Abstract
Background
A minority of HIV/HCV coinfected patients with opiate addiction undergo HCV treatment. HCV therapy for HCV-monoinfected methadone maintenance (MM) recipients is safe and effective. We evaluated treatment efficacy and adherence to pegylated interferon (pegIFN) among HIV/HCV coinfected MM recipients.
Methods
HCV treatment-naïve, HIV-infected persons 18–65 years with chronic HCV genotype 1 on MM were prospectively enrolled in an HCV treatment study at two HIV clinics. At weekly visits pegIFN alfa-2a injections were directly administered. Daily MM recipients had morning ribavirin delivered with methadone at off-site methadone clinics. Weekly take-home MM recipients took ribavirin unsupervised. Target enrollment was 30 participants.
Results
During 18 recruitment months, 11 participants were enrolled, 6 of whom received daily methadone. Mean age was 46, 64% were female, 5 were Caucasian, 4 Black and 2 Hispanic. At baseline, 82% had high HCV RNA and 55% had stage 2 fibrosis or greater. The majority (91%) were on HAART, and 82% had undetectable HIV RNA with a median CD4+ of 508 cells/μL. All had polysubstance use history, non-substance-based psychiatric diagnoses and were on psychotropic medications pre-enrollment.
Two (18%) participants achieved a Sustained Virologic Response (SVR). Two completed 48 treatment weeks, 5 were withdrawn due to adverse events, 2 dropped out prematurely and 2 had treatment discontinued for virologic non-response. Of on-treatment weeks, adherence to pegIFN was >99%.
Conclusions
SVR rate was comparable to historic controls for coinfected genotype 1 patients, with optimal pegIFN adherence. Adverse effects often prevented therapy completion in this population.
Keywords: Hepatitis C virus, Methadone maintenance therapy, HIV/HCV coinfection, Hepatitis C treatment, Integrated care
1. Introduction
Coinfection with HIV and hepatitis C virus (HCV) is a substantial public health problem driven largely by injection drug use (IDU) (Alter, 1997). Among HIV-infected U.S. persons reporting IDU as their route of exposure, HCV coinfection rates range from 26% to 85% (Piccolo et al., 2002; Sherman et al., 2002; Sulkowski and Thomas, 2003). As opioids are commonly injected, opioid dependence, HIV and HCV infection represent overlapping epidemics, and HIV/HCV coinfected patients may benefit from coordinated medical care.
HIV/HCV coinfected persons are at risk for accelerated liver disease progression compared to patients with HCV alone. With the advent of highly active antiretroviral therapy (HAART) controlling HIV, HCV-induced liver disease has emerged as a major cause of morbidity and mortality in coinfection (Bica et al., 2001; Koziel and Peters, 2007). Achievement of Sustained Virologic Response (SVR, undetectable serum HCV RNA 6 months post-treatment) with pegylated interferon (pegIFN) plus ribavirin (RBV) reduces liver-related morbidity and mortality in coinfection (Berenguer et al., 2009). Nevertheless, HCV treatment initiation rates in coinfection are low across varied settings (Fishbein et al., 2004; Fleming et al., 2003; Fultz et al., 2003; Grebely et al., 2009; Mehta et al., 2008).
Historically, opioid-dependent persons were considered poor HCV treatment candidates due to concerns about adherence, psychiatric effects of interferon, re-infection and the short-term threat of drug use (Davis and Rodrigue, 2001; Wagner et al., 2009). In 2002, IDU was retracted as a treatment contraindication by the National Institute of Health and in 2004 by the American Association for the Study of Liver Disease (National Institute of Health, 2002; Strader et al., 2004). HCV therapy for current and former IDUs is supported by a large body of literature (Backmund et al., 2001; Dalgard et al., 2002; Grebely et al., 2007; Hellard et al., 2009).
