Abstract
After primary subcutaneous immunization of rabbits with glycolipoprotein from Pseudomonas aeruginosa BI, indirect hemagglutinating and bacterial agglutinating activities appeared in the antiserum 6 days after immunization and reached a peak between 15 and 20 days. Both these in vitro activities paralleled in vivo antipseudomonas-induced leukopenia and mouse passive-protection activities. Further experiments indicated that a functional association exists between the hemagglutinating and passive-protection activities, and that passive protection depends on activity levels in the plasma rather than in the peritoneum. After intraperitoneal injection in mice, in vitro and in vivo activities of antiglycolipoprotein serum declined in the peritoneal cavity as the plasma levels increased. After intravenous injection of the antiglycolipoprotein serum, initially high levels of in vitro and in vivo activity declined at approximately equal rates. Immunoglobulin G (IgG) and immunoglobulin M (IgM) fractions from 15-day antiglycolipoprotein serum were assayed for biological activity. Most of the hemagglutinating and bacterial agglutinating activity and all of the mouse passive-protection activity were found in the IgM fraction. Assay of antiglycolipoprotein serum after 2-mercaptoethanol inactivation of IgM showed that most of the in vitro and all of the passive-protection activities had been destroyed, again locating these activities principally in the IgM fraction of the original antiserum.
Full text
PDF

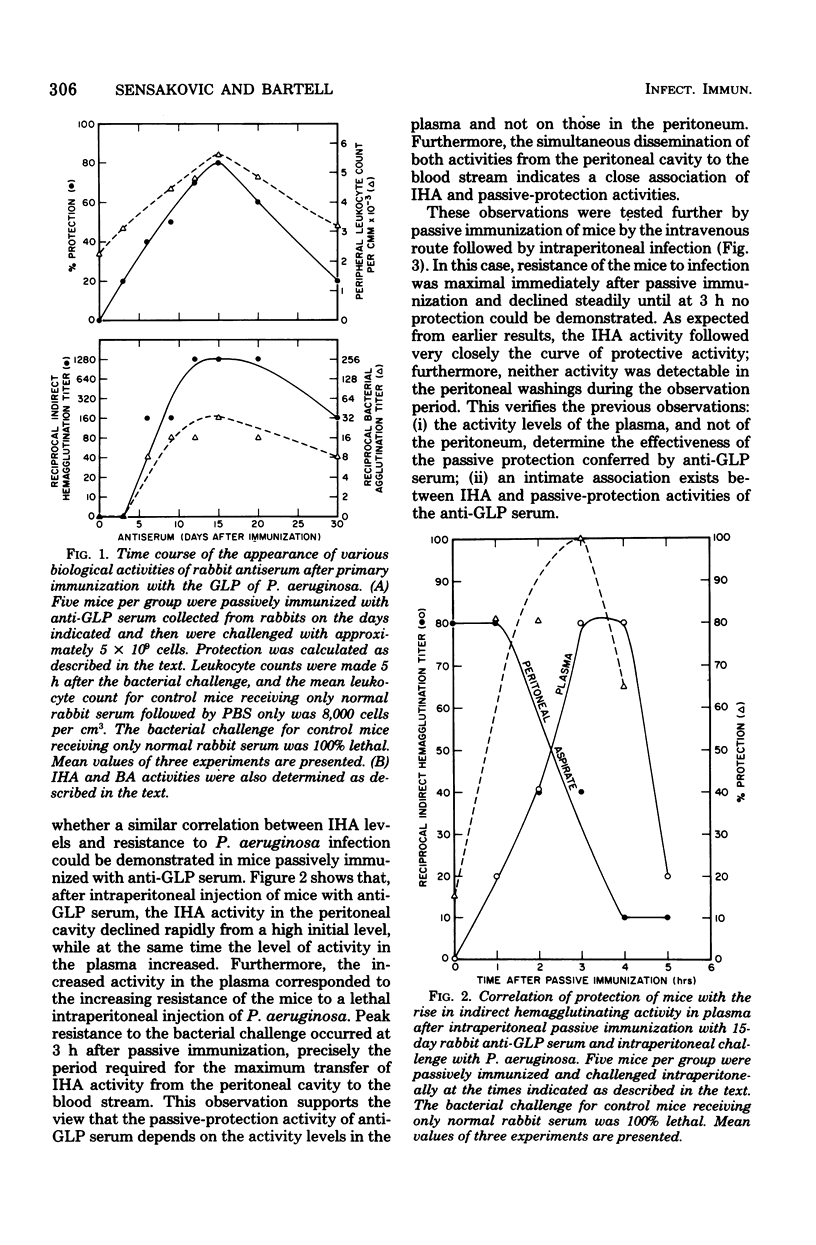
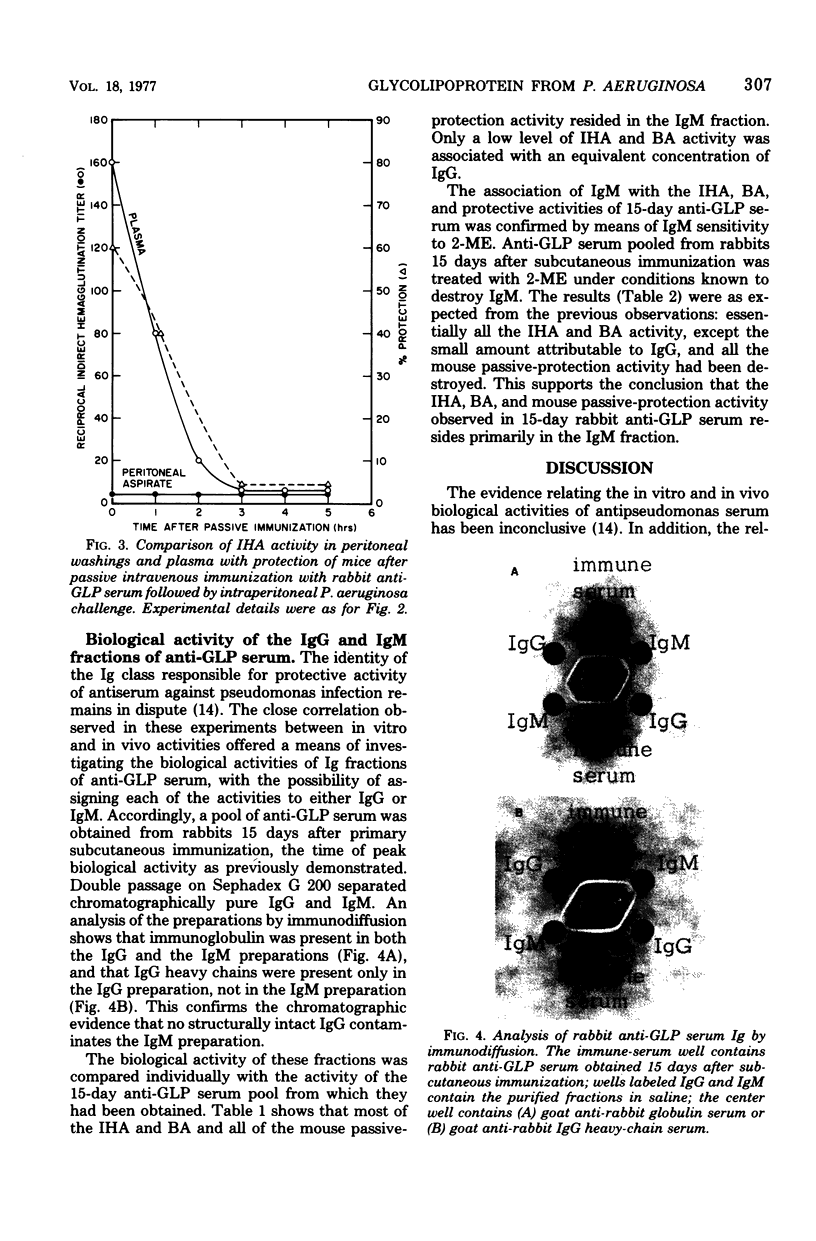


