abnormal TEG values (TEG:MA<50mm) after CPB but prior to DDAVP or placebo administration |
|
| Interventions |
|
|
| Outcomes | Number of patients transfused allogeneic blood (n) Amount of allogeneic blood transfused (units) Blood loss (ml) Myocardial infarction (n) Mortality (n) Re-operation for bleeding (n) |
|
| Notes | Quality assessment score (Schulz criteria): 5/7 Transfusion protocol used. |
|
| Risk of bias | ||
| Item | Authors’ judgement | Description |
| Adequate sequence generation? | Yes | A computer-generated random-number table was used to assign patients to treatment or control groups |
| Allocation concealment? | Unclear | B - Unclear |
| Blinding? All outcomes |
Yes | Double blind. |
| Methods | Methods of randomisation and allocation concealment were not described | |
| Participants | 66 patients undergoing coronary artery bypass graft surgery were randomised to one of two groups
|
|
| Interventions |
NB: Both groups were exposed to acute normovolemic hemodilution (ANH). Approximately 300-400 ml of autologous blood was taken from all patients before heparinisation, if the hematocrit level was above 40%, and was re-administered after protamine administration. One patient in the DDAVP group and two in the control group did not undergo this process |
|
| Outcomes | Number of patients transfused allogeneic blood (n) Amount of allogeneic blood transfused (units) Blood loss (ml) Re-operation for bleeding (n) |
|
| Notes | Quality assessment score (Schulz criteria): 4/7 Transfusion protocol used. |
|
| Risk of bias | ||
| Item | Authors’ judgement | Description |
| Adequate sequence generation? | Unclear | Method of randomisation was not described. |
| Allocation concealment? | Unclear | B - Unclear |
| Blinding? All outcomes |
Yes | Double blind. |
| Methods | Patients were randomised to treatment or placebo by means of a computer program. Study drugs were prepared by the hospital pharmacy and delivered in identical 20 ml syringes | |
| Participants | 100 patients undergoing primary coronary artery bypass graft surgery were randomly allocated to one of two groups
NB: Four patients from each group were not included in the final analysis |
|
| Interventions |
NB: Both groups were exposed to cell salvage (autotransfusion) and post-operative tranexamic acid |
|
| Outcomes | Number of patients transfused allogeneic blood (n) Number of patients transfused fresh frozen plasma (n) Number of patients transfused platelets (n) Amount of allogeneic blood transfused (units) Blood loss (ml) Amount of blood autotransfused (ml) Re-operation for bleeding (n) Renal failure (n) Pulmonary embolus (n) |
|
| Notes | Quality assessment score (Schulz criteria): 6/7 Transfusion protocol used. |
|
| Risk of bias | ||
| Item | Authors’ judgement | Description |
| Adequate sequence generation? | Yes | Patients were randomised to treatment or placebo by means of a computer program |
| Allocation concealment? | Yes | A - Adequate |
| Blinding? All outcomes |
Yes | Double blind. |
| Methods | Methods of randomisation and allocation concealment were not described | |
| Participants | 27 patients undergoing elective myocardial revascularisation or single-valve replacement surgery were randomised to one of two groups
|
|
| Interventions |
NB: Both groups were exposed to cell salvage (Haemonetics) in the pre-and post-CPB periods |
|
| Outcomes | Amount of allogeneic blood transfused (units) Blood loss (ml) Hypotension (n) |
|
| Notes | Quality assessment score (Schulz criteria): 4/7 Transfusion protocol not specified. |
|
| Risk of bias | ||
| Item | Authors’ judgement | Description |
| Adequate sequence generation? | Unclear | Method of randomisation was not described. |
| Allocation concealment? | Unclear | B - Unclear |
| Blinding? All outcomes |
Yes | Double blind. |
| Methods | Methods of randomisation and allocation concealment were not described | |
| Participants | 100 patients undergoing elective cardiac surgery requiring cardiopulmonary bypass (CPB) were randomised to one of two groups
|
|
| Interventions |
|
|
| Outcomes | Amount of allogeneic blood transfused (units) Blood loss (ml) Mortality (n) |
|
| Notes | Quality assessment score (Schulz criteria): 4/7 Transfusion protocol not specified. |
|
| Risk of bias | ||
| Item | Authors’ judgement | Description |
| Adequate sequence generation? | Unclear | Method of randomisation was not described. |
| Allocation concealment? | Unclear | B - Unclear |
| Blinding? All outcomes |
Unclear | Double blind. |
| Methods | Methods of randomisation and allocation concealment were not described | |
| Participants | 109 of 122 eligible patients scheduled for coronary bypass graft surgery or mixed cardiac surgery were randomised to one of four different groups
NB: Of the total number of patients enrolled in the study, 13 were excluded from the subsequent analysis because they did not complete the study (four in the placebo group and nine in the treatment group) |
|
| Interventions |
|
|
| Outcomes | Number of patients transfused allogeneic blood (n) Amount of allogenic blood transfused (units) Blood loss (ml) Mortality (n) Thrombosis (n) |
|
| Notes | Quality assessment score (Schulz criteria): 1/7 Transfusion protocol not specified. |
|
| Risk of bias | ||
| Item | Authors’ judgement | Description |
| Adequate sequence generation? | Unclear | Method of randomisation was not described. |
| Allocation concealment? | Unclear | B - Unclear |
| Blinding? All outcomes |
Yes | Single blind. |
| Methods | Patients were randomly assigned to one of two patient groups according to a system which used a series of sealed envelopes. The method of generating allocation sequences was not described | |