Methadone maintenance (MM), the most common form of opioid replacement therapy in the U.S., reduces illicit opioid use, opioid-related morbidity and mortality, HIV acquisition and illegal activity for the pursuit of opiates (Kleber, 2008). Federal regulations govern prescription of MM, which is primarily dispensed by certified programs. High HCV seroprevalence exists at these methadone maintenance programs (MMPs), ranging from 67% to 96% (McCarthy and Flynn, 2001; Piccolo et al., 2002). Research confirms the feasibility, efficacy, safety and tolerability of HCV therapy for HCV-monoinfected MM patients (Bonkovsky et al., 2008; Litwin et al., 2009; Mauss et al., 2004; Sylvestre and Clements, 2007). Less is known about HCV treatment for HIV/HCV coinfected MM recipients, and about HCV treatment when pegIFN is not delivered on-site at the MMP. HCV therapy in coinfection is more complicated than in HCV monoinfection due to drug interactions with HAART, and adverse events being more common and severe.
Directly administered therapy effectively delivers HAART to HIV-infected patients in the MMP setting (Conway et al., 2004) and may optimize HCV medication adherence. In the Caring for HIV/HCV at Methadone Programs (CHAMP) study, we examined HCV treatment efficacy and pegIFN adherence among coinfected MM recipients, where HCV therapy is delivered in the HIV care setting apart from a MMP.
2. Methods
Participants were prospectively enrolled at two Providence, RI HIV clinics. Eligible participants were 18–65 years of age with chronic HCV genotype 1 infection, who were interferon-naïve and enrolled in a MMP. Laboratory criteria included a CD4+ cell count greater than 100 cells/μL, or HIV RNA less than 10,000 copies/ml for CD4+ cell count less than or equal to 200 cells/μL. A liver biopsy was required within 2 years of enrollment unless cirrhosis was clinically evident. Biopsies were scored according to the Batts and Ludwig staging system (Batts and Ludwig, 1995).
Individuals meeting any of the following criteria were excluded: currently psychotic, suicidal or with severe depression (Beck Depression Inventory II (BDI-II) score greater than 28) (Beck et al., 1996); having retinopathy, seizure disorder, or coronary artery, autoimmune, cerebrovascular, poorly controlled thyroid or severe chronic obstructive lung disease; hemoglobin less than 10 g/ml, absolute neutrophil count (ANC) less than 1000 cells/mm3, platelet count less than 70,000 cells/mm3, creatinine greater than 1.5 mg/dl, creatinine clearance less than 50 ml/h or significant proteinuria; decompensated cirrhosis, hepatic disease from other causes, hepatocellular carcinoma; acute opportunistic infection or malignancy; active alcohol use; unwillingness to switch didanosine to an alternate medication. Women were excluded if they were pregnant, breastfeeding or unable to use contraception, as were male sexual partners of pregnant or breastfeeding women. Individuals were not excluded or discontinued from treatment for active drug use.
Recruitment occurred through posters at two HIV clinics and eight freestanding MMPs, on buses and via advertisements on local list serves. Intended study enrollment was thirty participants. Sample size was empiric, determined by cost of laboratory testing and limited size of the population available. In RI, there are approximately 200 coinfected out of 4000 MM patients.
The study was a prospective, single-arm observational study. Nurses administered pegIFN alfa 2a (Pegasys, Roche) 180 μg subcutaneous injections at weekly HIV clinic visits. Participants weighing less than 75 kg took RBV (Copegus, Roche) 600 mg each morning and 400 mg each evening. Participants weighing 75 kg or more took 600 mg twice daily. For participants receiving daily methadone, the morning RBV dose was directly observed with the morning methadone dose seven days per week. Evening RBV doses were distributed daily by MMP nurses, with instructions to take RBV at home with food. Some MM recipients were allowed to take home some of their methadone doses upon demonstrating excellent adherence to MMP visits and drug abstinence. Participants with MM “take home” status took RBV unsupervised.