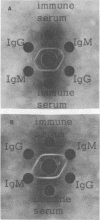
Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alexander J. W., Fisher M. W., MacMillan B. G. Immunological control of Pseudomonas infection in burn patients: a clinical evaluation. Arch Surg. 1971 Jan;102(1):31–35. doi: 10.1001/archsurg.1971.01350010033008. [DOI] [PubMed] [Google Scholar]
- Bartell P. F., Orr T. E., Chudio B. Purification and Chemical Composition of the Protective Slime Antigen of Pseudomonas aeruginosa. Infect Immun. 1970 Nov;2(5):543–548. doi: 10.1128/iai.2.5.543-548.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartell P. F., Orr T. E., Lam G. K. Polysaccharide depolymerase associated with bacteriophage infection. J Bacteriol. 1966 Jul;92(1):56–62. doi: 10.1128/jb.92.1.56-62.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bjornson A. B., Michael J. G. Biological Activities of Rabbit Immunoglobulin M and Immunoglobulin G Antibodies to Pseudomonas aeruginosa. Infect Immun. 1970 Oct;2(4):453–461. doi: 10.1128/iai.2.4.453-461.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DEUTSCH H. F., MORTON J. I. Dissociation of human serum macroglobulins. Science. 1957 Mar 29;125(3248):600–601. doi: 10.1126/science.125.3248.600. [DOI] [PubMed] [Google Scholar]
- Dimitracopoulos G., Sensakovic J. W., Bartell P. F. Slime of Pseudomonas aeruginosa: in vivo production. Infect Immun. 1974 Jul;10(1):152–156. doi: 10.1128/iai.10.1.152-156.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FLODIN P., KILLANDER J. Fractionation of human-serum proteins by gel filtration. Biochim Biophys Acta. 1962 Oct 8;63:402–410. doi: 10.1016/0006-3002(62)90104-x. [DOI] [PubMed] [Google Scholar]