| Participants | 70 patients undergoing various cardiac operations requiring cardiopulmonary bypass (CPB) were
randomly assigned to one of two groups
NB: One patient died in the operating room before receiving the study drug, and a second was withdrawn from the study at the request of the surgeon after randomisation but before treatment with the study drug. Neither of these patients were included in the analysis of the data |
|
| Interventions |
|
|
| Outcomes | Amount of allogeneic blood transfused (units) Blood loss (ml) Mortality (n) Myocardial infarction (n) Re-operation for bleeding (n) Cardiac arrest (n) Stroke (n) |
|
| Notes | Quality assessment score (Schulz criteria): 4/7 Transfusion protocol not specified. |
|
| Risk of bias | ||
| Item | Authors’ judgement | Description |
| Adequate sequence generation? | Unclear | Method of randomisation was not described. |
| Allocation concealment? | No | C - Inadequate |
| Blinding? All outcomes |
Yes | Double blind. |
| Methods | Methods of randomisation and allocation concealment were not described | |
| Participants | 80 patients scheduled for elective total hip replacement (THR) surgery for primary coxarthrosis were randomly allocated to one of two groups
NB: One patient was excluded from the study. |
|
| Interventions |
|
|
| Outcomes | Amount of allogeneic blood transfused (units) Blood loss (ml) Angina pectoris (n) Pulmonary embolus (n) Use of ephidrine (n) Laboratory/biochemistry |
|
| Notes | Quality assessment score (Schulz criteria): 3/7 Transfusion protocol used. Heterologous erythrocyte concentrate combined with 3% dextran 60 in a 1:1 ratio was transfused to correct the erythrocyte volume fraction (EVF) >27% |
|
| Risk of bias | ||
| Item | Authors’ judgement | Description |
| Adequate sequence generation? | Unclear | Method of randomisation was not described. |
| Allocation concealment? | Unclear | B - Unclear |
| Blinding? All outcomes |
Yes | Double blind. |
| Methods | Method of randomisation and allocation concealment was not described | |
| Participants | 44 patients who had taken acetylsalicylic acid within 7 days of scheduled coronary bypass surgery were randomly assigned to one of two groups:
|
|
| Interventions |
|
|
| Outcomes | Number of patients transfused allogeneic blood (n) Amount of allogeneic blood transfused (units) Number of patients transfused fresh frozen plasma (n) Number of patients transfused platelets (n) Blood loss (mls) Mortality (n) Stroke (n) |
|
| Notes | Quality assessment score (Schulz criteria): 4/7 Transfusion protocol not specified. |
|
| Risk of bias | ||
| Item | Authors’ judgement | Description |
| Adequate sequence generation? | Unclear | Method of randomisation was not described. |
| Allocation concealment? | Unclear | B - Unclear |
| Blinding? All outcomes |
Yes | Double blind. |
| Methods | Method of randomisation was not described. Study drugs were supplied in numbered but otherwise identical vials (Ferring Pharmaceuticals, Feltham, Middlesex, UK) | |
| Participants | 100 patients undergoing elective cardiopulmonary bypass for coronary artery bypass graft surgery were randomly assigned to one of two groups
|
|
| Interventions |
|
|
| Outcomes | Number of patients transfused allogeneic blood (n) Amount of allogeneic blood transfused (units) Blood loss (ml) |
|
| Notes | Quality assessment score (Schulz criteria): 5/7 Transfusion protocol used. |
|
| Risk of bias | ||
| Item | Authors’ judgement | Description |
| Adequate sequence generation? | Unclear | Method of randomisation was not described. |
| Allocation concealment? | Yes | A - Adequate |
| Blinding? All outcomes |
Yes | Double blind. |
| Methods | The pharmacy provided bottles containing placebo or desmopressin (DDAVP) that were identical in appearance. A card drawn at random at the beginning of the operation determined which vial was to be used for the patient. The method used to generate allocation sequences was not described | |
| Participants | 83 consecutive patients having open heart surgery were randomly allocated to one of two groups
NB: No patient demographic data were reported. |
|
| Interventions |
|
|
| Outcomes | Number of patients transfused allogeneic blood (n) Number of patients transfused fresh frozen plasma (n) Number of patients transfused platelets (n) Blood loss (ml) Amount of autotransfusion (ml) Number of patients treated with EACA |
|
| Notes | Quality assessment score (Schulz criteria): 4/7 Transfusion protocol not specified. |
|
| Risk of bias | ||
| Item | Authors’ judgement | Description |
| Adequate sequence generation? | Unclear | Method of randomisation was not described. |
| Allocation concealment? | Unclear | B - Unclear |
| Blinding? All outcomes |
Yes | Double blind. |
| Methods | Patient randomisation was by drawing a sealed envelope specifying a prescription for either desmopressin or placebo, which was then prepared by an independent investigator; blinded to the patient, attending anaesthesiologist, and surgeon | |
| Participants | 60 patients undergoing elective hepatectomy were randomly assigned to one of two groups
|
|
| Interventions |
|
|
| Outcomes | Number of patients transfused allogeneic blood transfusion (n) Amount of allogeneic blood transfused (units) Blood loss (ml) |
|
| Notes | Quality assessment score (Schulz criteria): 4/7 Transfusion protocol used. |
|
| Risk of bias | ||
| Item | Authors’ judgement | Description |
| Adequate sequence generation? | Unclear | Method of randomisation was not described. |
| Allocation concealment? | No | C - Inadequate |
| Blinding? All outcomes |
Yes | Double blind. |
DATA AND ANALYSES
Comparison 1. Desmopressin versus control (blood transfused and blood loss).