HCV RNA levels were measured at weeks 1, 12, 24, and 48 during therapy, and 6 months after treatment completion or discontinuation to determine attainment of SVR. Participants with detectable week 24 HCV RNA had treatment discontinued due to virologic failure. This protocol was established prior to the week 12 “stopping rule” for nonresponse, defined as <2 log10 drop in HCV RNA by week 12 (termed lack of Early Virologic Response, EVR) (Ghany et al., 2009). HCV RNA levels were measured using the Siemens bDNA quantitative assay, with a lower limit of detection of 615 IU/mL and upper limit of 7,700,000 IU/mL.
Safety was assessed at weekly HIV clinic visits through physical examination, assessment of side effects, serum chemical and hematologic evaluations and administration of the BDI-II, throughout treatment and 4, 12 and 24 weeks post-treatment. Participants received an ophthalmologic examination at baseline, 3 and 6 months, and end of treatment. Erythropoietin and neupogen were utilized before dose reductions of RBV and/or pegIFN for hematologic adverse events. If hemoglobin declined by 2 g/dl from baseline or to 10 g/dl, erythropoeitin was initiated at 40,000 IU/ml weekly and hemoglobin was rechecked weekly until stable. If hemoglobin continued to decline despite erythropoietin administration, the RBV dose was decreased by 200 mg/wk and erythropoietin continued at 40,000 IU/ml weekly. If hemogloblin continued to decline with erythropoietin 40,000 IU/ml weekly and RBV 600 mg/day, RBV was suspended and erythropoietin continued until hemoglobin increased to baseline or to maximum of 14 g/dl. For participants whose RBV was withheld, RBV was then restarted at 600 mg and increased to a maximum of 800 mg daily. RBV was permanently discontinued for hemoglobin less than 8.5 g/dl.
Monitoring for suicidality was part of the rationale for directly administered pegIFN with weekly BDI-IIs. Participants with BDI-II ≥29 or ≥20 (if this represented doubling of baseline BDI-II score) met with a therapist prior to leaving clinic. A study nurse spoke with these participants via telephone within several days. Participants with two consecutive weeks of BDI-II ≥29 or≥20 (if this represented a doubling of baseline score) were assessed for major depression using a major depressive disorder checklist and referred to the study psychiatrist. A study clinician assessed participants who endorsed suicidality (BDI-II ≥1 on Item 9) before they could leave clinic. Further steps were taken depending on level of suicide risk.
For a serious adverse event (SAE), participants who discontinued treatment remained in the study and continued with quarterly clinic visits (or more frequently if the SAE required) until the end of the 48-week treatment period, and for 6 months following the treatment period.
Primary end point was SVR. Analyses were performed on intent to treat basis, including data from all participants who received at least one pegIFN injection. A secondary end point was adherence to pegIFN. SVR was compared to a historical control group, AIDS Clinical Trial Group (ACTG) A5071 (Chung et al., 2004). ACTG A5071 is the only randomized trial of coinfected U.S. subjects utilizing pegIFN/RBV, including a racially diverse population similar to RI’s. coinfected population; one third of the ACTG A5071 study group was Black. This trial was also selected as a comparator because the study design incorporated RBV escalation from 600 mg to 1000 mg daily. In A5071, 14% (7 of 51) of genotype 1 subjects achieved SVR. Of 133 A5071 subjects, 9 were taking MM at entry, and 3 achieved SVR, consistent with the overall ACTG study SVR rate of 27% (Chung Personal Communication, 2009).