- Lynn M., Sensakovic J. W., Bartell P. F. In vivo distribution of Pseudomonas aeruginosa slime glycolipoprotein: association with leukocytes. Infect Immun. 1977 Jan;15(1):109–114. doi: 10.1128/iai.15.1.109-114.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olitzki L. MUCIN AS A RESISTANCE-LOWERING SUBSTANCE. Bacteriol Rev. 1948 Jun;12(2):149–172. doi: 10.1128/br.12.2.149-172.1948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pseudomonas aeruginosa infections: persisting problems and current research to find new therapies. Ann Intern Med. 1975 Jun;82(6):819–831. doi: 10.7326/0003-4819-82-6-819. [DOI] [PubMed] [Google Scholar]
- Sensakovic J. W., Bartell P. F. Biological activity of fragments derived from the extracellular slime glycolipoprotein of Pseudomonas aeruginosa. Infect Immun. 1975 Oct;12(4):808–812. doi: 10.1128/iai.12.4.808-812.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sensakovic J. W., Bartell P. F. The slime of Pseudomonas aeruginosa: biological characterization and possible role in experimental infection. J Infect Dis. 1974 Feb;129(2):101–109. doi: 10.1093/infdis/129.2.101. [DOI] [PubMed] [Google Scholar]
- Tapper M. L., Armstrong D. Bacteremia due to Pseudomonas aeruginosa complicating neoplastic disease: a progress report. J Infect Dis. 1974 Nov;130 (Suppl)(0):S14–S23. doi: 10.1093/infdis/130.supplement.s14. [DOI] [PubMed] [Google Scholar]
- Young L. S. Role of antibody in infections due to Pseudomonas aeruginosa. J Infect Dis. 1974 Nov;130 (Suppl)(0):S111–S118. doi: 10.1093/infdis/130.supplement.s111. [DOI] [PubMed] [Google Scholar]