| Outcome or subgroup title | No. of studies |
No. of participants |
Statistical method | Effect size |
|---|---|---|---|---|
| 1 No. exposed to allogeneic blood - All studies |
19 | 1387 | Risk Ratio (M-H, Random, 95% CI) | 0.96 [0.87, 1.06] |
| 2 No. exposed to allogeneic blood - Type of surgery |
19 | 1387 | Risk Ratio (M-H, Random, 95% CI) | 0.96 [0.87, 1.06] |
| 2.1 Cardiac | 15 | 1196 | Risk Ratio (M-H, Random, 95% CI) | 0.95 [0.84, 1.07] |
| 2.2 Miscellaneous | 4 | 191 | Risk Ratio (M-H, Random, 95% CI) | 1.01 [0.81, 1.26] |
| 3 No. exposed to allogeneic blood - Cardiac surgery |
15 | 1196 | Risk Ratio (M-H, Random, 95% CI) | 0.95 [0.84, 1.07] |
| 3.1 Primary CABG surgery | 9 | 586 | Risk Ratio (M-H, Random, 95% CI) | 0.85 [0.73, 0.99] |
| 3.2 CABG + valve +/− combination / redo surgery |
6 | 610 | Risk Ratio (M-H, Random, 95% CI) | 1.03 [0.88, 1.19] |
| 4 No. exposed to allogeneic blood - Cardiac surgery - ASA use |
10 | 685 | Risk Ratio (M-H, Random, 95% CI) | 0.84 [0.69, 1.01] |
| 4.1 ASA use within 7 days prior to surgery |
6 | 399 | Risk Ratio (M-H, Random, 95% CI) | 0.89 [0.64, 1.23] |
| 4.2 No ASA use within 7 days of surgery |
4 | 286 | Risk Ratio (M-H, Random, 95% CI) | 0.79 [0.62, 1.01] |
| 5 No. exposed to allogeneic blood - Transfusion protocol |
19 | 1387 | Risk Ratio (M-H, Random, 95% CI) | 0.96 [0.87, 1.06] |
| 5.1 Transfusion Protocol | 10 | 736 | Risk Ratio (M-H, Random, 95% CI) | 0.90 [0.77, 1.04] |
| 5.2 No Transfusion Protocol | 9 | 651 | Risk Ratio (M-H, Random, 95% CI) | 1.03 [0.93, 1.14] |
| 6 No. exposed to allogeneic blood - Use of autologous techniques (ANH, PAD, CS) |
19 | 1387 | Risk Ratio (M-H, Random, 95% CI) | 0.96 [0.87, 1.06] |
| 6.1 No autologous techniques used |
10 | 732 | Risk Ratio (M-H, Random, 95% CI) | 0.91 [0.78, 1.07] |
| 6.2 Autologous technique/s used (ANH, PAD, CS) |
9 | 655 | Risk Ratio (M-H, Random, 95% CI) | 1.00 [0.84, 1.19] |
| 7 Trial quality - Allocation concealment (Cochrane scale) | 19 | 1387 | Risk Ratio (M-H, Random, 95% CI) | 0.96 [0.87, 1.06] |
| 7.1 Quality A | 3 | 249 | Risk Ratio (M-H, Random, 95% CI) | 0.97 [0.75, 1.24] |
| 7.2 Quality B | 11 | 766 | Risk Ratio (M-H, Random, 95% CI) | 0.88 [0.75, 1.03] |
| 7.3 Quality C | 5 | 372 | Risk Ratio (M-H, Random, 95% CI) | 1.11 [0.94, 1.33] |
| 8 Units of blood transfused - All patients |
14 | 885 | Mean Difference (IV, Random, 95% CI) | −0.30 [−0.60, −0.01] |
| 9 Units of blood transfused - Type of surgery |
14 | 885 | Mean Difference (IV, Random, 95% CI) | −0.30 [−0.60, −0.01] |
| 9.1 Cardiac | 10 | 621 | Mean Difference (IV, Random, 95% CI) | −0.39 [−0.77, −0.01] |
| 9.2 Orthopaedic | 2 | 129 | Mean Difference (IV, Random, 95% CI) | −0.15 [−0.64, 0.33] |
| 9.3 Vascular | 2 | 135 | Mean Difference (IV, Random, 95% CI) | 0.06 [−0.89, 1.02] |
| 10 Units of blood transfused - All patients - Use of autologous techniques (ANH, PAD, CS) |
14 | 885 | Mean Difference (IV, Random, 95% CI) | −0.30 [−0.60, −0.01] |
| 10.1 No autologous techniques used |
10 | 734 | Mean Difference (IV, Random, 95% CI) | −0.22 [−0.55, 0.10] |
| 10.2 Autologous technique/s used (ANH, PAD, CS) |
4 | 151 | Mean Difference (IV, Random, 95% CI) | −0.47 [−1.15, 0.20] |
| 11 Units of blood transfused - In those transfused |
5 | 211 | Mean Difference (IV, Random, 95% CI) | −0.49 [−0.94, −0.04] |
| 12 Blood loss - All surgery | 22 | 2363 | Mean Difference (IV, Random, 95% CI) | −113.05 [−160.27, − 65.84] |
| 12.1 Intra-operative blood loss |
7 | 493 | Mean Difference (IV, Random, 95% CI) | −90.07 [−199.56, 19. 42] |
| 12.2 Post-operative blood loss | 18 | 1201 | Mean Difference (IV, Random, 95% CI) | −92.98 [−149.86, − 36.11] |
| 12.3 Total blood loss - intra+post-operative blood loss |
10 | 669 | Mean Difference (IV, Random, 95% CI) | −241.78 [−387.55, − 96.01] |
| 13 Blood loss - All surgery (time period) |
22 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 13.1 Intra-operative blood loss |
7 | 493 | Mean Difference (IV, Random, 95% CI) | −90.07 [−199.56, 19. 42] |
| 13.2 0-6 hours post-operative blood loss |
1 | 59 | Mean Difference (IV, Random, 95% CI) | −98.0 [−304.99, 108. 99] |
| 13.3 0-12 hours post operative blood loss |
3 | 233 | Mean Difference (IV, Random, 95% CI) | −114.05 [−269.46, 41.36] |
| 13.4 0-16 hours post operative blood loss |
2 | 122 | Mean Difference (IV, Random, 95% CI) | −18.01 [−113.34, 77. 32] |
| 13.5 0-24 hours post operative blood loss |
12 | 787 | Mean Difference (IV, Random, 95% CI) | −100.41 [−176.48, − 24.34] |
| 13.6 Total blood loss - intra+post-operative blood loss |
10 | 669 | Mean Difference (IV, Random, 95% CI) | −239.02 [−388.58, − 89.46] |
| 14 Blood loss - Cardiac surgery | 18 | 1832 | Mean Difference (IV, Random, 95% CI) | −116.81 [−172.63, − 60.99] |
| 14.1 Intra-operative blood loss |
3 | 229 | Mean Difference (IV, Random, 95% CI) | −119.79 [−314.57, 75.00] |
| 14.2 Post-operative blood loss | 16 | 1107 | Mean Difference (IV, Random, 95% CI) | −96.58 [−163.04, − 30.12] |
| 14.3 Total blood loss - intra+post-operative |
7 | 496 | Mean Difference (IV, Random, 95% CI) | −237.92 [−413.43, − 62.40] |
| 15 Blood loss (Post-operative) - Cardiac surgery - ASA use |
13 | 854 | Mean Difference (IV, Random, 95% CI) | −108.10 [−175.60, − 40.60] |