3. Results
During the 18-month recruitment period, 11 participants from three MMPs were enrolled (Table 1). The majority (7) were women, mean age was 46 and 6 were Black or Hispanic. Most (9) had HCV RNA levels of 400,000 IU/ml or more, and 6 had at least stage 2 fibrosis including 2 with cirrhosis. Ten were on HAART, with 5 taking zidovidine (AZT, 2 female, 3 male). Nine had undetectable HIV RNA. Median CD4+ was 508 cells/μL. All participants had polysubstance use history including opioids in addition to cocaine, alcohol, marijuana and/or benzodiazepines. All were taking psychotropic medications for pre-existing non-substance-based psychiatric diagnoses. Six received daily methadone and had morning RBV directly administered at their MMP, while 5 participants had “take home” MM status and took all RBV unsupervised. No person who was clinically eligible declined participation.
Table 1.
Characteristics of 11 HIV/HCV coinfected genotype 1 methadone maintenance study participants at start of HCV treatment.
| Characteristic | Value |
|---|---|
| Demographic | |
| Age, mean (range) (years) | 46 (37–61) |
| Female | 7 (64) |
| Race | |
| Black, non-Hispanic | 4 |
| White, non-Hispanic | 5 |
| Hispanic | 2 |
| Substance use history | |
| Injection drug use | 11 (100) |
| Polysubstance drug use | 11 (100) |
| Psychiatric history | 11 (100) |
| Prescribed psychotropic medications pre-study | 11 (100) |
| Depression | 9 (82) |
| Anxiety | 6 (55) |
| Panic disorder | 1 (9) |
| Schizoaffective disorder | 1 (9) |
| Post-traumatic stress disorder | 1 (9) |
| HCV-related clinical values | |
| Biopsy stage (Batts and Ludwig staging system) | |
| Stage 1 | 1 (9) |
| Stage1–2 or 2 | 5 (45) |
| Stage 3 | 1 (9) |
| Stage 3–4 or 4 | 4 (36) |
| High HCV RNA (>400,000 IU/ml) | 9 (82) |
| HIV-related clinical values | |
| On HAART | 10 (91) |
| CD4+ cell count, mean (range) (cells/μL) | 498 (210–868) |
| Undetectable HIV RNA (<75 copies/ml) | 9 (82) |
Note: Data are no. (%) of participants, except where noted.
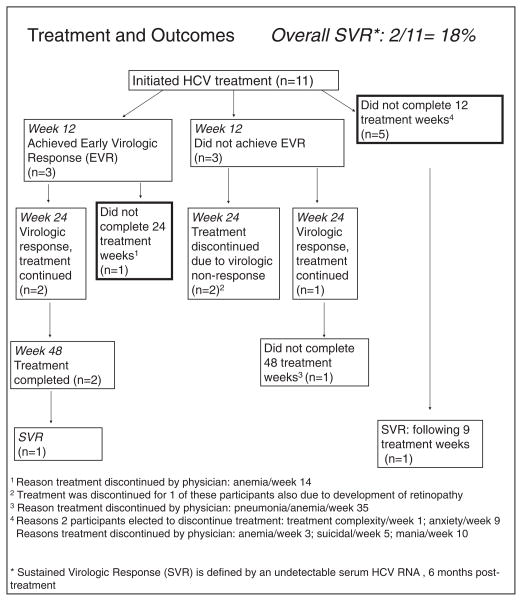
Two (18%) participants achieved SVR (Fig. 1). Of these, 1 completed 48 treatment weeks following EVR at week 12. The other, with low baseline HCV RNA level, chose to stop treatment at week 9 due to anxiety but achieved SVR. Two additional participants achieved EVR but not subsequent SVR; of these, 1 had treatment discontinued at week 14 for anemia and the other completed 48 weeks. Three participants not achieving EVR continued treatment and subsequently had treatment discontinued before week 48; of these, 1 was discontinued due to pneumonia and anemia, while 2 were discontinued due to week 24 virologic nonresponse. One of these week 24 virologic non-responders also developed retinopathy at week 24, which resolved with treatment discontinuation. Four participants completed less than 12 weeks; 3 were discontinued due to SAEs (anemia, suicidal ideation, mania) while 1 elected to discontinue treatment after one week.
Fig. 1.
Outcomes for the enrolled patients.
Two participants completed 48 treatment weeks, 2 elected to stop treatment prematurely, and 7 had treatment discontinued by the physician due to SAEs (5) and virologic nonresponse (2). Most participants (7) chose to stop treatment or had treatment discontinued prior to 48 weeks for reasons other than virologic non-response, including 3 for psychiatric adverse events (suicidality, mania, anxiety), 3 men for anemia (with 1 of these having pneumonia plus anemia), and 1 after one injection. Two of the 3 participants stopping for anemia were taking AZT. Most participants received growth factors during treatment, either erythropoietin (9) or neupogen (1).
Of on-treatment weeks, adherence to pegIFN was >99%, with 1 out of the total 220 scheduled injections being missed.
4. Discussion
Our findings reflect the benefits and complexities of extending HCV treatment to coinfected MM patients. To our knowledge, CHAMP is the first study of HCV treatment in coinfected MM patients with directly administered pegIFN when MM is received off-site. In the U.S. it is more likely that coinfected MM patients do not receive methadone, HCV and HIV services at a single site, making study findings more generalizable.
Our 18% (2 of 11) SVR rate was comparable to a historic control of coinfected genotype 1 persons (14%), most of whom were not on MM, despite poor prognostic factors including high HCV RNA, advanced fibrosis, age greater than 40 years, Black race and Hispanic ethnicity. Our participants also had complex comorbidities including polysubstance use and other psychiatric diagnoses. It is promising that during on-treatment weeks, participants received over 99% of pegIFN injections; treatment failure did not result from non-adherence to the backbone of the regimen. Most participants had non-detectable serum HIV RNA at enrollment, consistent with evidence demonstrating that drug-involved patients may adhere to complex antiviral therapies.
Most participants discontinued treatment prematurely due to SAEs including psychiatric effects and anemia. Concern for potential premature treatment cessation should not however be a reason to withhold treatment. In a prospective controlled study, Mauss et al. (2004) reported that MM patients discontinuing HCV therapy prematurely did so early, minimizing the cost of an unsuccessful treatment. In CHAMP, among the 10 participants not completing treatment, 5 were withdrawn or dropped out within the first 12 weeks; a sixth was withdrawn at week 14. With on-treatment viral kinetics to motivate or appropriately dissuade patients, and testing for polymorphisms in the IL28B (interleukin-28B) gene region to predict genetic predisposition to SVR on the horizon, tailored therapy duration is increasingly possible. Even when treatment is discontinued early, SVR may be achieved, consistent with previous reports (Rachline et al., 2010); 1 CHAMP participant with low baseline HCV RNA achieved SVR with only 9 treatment weeks.
What has changed since CHAMP is that AZT is now contraindicated with RBV due to appreciation of a synergistic effect exacerbating anemia. Were the study repeated, we would avoid AZT, possibly curtailing discontinuation for anemia. Optimal RBV dosing in coinfection remains under investigation, and weight-based RBV may have been contributory. However AZT use rather than RBV exposure was likely the main determinant of anemia (Alvarez et al., 2006; Soriano et al., 2007). Hopefully pegIFN and thus most psychiatric SAEs may be eliminated with future HCV regimens.
Recruitment for this study was slow. Our difficulty with enrollment reflects a problem within the HIV/HCV community at large. HCV treatment eligibility in coinfection is limited, due to advanced AIDS, late stage of liver disease at presentation and other medical contraindications (Butt et al., 2009; Fleming et al., 2003; Fultz et al., 2003; Rauch et al., 2005; Taylor et al., 2002). Local conditions posed additional constraints, given the small potential pool of 200 coinfected MM patients in RI at the time of enrollment. With estimated 70% genotype 1 (140 individuals), our sample size is consistent with reported HCV treatment uptake rates (Fultz et al., 2003; Mehta et al., 2006).