| 15.1 ASA use within 7 days prior to surgery |
10 | 633 | Mean Difference (IV, Random, 95% CI) | −109.57 [−200.11, − 19.03] |
| 15.2 No ASA use within 7 days of surgery |
3 | 221 | Mean Difference (IV, Random, 95% CI) | −112.69 [−227.59, 2. 22] |
| 16 Blood loss - Cardiac surgery (time period) |
18 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 16.1 Intra-operative blood loss |
3 | 229 | Mean Difference (IV, Random, 95% CI) | −119.79 [−314.57, 75.00] |
| 16.2 0-6 hours post-operative blood loss |
1 | 59 | Mean Difference (IV, Random, 95% CI) | −98.0 [−304.99, 108. 99] |
| 16.3 0-12 hours post operative blood loss |
3 | 233 | Mean Difference (IV, Random, 95% CI) | −114.05 [−269.46, 41.36] |
| 16.4 0-16 hours post operative blood loss |
2 | 122 | Mean Difference (IV, Random, 95% CI) | −18.01 [−113.34, 77. 32] |
| 16.5 0-24 hours post operative blood loss |
10 | 693 | Mean Difference (IV, Random, 95% CI) | −107.46 [−207.12, − 7.80] |
| 16.6 Total blood loss - intra+post-operative blood loss |
7 | 496 | Mean Difference (IV, Random, 95% CI) | −237.92 [−413.43, − 62.40] |
| 17 Blood loss - Post-operative blood loss by CPB times |
15 | 1024 | Mean Difference (IV, Random, 95% CI) | −93.34 [−162.24, − 24.44] |
| 17.1 Less than 80 minutes | 3 | 198 | Mean Difference (IV, Random, 95% CI) | −41.22 [−157.25, 74. 80] |
| 17.2 Between 80-100 minutes | 5 | 330 | Mean Difference (IV, Random, 95% CI) | −104.18 [−184.75, − 23.61] |
| 17.3 Between 101-120 minutes |
3 | 129 | Mean Difference (IV, Random, 95% CI) | 53.08 [−156.33, 262. 50] |
| 17.4 Between 121-140 minutes |
2 | 196 | Mean Difference (IV, Random, 95% CI) | −46.53 [−366.29, 273.23] |
| 17.5 Greater than 140 minutes |
2 | 171 | Mean Difference (IV, Random, 95% CI) | −344.74 [−478.50, − 210.97] |
Comparison 2. Adverse events and other outcomes.
| Outcome or subgroup title | No. of studies |
No. of participants |
Statistical method | Effect size |
|---|---|---|---|---|
| 1 Re-operation for bleeding | 11 | 778 | Risk Ratio (M-H, Random, 95% CI) | 0.69 [0.26, 1.83] |
| 2 Mortality | 12 | 1061 | Risk Ratio (M-H, Random, 95% CI) | 1.72 [0.68, 4.33] |
| 3 Myocardial infarction | 12 | 876 | Risk Ratio (M-H, Random, 95% CI) | 1.38 [0.77, 2.50] |
| 4 Stroke (CVA) | 5 | 360 | Risk Ratio (M-H, Random, 95% CI) | 2.40 [0.68, 8.43] |
| 5 Any thrombosis | 9 | 691 | Risk Ratio (M-H, Random, 95% CI) | 1.46 [0.64, 3.35] |
| 6 Hypotension - during DDAVP/ Placebo infusion requiring treatment (fluids and/or vasoactive drugs) |
5 | 183 | Risk Ratio (M-H, Random, 95% CI) | 2.81 [1.50, 5.27] |
ADDITIONAL TABLES
Table 1. Duration of cardiac surgery (min).
| Study | DDAVP (n) | DDAVP - duration | Control (n) | Control - duration |
|---|---|---|---|---|
| Pleym 2004 | 46 | 133.00 (27.00) | 46 | 135.00 (31.00) |
| Casas 1995 | 50 | 188.00 (41.00) | 51 | 184.00 (55.00) |
| Dilthey 1993 | 19 | 240.00 (37.00) | 20 | 248.00 (48.00) |
| Kuitunen 1992 | 15 | 247.00 (42.00) | 15 | 244.00 (39.00) |
| Ansell 1992 | 41 | 261.20 (70.00) | 42 | 242.50 (61.40) |
| Hackmann 1989 | 74 | 306.00 (89.00) | 76 | 318.00 (114.00) |
| Salzman 1986 | 35 | 373.00 (132.00) | 35 | 392.00 (145.00) |
Table 2. Post-operative blood loss (ml) and duration of CPB (min) - mean (SD).
| Study | DDAVP (n) | DDAVP - Blood loss |
DDAVP - CPB times |
Control (n) | Control - Blood loss |
Control - CPB times |
|---|---|---|---|---|---|---|
| Horrow 1991 | 38 | 312.40 (169.50) | 92.00 (34.00) | 44 | 440.00 (195.78) | 98.00 (33.00) |
| Ozkisacik 2001 | 33 | 405.27 (102.56) | 73.20 (32.41) | 33 | 524.22 (174.50) | 65.10 (26.72) |
| Rocha 1988 | 50 | 421.83 (242.25) | 93.00 (43.00) | 50 | 428.52 (377.69) | 94.00 (40.00) |
| Salzman 1986 | 35 | 510.00 (289.00) | 144.00 (38.00) | 35 | 803.00 (471.00) | 159.00 (66.00) |
| Hedderich 1990 | 29 | 544.00 (390.00) | 92.00 (21.00) | 30 | 642.00 (421.00) | 89.00 (24.00) |
| Pleym 2004 | 46 | 606.00 (237.00) | 59.00 (16.00) | 46 | 601.00 (301.00) | 56.00 (20.00) |
| Despotis 1999 | 50 | 624.00 (209.00) | 147.00 (49.00) | 51 | 1028.00 (682.00) | 146.00 (38.00) |
| Reich 1991 | 14 | 624.00 (351.00) | 118.00 (30.00) | 13 | 729.00 (200.00) | 123.00 (26.00) |
| Frankville 1991 | 20 | 790.00 (581.38) | 50.80 (14.27) | 20 | 687.00 (223.60) | 50.70 (10.73) |
| Mongan 1992 | 57 | 795.05 (355.79) | 128.44 (37.12) | 58 | 999.73 (517.96) | 132.91 (33.43) |
| Gratz 1992 | 29 | 833.00 (311.00) | 80.40 (29.00) | 30 | 1176.00 (674.00) | 95.40 (25.60) |
| Brown 1989 | 10 | 879.00 (353.00) | 109.00 (33.00) | 9 | 803.00 (405.00) | 89.00 (38.00) |
| Rocha 1994 | 53 | 908.98 (475.42) | 127.26 (37.07) | 28 | 787.21 (406.23) | 121.30 (36.20) |
| Kuitunen 1992 | 15 | 950.00 (185.00) | 94.00 (19.00) | 15 | 1034.00 (321.00) | 103.00 (23.00) |
| Ansell 1992 | 41 | 1064.80 (647. 10) |
118.60 (39.00) | 42 | 844.40 (507.60) | 111.80 (41.50) |
WHAT’S NEW
Last assessed as up-to-date: 20 April 2008.
| Date | Event | Description |
|---|---|---|
| 13 June 2008 | Amended | Converted to new review format. |
| 21 April 2008 | New search has been performed | New studies found and included or excluded. |
HISTORY
Protocol first published: Issue 1, 2000
Review first published: Issue 2, 2001
Footnotes
DECLARATIONS OF INTEREST
None known.