We did not require a period of pre-treatment abstinence. Because addiction is a chronic, relapsing illness, six months of abstinence does not predict future abstinence, just as six months of drug use does not preclude future abstinence (Cami and Farre, 2003; Schaefer et al., 2003). Duration of abstinence does not affect HCV treatment discontinuation (Schaefer et al., 2003). Successful HCV treatment occurs among individuals with ongoing substance use (Sylvestre et al., 2004; Sylvestre and Zweben, 2007). In CHAMP, ongoing drug use did not specifically lead to premature HCV treatment discontinuation to our knowledge.
Several limitations should be noted. We did not adequately monitor the evening RBV dose, so the impact of RBV adherence cannot be assessed. We did not systematically collect data on continuing drug use. Discussions about and assistance with ongoing drug use occurred in the clinical setting along with discussions of other health issues.
Our treatment completion rate (1 of 11, 9%) is lower than reported in HCV-monoinfected MM patients (Novick and Kreek, 2008) and in HIV/HCV coinfection (Carrat et al., 2004; Chung et al., 2004; Soriano et al., 2007; Torriani et al., 2004). AZT use contributed to treatment discontinuation and modification, highlighting that antiretroviral agents impact SAEs during HCV therapy (Bhattacharya et al., 2010). Real-world coinfected MM patients are difficult to treat yet have great need for treatment. Specifically targeted antiviral therapy for HCV should be studied in coinfection to improve efficacy with the hope of shorter therapy duration and improved tolerability, and with methadone to evaluate whether the stability provided by MM may ease the use of complex novel therapies for opiate-dependent individuals.
Acknowledgments
Role of funding source
Funding for this study was provided by Roche Laboratories, Inc.; Roche had no further role in study design; in the collection, analysis and interpretation of data; in the writing of the report; or in the decision to submit the paper for publication.
This publication resulted (in part) from Dr. Taylor’s research supported by Award Number K23DA020383-01 from the National Institute on Drug Abuse. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institute on Drug Abuse or the National Institutes of Health. This research has been facilitated by the infrastructure and resources provided by the Lifespan/Tufts/Brown Center for AIDS Research, an NIH funded program #P30 AI42853.
We thank Raymond S. Koff, M.D. for his early support of the CHAMP Study; Raymond T. Chung, M.D., who kindly provided data on methadone maintenance use in AIDS Clinical Trial Group A5071; Steven Peligian, M.D., Medical Director, and the staff of CODAC Behavioral Healthcare, Cranston, Rhode Island; the staff of Discovery House, Providence and Woonsocket, Rhode Island; and the patients of the Miriam Hospital Immunology Center and Rhode Island Hospital HIV Clinic.
Footnotes
Contributors
Author McGovern designed the study. Authors McGovern and Taylor wrote the protocol. Authors Taylor, Bowman and Maynard managed the literature searches and summaries of previous related work. Authors Taylor, Stein, Chapman, Zaller and Cioe implemented the study protocol and participated in subject selection. Authors Taylor, Stein, Chapman and Cioe provided clinical care to study participants. Author Zaller led fieldwork with community-based methadone maintenance programs. Authors Taylor, Zaller and Chapman were involved in the study’s daily running. Authors Chapman and Cioe oversaw data collection. Authors Taylor and Bowman undertook the data analysis and wrote the first draft of the manuscript. All authors contributed to and have approved the final manuscript.
Conflict of interest statement
Author Taylor has consulted for Vertex Pharmaceuticals, is on the Speakers Bureau for Genentech and has received grant support from Roche and Vertex. Author McGovern was on the Speakers Bureau for Roche and is on the Advisory Board for Vertex and Merck. Author Cioe is on the Speakers Bureau for Genentech and Schering Plough and has consulted for Vertex. Authors Bowman, Chapman, Zaller, Maynard and Stein declare that they have no conflicts of interest.