References to studies included in this review
*Indicates the major publication for the study
- Ansell 1992 {published data only} .Ansell J, Klassen V, Lew R, Ball S, Weinstein M, VanderSalm T, et al. Does desmopressin acetate prophylaxis reduce blood loss after valvular heart operations? A randomized, double-blind study. Journal of Thoracic and Cardiovascular Surgery. 1992;104(1):117–23. [PubMed] [Google Scholar]
- Brown 1989 {published data only} .Brown MR, Swygert TH, Whitten CW, Hebeler R. Desmopressin acetate following cardiopulmonary bypass: evaluation of coagulation parameters. Journal of Cardiothoracic Anesthesia. 1989;3(6):726–9. doi: 10.1016/s0888-6296(89)94790-x. [DOI] [PubMed] [Google Scholar]
- Casas 1995 {published data only} .Casas JI, Zuazu-Jausoro I, Mateo J, Oliver A, Litvan H, Muniz-Diaz E, Aris A, Caralps JM, Fontcuberta J. Aprotinin versus desmopressin for patients undergoing operations with cardiopulmonary bypass. A double-blind placebo-controlled study. Journal of Thoracic and Cardiovascular Surgery. 1995;110(4 Pt 1):1107–17. doi: 10.1016/s0022-5223(05)80180-1. [DOI] [PubMed] [Google Scholar]
- Clagett 1995 {published data only} .Clagett GP, Valentine RJ, Myers SI, Chervu A, Heller J. Does desmopressin improve hemostasis and reduce blood loss from aortic surgery? A randomized, double-blind study. Journal of Vascular Surgery. 1995;22(3):223–30. doi: 10.1016/s0741-5214(95)70134-6. [DOI] [PubMed] [Google Scholar]
- Despotis 1999 {published data only} .Despotis GJ, Levine V, Saleem R, Spitznagel E, Joist JH. Use of point-of-care test in identification of patients who can benefit from desmopressin during cardiac surgery: a randomised controlled trial [see comments] Lancet. 1999;354(9173):106–10. doi: 10.1016/S0140-6736(98)12494-7. [DOI] [PubMed] [Google Scholar]
- Dilthey 1993 {published data only} .Dilthey G, Dietrich W, Spannagl M, Richter JA. Influence of desmopressin acetate on homologous blood requirements in cardiac surgical patients pretreated with aspirin. Journal of Cardiothoracic and Vascular Anesthesia. 1993;7(4):425–30. doi: 10.1016/1053-0770(93)90164-g. [DOI] [PubMed] [Google Scholar]
- Ellis 2001 {published data only} .Ellis MH, Fredman B, Zohar E, Ifrach N, Jedeikin R. The effect of tourniquet application, tranexamic acid, and desmopressin on the procoagulant and fibrinolytic systems during total knee replacement. Journal of Clinical Anesthesia. 2001;13(7):509–13. doi: 10.1016/s0952-8180(01)00319-1. [DOI] [PubMed] [Google Scholar]
- Flordal 1992 {published data only} .Flordal PA, Ljungstrom KG, Ekman B, Neander G. Effects of desmopressin on blood loss in hip arthroplasty. Controlled study in 50 patients. Acta Orthopaedica Scandinavica. 1992;63(4):381–5. doi: 10.3109/17453679209154749. [DOI] [PubMed] [Google Scholar]
- Frankville 1991 {published data only} .Frankville DD, Harper GB, Lake CL, Johns RA. Hemodynamic consequences of desmopressin administration after cardiopulmonary bypass. Anesthesiology. 1991;74(6):988–96. doi: 10.1097/00000542-199106000-00004. [DOI] [PubMed] [Google Scholar]
- Gratz 1992 {published data only} .Gratz I, Koehler J, Olsen D, Afshar M, DeCastro N, Spagna PM, et al. The effect of desmopressin acetate on postoperative hemorrhage in patients receiving aspirin therapy before coronary artery bypass operations. Journal of Thoracic and Cardiovascular Surgery. 1992;104(5):1417–22. [PubMed] [Google Scholar]
- Guyuron 1996 {published data only} .Guyuron B, Vaughan C, Schlecter B. The role of DDAVP (desmopressin) in orthognathic surgery. Annals of Plastic Surgery. 1996;37(5):516–9. doi: 10.1097/00000637-199611000-00010. [DOI] [PubMed] [Google Scholar]
- Hackmann 1989 {published data only} .Hackmann T, Gascoyne RD, Naiman SC, Growe GH, Burchill LD, Jamieson WR, et al. A trial of desmopressin (1-desamino-8-D-arginine vasopressin) to reduce blood loss in uncomplicated cardiac surgery [see comments] New England Journal of Medicine. 1989;321(21):1437–43. doi: 10.1056/NEJM198911233212104. [DOI] [PubMed] [Google Scholar]
- Hedderich 1990 {published data only} .Hedderich GS, Petsikas DJ, Cooper BA, Leznoff M, Guerraty AJ, Poirier NL, et al. Desmopressin acetate in uncomplicated coronary artery bypass surgery: a prospective randomized clinical trial [comment] Canadian Journal of Surgery. 1990;33(1):33–6. [PubMed] [Google Scholar]
- Horrow 1991 {published data only} .Horrow JC, Van Riper DF, Strong MD, Brodsky I, Parmet JL. Hemostatic effects of tranexamic acid and desmopressin during cardiac surgery. Circulation. 1991;84(5):2063–70. doi: 10.1161/01.cir.84.5.2063. [DOI] [PubMed] [Google Scholar]
- Kuitunen 1992 {published data only} .Kuitunen AH. Haemostatic responses to desmopressin acetate after primary coronary artery bypass surgery. Annales Chirurgiae et Gynaecologiae. 1992;81(1):11–8. [PubMed] [Google Scholar]
- Lethagen 1991 {published data only} .Lethagen S, Rugarn P, Bergqvist D. Blood loss and safety with desmopressin or placebo during aorto-iliac graft surgery. European Journal of Vascular Surgery. 1991;5(2):173–8. doi: 10.1016/s0950-821x(05)80684-x. [DOI] [PubMed] [Google Scholar]
- Marquez 1992 {published data only} .Marquez J, Koehler S, Strelec SR, Benckart DH, Spero JA, Cottington EM, Torpey DJ., Jr. Repeated dose administration of desmopressin acetate in uncomplicated cardiac surgery: a prospective, blinded, randomized study. Journal of Cardiothoracic and Vascular Anesthesia. 1992;6(6):674–6. doi: 10.1016/1053-0770(92)90049-d. [DOI] [PubMed] [Google Scholar]
- Mongan 1992 {published data only} .Mongan PD, Hosking MP. The role of desmopressin acetate in patients undergoing coronary artery bypass surgery. A controlled clinical trial with thromboelastographic risk stratification. Anesthesiology. 1992;77(1):38–46. doi: 10.1097/00000542-199207000-00007. [DOI] [PubMed] [Google Scholar]
- Ozkisacik 2001 {published data only} .Ozkisacik E, Islamoglu F, Posacioglu H, Yagdi T, Basarir S, Omay SB, et al. Desmopressin usage in elective cardiac surgery. Journal of Cardiovascular Surgery (Torino) 2001;42(6):741–4. [PubMed] [Google Scholar]
- Pleym 2004 {published data only} .Pleym H, Stenseth R, Wahba A, Bjella L, Tromsdal A, Karevold A, Dale O. Prophylactic treatment with desmopressin does not reduce postoperative bleeding after coronary surgery in patients treated with aspirin before surgery. Anesthesia and Analgesia. 2004;98(3):578–84. doi: 10.1213/01.ane.0000100682.84799.e8. [DOI] [PubMed] [Google Scholar]
- Reich 1991 {published data only} .Reich DL, Hammerschlag BC, Rand JH, Weiss-Bloom L, Perucho H, Galla J, Thys DM. Desmopressin acetate is a mild vasodilator that does not reduce blood loss in uncomplicated cardiac surgical procedures. Journal of Cardiothoracic and Vascular Anesthesia. 1991;5(2):142–5. doi: 10.1016/1053-0770(91)90327-p. [DOI] [PubMed] [Google Scholar]
- Rocha 1988 {published data only} .Rocha E, Llorens R, Paramo JA, Arcas R, Cuesta B, Trenor AM. Does desmopressin acetate reduce blood loss after surgery in patients on cardiopulmonary bypass? Circulation. 1988;77(6):1319–23. doi: 10.1161/01.cir.77.6.1319. [DOI] [PubMed] [Google Scholar]
- Rocha 1994 {published data only} .Rocha E, Hidalgo F, Llorens R, Melero JM, Arroyo JL, Paramo JA. Randomized study of aprotinin and DDAVP to reduce postoperative bleeding after cardiopulmonary bypass surgery. Circulation. 1994;90(2):921–7. doi: 10.1161/01.cir.90.2.921. [DOI] [PubMed] [Google Scholar]
- Salzman 1986 {published data only} .Salzman EW, Weinstein MJ, Weintraub RM, Ware JA, et al. Treatment with desmopressin acetate to reduce blood loss after cardiac surgery. New England Journal of Medicine. 1986;314:1402–6. doi: 10.1056/NEJM198605293142202. [DOI] [PubMed] [Google Scholar]
- Schott 1995 {published data only} .Schott U, Sollen C, Axelsson K, Rugarn P, Allvin I. Desmopressin acetate does not reduce blood loss during total hip replacement in patients receiving dextran. Acta Anaesthesiologica Scandinavica. 1995;39(5):592–8. doi: 10.1111/j.1399-6576.1995.tb04133.x. [DOI] [PubMed] [Google Scholar]
- Sheridan 1994 {published data only} .Sheridan DP, Card RT, Pinilla JC, Harding SM, Thomson DJ, Gauthier L, Drotar D. Use of desmopressin acetate to reduce blood transfusion requirements during cardiac surgery in patients with acetylsalicylic-acid-induced platelet dysfunction. Canadian Journal of Surgery. 1994;37(1):33–6. [PubMed] [Google Scholar]
- Spyt 1990 {published data only} .Spyt TJ, Weerasena NA, Bain WH, Lowe GDO, Rumley A. The effects of desmopressin acetate (DDAVP) on haemostasis and blood loss in routine coronary artery bypass surgery: A randomized, double-blind trial. Perfusion. 1990;5:57–61. [Google Scholar]
- Temeck 1994 {published data only} .Temeck BK, Bachenheimer LC, Katz NM, Coughlin SS, Wallace RB. Desmopressin acetate in cardiac surgery: a double-blind, randomized study. [Review] Southern Medical Journal. 1994;87(6):611–5. doi: 10.1097/00007611-199406000-00006. [DOI] [PubMed] [Google Scholar]
- Wong 2003 {published data only} .Wong AYC, Irwin MG, Hui TWC, Fung SKY, Fan ST, Ma ESK. Desmopressin does not decrease blood loss and transfusion requirements in patients undergoing hepatectomy. Canadian Journal of Anaesthesia. 2003;50(1):14–20. doi: 10.1007/BF03020180. [DOI] [PubMed] [Google Scholar]
Additional references
- Bryson 1998 .Bryson GL, Laupacis A, Wells GA. Does acute normovolemic hemodilution reduce perioperative allogeneic transfusion? A meta-analysis. The International Study of Perioperative Transfusion. Anesthesia and Analgesia. 1998;86(1):9–15. doi: 10.1213/00000539-199801000-00003. [DOI] [PubMed] [Google Scholar]
- Carless 2002 .Carless PA, Anthony DM, Henry DA. Systematic review of the use of fibrin sealant to minimize perioperative allogeneic blood transfusion. [Review] [41 refs] British Journal of Surgery. 2002;89(6):695–703. doi: 10.1046/j.1365-2168.2002.02098.x. [DOI] [PubMed] [Google Scholar]
- Carless 2003 .Carless PA, Rubens FD, Anthony DM, O’Connell D, Henry DA. Platelet-rich-plasmapheresis for minimising peri-operative allogeneic blood transfusion. Cochrane Database of Systematic Reviews. 2003;(2) doi: 10.1002/14651858.CD004172. DOI: 10.1002/14651858.CD004172. [DOI] [PubMed] [Google Scholar]