References
- Alter M. Epidemiology of hepatitis C. Hepatology. 1997;26:62S–65S. doi: 10.1002/hep.510260711. [DOI] [PubMed] [Google Scholar]
- Alvarez D, Dieterich DT, Brau N, Moorehead L, Ball L, Sulkowski MS. Zidovudine use but not weight-based ribavirin dosing impacts anaemia during HCV treatment in HIV-infected persons. J Viral Hepat. 2006;13:683–689. doi: 10.1111/j.1365-2893.2006.00749.x. [DOI] [PubMed] [Google Scholar]
- Backmund M, Meyer K, Von Zielonka M, Eichenlaub D. Treatment of hepatitis C infection in injection drug users. Hepatology. 2001;34:188–193. doi: 10.1053/jhep.2001.25882. [DOI] [PubMed] [Google Scholar]
- Batts K, Ludwig J. Chronic hepatitis—an update on terminology and reporting. Am J Surg Pathol. 1995;19:1409–1417. doi: 10.1097/00000478-199512000-00007. [DOI] [PubMed] [Google Scholar]
- Beck AT, Steer RA, Brown GK. Manual for Beck Depression Inventory-II. Psychological Corporation; San Antonio, TX: 1996. [Google Scholar]
- Berenguer J, Alvarez-Pellicer J, Martin PM, Lopez-Aldeguer J, Von-Wichmann MA, Quereda C, Mallolas J, Sanz J, Tural C, Bellon JM, Gonzalez-Garcia J. Sustained virological response to interferon plus ribavirin reduces liver-related complications and mortality in patients coinfected with human immunodeficiency virus and hepatitis C virus. Hepatology. 2009;50:407–413. doi: 10.1002/hep.23020. [DOI] [PubMed] [Google Scholar]
- Bhattacharya D, Umbleja T, Carrat F, Chung RT, Peters MG, Torriani F, Andersen J, Currier JS. Women experience higher rates of adverse events during hepatitis C virus therapy in HIV infection: a meta-analysis. J Acquir Immune Defic Syndr. 2010;55:170–175. doi: 10.1097/QAI.0b013e3181e36420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bica I, McGovern B, Dhar R, Stone D, McGowan K, Scheib R, Snydman D. Increasing mortality due to end-stage liver disease in patients with human immunodeficiency virus infection. Clin Infect Dis. 2001;32:492–497. doi: 10.1086/318501. [DOI] [PubMed] [Google Scholar]
- Bonkovsky H, Tice A, Yapp R, Bodenheimer HJ, Monto A, Rossi S, Sulkowski M. Efficacy and safety of peginterferon alfa-2a/ribavirin in methadone maintenance patients: randomized comparison of direct observed therapy and self-administration. Am J Gastroenterol. 2008;103:2757–2765. doi: 10.1111/j.1572-0241.2008.02065.x. [DOI] [PubMed] [Google Scholar]
- Butt AA, Khan UA, Shaikh OS, McMahon D, Dorey-Stein Z, Tsevat J, Lo Re V. Rates of HCV treatment eligibility among HCV-monoinfected and HCV/HIV-coinfected patients in tertiary care referral centers. HIV Clin Trials. 2009;10:25–32. doi: 10.1310/hct1001-025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cami J, Farre M. Drug addiction. N Engl J Med. 2003;349:975–986. doi: 10.1056/NEJMra023160. [DOI] [PubMed] [Google Scholar]
- Carrat F, Bani-Sadr F, Pol S, Rosenthal E, Lunel-Fabiani F, Benzekri A, Morand P, Goujard C, Pialoux G, Piroth L, Salmon-Céron D, Degott C, Cacoub P, Perronne C. Pegylated interferon alfa-2b vs standard interferon alfa-2b, plus ribavirin, for chronic hepatitis C in HIV-infected patients: a randomized controlled trial. JAMA. 2004;292:2839–2848. doi: 10.1001/jama.292.23.2839. [DOI] [PubMed] [Google Scholar]