- Carless 2004 .Carless PA, Henry DA, Moxey AJ, O’Connell D, McClelland B, Henderson KM, Sly K, Laupacis A, Fergusson D. Desmopressin for minimising perioperative allogeneic blood transfusion. Cochrane Database of Systematic Reviews. 2004;(1) doi: 10.1002/14651858.CD001884.pub2. DOI: 10.1002/14651858.CD001884.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carless 2006 .Carless PA, Henry DA, Moxey AJ, O’connell DL, Brown T, Fergusson DA. Cell salvage for minimising perioperative allogeneic blood transfusion. Cochrane Database of Systematic Reviews. 2006;(4) doi: 10.1002/14651858.CD001888.pub2. DOI: 10.1002/14651858.CD001888.pub2. [DOI] [PubMed] [Google Scholar]
- Carson 1998 .Carson JL, Terrin ML, Barton FB, Aaron R, Greenburg AG, Heck DA, et al. A pilot randomized trial comparing symptomatic vs. hemoglobin-level-driven red blood cell transfusions following hip fracture. Transfusion. 1998;38(6):522–9. doi: 10.1046/j.1537-2995.1998.38698326331.x. [DOI] [PubMed] [Google Scholar]
- Cattaneo 1995 .Cattaneo M, Harris AS, Stromberg U, Mannucci PM. The effect of desmopressin on reducing blood loss in cardiac surgery--a meta-analysis of double-blind, placebo-controlled trials. Thrombosis and Haemostasis. 1995;74(4):1064–70. [PubMed] [Google Scholar]
- Cattaneo 2008 .Cattaneo M. The use of desmopressin in open-heart surgery. Haemophilia. 2008;14(Suppl 1):40–7. doi: 10.1111/j.1365-2516.2007.01608.x. [DOI] [PubMed] [Google Scholar]
- Coyle 1999 .Coyle D, Lee KM, Fergusson DA, Laupacis A. Economic analysis of erythropoietin use in orthopaedic surgery. Transfusion Medicine. 1999;9(1):21–30. doi: 10.1046/j.1365-3148.1999.009001021.x. [DOI] [PubMed] [Google Scholar]
- DerSimonian 1986 .DerSimonian R, Laird N. Meta-analysis in clinical trials. Controlled Clinical Trials. 1986;7(3):177–88. doi: 10.1016/0197-2456(86)90046-2. [DOI] [PubMed] [Google Scholar]
- Dickersin 1994 .Dickersin K, Scherer R, Lefebvre C. Identifying relevant studies for systematic reviews. BMJ. 1994;309(6964):1286–91. doi: 10.1136/bmj.309.6964.1286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Federici 2008 .Federici AB. The use of desmopressin in von Willebrand disease: The experience of the first 30 years (1977-2007) Haemophilia. 2008;14(Suppl 1):5–14. doi: 10.1111/j.1365-2516.2007.01610.x. [DOI] [PubMed] [Google Scholar]
- Fergusson 1999 .Fergusson D, van Walraven C, Coyle D, Laupacis A. Economic evaluations of technologies to minimize perioperative transfusion: a systematic review of published studies. International Study of Peri-operative Transfusion (ISPOT) investigators. Transfusion Medicine Reviews. 1999;13(2):106–17. doi: 10.1016/s0887-7963(99)80005-4. [DOI] [PubMed] [Google Scholar]
- Fergusson 2008 .Fergusson DA, Hebert PC, Mazer CD, Fremes SE, MacAdams C, Murkin JM, et al. A comparison aprotinin and lysine analogues in high-risk cardiac surgery. New England Journal of Medicine. 2008;358(22):2319–31. doi: 10.1056/NEJMoa0802395. [DOI] [PubMed] [Google Scholar]
- Ferraris 2007 .Ferraris VA. Perioperative Blood Transfusion and Blood Conservation in Cardiac Surgery: The Society of Thoracic Surgeons and The Society of Cardiovascular Anesthesiologists Clinical Practice Guideline. Annals of Thoracic Surgery. 2007;83(Suppl 5):27–86. doi: 10.1016/j.athoracsur.2007.02.099. [DOI] [PubMed] [Google Scholar]
- Forgie 1998 .Forgie MA, Wells PS, Laupacis A, Fergusson D. Preoperative autologous donation decreases allogeneic transfusion but increases exposure to all red blood cell transfusion: results of a meta-analysis. International Study of Perioperative Transfusion (ISPOT) Archives of Internal Medicine. 1998;158(6):610–6. doi: 10.1001/archinte.158.6.610. [DOI] [PubMed] [Google Scholar]
- Franchini 2007 .Franchini M. The use of desmopressin as a hemostatic agent: A concise review. American Journal of Hematology. 2007;82(8):731–5. doi: 10.1002/ajh.20940. [DOI] [PubMed] [Google Scholar]
- Fremes 1994 .Fremes SE, Wong BI, Lee E, Mai R, Christakis GT, McLean RF, et al. Metaanalysis of prophylactic drug treatment in the prevention of postoperative bleeding. Annals of Thoracic Surgery. 1994;58(6):1580–8. doi: 10.1016/0003-4975(94)91636-5. [DOI] [PubMed] [Google Scholar]
- Hebert 1995 .Hebert PC, Wells G, Marshall J, Martin C, Tweeddale M, Pagliarello G, Blajchman M. Transfusion requirements in critical care. A pilot study. JAMA. 1995;273(18):1439–44. doi: 10.1001/jama.1995.03520420055038. [DOI] [PubMed] [Google Scholar]
- Hebert 1999 .Hebert PC, Wells G, Blajchman MA, Marshall J, Martin C, Pagliarello G, et al. A multicenter, randomized, controlled clinical trial of transfusion requirements in critical care. Transfusion Requirements in Critical Care Investigators, Canadian Critical Care Trials Group. New England Journal of Medicine. 1999;340(6):409–17. doi: 10.1056/NEJM199902113400601. [DOI] [PubMed] [Google Scholar]
- Henry 2002 .Henry DA, Carless PA, Moxey AJ, O’Connell D, Forgie MA, Wells PS, Fergusson D. Pre-operative autologous donation for minimising perioperative allogeneic blood transfusion. Cochrane Database of Systematic Reviews. 2002;(2) doi: 10.1002/14651858.CD003602. DOI: 10.1002/14651858.CD003602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henry 2007 .Henry DA, Carless PA, Moxey AJ, O’Connell, Stokes BJ, McClelland B, Laupacis A, Fergusson D. Anti-fibrinolytic use for minimising perioperative allogeneic blood transfusion. Cochrane Database of Systematic Reviews. 2007;(4) doi: 10.1002/14651858.CD001886.pub2. DOI: 10.1002/14651858.CD001886.pub2. [DOI] [PubMed] [Google Scholar]
- Higgins 2002 .Higgins JP, Thompson SG, Higgins JPT, Thompson SG. Quantifying heterogeneity in a meta-analysis. Statistics in Medicine. 2002;21(11):1539–58. doi: 10.1002/sim.1186. [DOI] [PubMed] [Google Scholar]
- Higgins 2003 .Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ. 2003;327(7414):557–60. doi: 10.1136/bmj.327.7414.557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huet 1999 .Huet C, Salmi LR, Fergusson D, Koopman-van Gemert AW, Rubens F, Laupacis A. A meta-analysis of the effectiveness of cell salvage to minimize perioperative allogeneic blood transfusion in cardiac and orthopedic surgery. International Study of Perioperative Transfusion (ISPOT) Investigators. Anesthesia and Analgesia. 1999;89(4):861–9. doi: 10.1097/00000539-199910000-00009. [DOI] [PubMed] [Google Scholar]
- Karkouti 2006 .Karkouti K, Beattie WS, Dattilo KM, McCluskey SA, Ghannam M, Hamdy A, et al. A propensity score case-control comparison of aprotinin and tranexamic acid in high-transfusion-risk cardiac surgery. Transfusion. 2006;46(3):327–38. doi: 10.1111/j.1537-2995.2006.00724.x. [DOI] [PubMed] [Google Scholar]
- Laupacis 1997 .Laupacis A, Fergusson D. Drugs to minimise perioperative blood loss in cardiac surgery: Meta-analyses using periopreative blood transfusion as the outcome. Anaesthesia and Analgesia. 1997;85:1258–67. doi: 10.1097/00000539-199712000-00014. [DOI] [PubMed] [Google Scholar]
- Levi 1999 .Levi M, Cromheecke ME, de Jonge E, Prins MH, Mol de BJM, Briet E, Buller HR. Pharmacological strategies to decrease excessive blood loss in cardiac surgery: a meta-analysis of clinically relevant endpoints. Lancet. 1999;354(9194):1940–7. doi: 10.1016/S0140-6736(99)01264-7. [DOI] [PubMed] [Google Scholar]
- Mangano 2006 .Mangano DT, Tudor IC, Dietzel C. Multicenter Study of Perioperative Ischemia Research Group, Ischemia Research and Education Foundation. The risk associated with aprotinin in cardiac surgery. New England Journal of Medicine. 2006;354(4):353–65. doi: 10.1056/NEJMoa051379. [DOI] [PubMed] [Google Scholar]
- Mangano 2007 .Mangano DT, Miao Y, Vuylsteke A, Tudor IC, Juneja R, Filipescu D, et al. Investigators of The Multicenter Study of Perioperative Ischemia Research Group, Ischemia Research and Education Foundation. Mortality associated with aprotinin during 5 years following coronary artery bypass graft surgery. JAMA. 2007;297(5):471–9. doi: 10.1001/jama.297.5.471. [DOI] [PubMed] [Google Scholar]
- Mannucci 1977 .Mannucci PM, Ruggeri ZM, Pareti FI, Capitanio A. 1-Deamino-8-d-arginine vasopressin: a new pharmacological approach to the management of haemophilia and von Willebrands’ diseases. Lancet. 1977;1(8017):869–72. doi: 10.1016/s0140-6736(77)91197-7. [DOI] [PubMed] [Google Scholar]
- Mannucci 1998 .Mannucci PM. Drug Therapy: Hemostatic Drugs. The New England Journal of Medicine. 1998;339(4):245–53. doi: 10.1056/NEJM199807233390407. [DOI] [PubMed] [Google Scholar]
- Mannucci 2008 .Mannucci PM. Desmopressin: An historical introduction. Haemophilia. 2008;14(Suppl 1):1–4. doi: 10.1111/j.1365-2516.2007.01609.x. [DOI] [PubMed] [Google Scholar]
- McFarland 1997 .McFarland W, Mvere D, Shandera W, Reingold A. Epidemiology and prevention of transfusion-associated human immunodeficiency virus transmission in sub-Saharan Africa. Vox Sanguinis. 1997;72(2):85–92. doi: 10.1046/j.1423-0410.1997.7220085.x. [DOI] [PubMed] [Google Scholar]
- Review Manager (RevMan) .The Nordic Cochrane Centre. The Cochrane Collaboration . Review Manager (RevMan). 5.0. The Nordic Cochrane Centre, The Cochrane Collaboration; Copenhagen: 2008. [Google Scholar]
- Robinson 2002 .Robinson KA, Dickersin K. Development of a highly sensitive search strategy for the retrieval of reports of controlled trials using PubMed. International Journal of Epidemiology. 2002;31(1):150–3. doi: 10.1093/ije/31.1.150. [DOI] [PubMed] [Google Scholar]
- Schulz 1995 .Schulz KF, Chalmers I, Hayes RJ, Altman DJ. Empirical evidence of bias. Dimensions of methodological quality associated with estimates of treatment effects in controlled trials. JAMA. 1995;273:408–12. doi: 10.1001/jama.273.5.408. [DOI] [PubMed] [Google Scholar]
- Whyte 1997 .Whyte GS, Savoia HF. The risk of transmitting HCV, HBV or HIV by blood transfusion in Victoria. Medical Journal of Australia. 1997;166(11):584–6. doi: 10.5694/j.1326-5377.1997.tb123269.x. [DOI] [PubMed] [Google Scholar]