- Chung R, Andersen J, Volberding P, Robbins G, Liu T, Sherman K, Peters M, Koziel M, Bhan A, Alston B, Colquhoun D, Nevin T, Harb G, van der Horst C. Peginterferon Alfa-2a plus ribavirin versus interferon alfa-2a plus ribavirin for chronic hepatitis C in HIV-coinfected persons. N Engl J Med. 2004;351:451–459. doi: 10.1056/NEJMoa032653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conway B, Prasad J, Reynolds R, Farley J, Jones M, Jutha S, Smith N, Mead A, DeVlaming S. Directly observed therapy for the management of HIV-infected patients in a methadone program. Clin Infect Dis. 2004;38 (Suppl 5):S402–408. doi: 10.1086/421404. [DOI] [PubMed] [Google Scholar]
- Dalgard O, Bjøro K, Hellum K, Myrvang B, Skaug K, Gutigard B, Bell H. Treatment of chronic hepatitis C in injecting drug users: 5 year’s follow-up. Eur Addict Res. 2002;8:45–49. doi: 10.1159/000049487. [DOI] [PubMed] [Google Scholar]
- Davis G, Rodrigue J. Treatment of chronic hepatitis C in active drug users. N Engl J Med. 2001;345:215–217. doi: 10.1056/NEJM200107193450312. [DOI] [PubMed] [Google Scholar]
- Fishbein DA, Lo Y, Reinus JF, Gourevitch MN, Klein RS. Factors associated with successful referral for clinical care of drug users with chronic hepatitis C who have or are at risk for HIV infection. J Acquir Immune Defic Syndr. 2004;37:1367–1375. doi: 10.1097/01.qai.0000131932.21612.49. [DOI] [PubMed] [Google Scholar]
- Fleming C, Craven D, Thornton D, Tumilty S, Nunes D. Hepatitis C virus and human immunodeficiency virus coinfection in an urban population: low eligibility for interferon treatment. Clin Infect Dis. 2003;36:97–100. doi: 10.1086/344907. [DOI] [PubMed] [Google Scholar]
- Fultz S, Justice A, Butt A, Rabeneck L, Weissman S, Rodriguez-Barradas M. Testing, referral, and treatment patterns for hepatitis C virus coinfection in a cohort of veterans with human immunodeficiency virus infection. Clin Infect Dis. 2003;36:1039–1046. doi: 10.1086/374049. [DOI] [PubMed] [Google Scholar]
- Ghany M, Strader D, Thomas D, Seeff L. Diagnosis, management, and treatment of hepatitis C: an update. Hepatology. 2009;49:1335–1374. doi: 10.1002/hep.22759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grebely J, Raffa J, Meagher C, Duncan F, Genoway K, Khara M, McLean M, Mead A, Viljoen M, DeVlaming S, Fraser C, Conway B. Directly observed therapy for the treatment of hepatitis C virus infection in current and former injection drug users. J Gastroenterol Hepatol. 2007;22:1519–1525. doi: 10.1111/j.1440-1746.2007.05032.x. [DOI] [PubMed] [Google Scholar]
- Grebely J, Raffa JD, Lai C, Krajden M, Kerr T, Fischer B, Tyndall MW. Low uptake of treatment for hepatitis C virus infection in a large community-based study of inner city residents. J Viral Hepat. 2009;16:352–358. doi: 10.1111/j.1365-2893.2009.01080.x. [DOI] [PubMed] [Google Scholar]
- Hellard M, Sacks-Davis R, Gold J. Hepatitis C treatment for injection drug users: a review of the available evidence. Clin Infect Dis. 2009;49:561–573. doi: 10.1086/600304. [DOI] [PubMed] [Google Scholar]
- Kleber H. Methadone maintenance 4 decades later: thousands of lives saved but still controversial. JAMA. 2008;300:2303–2305. doi: 10.1001/jama.2008.648. [DOI] [PubMed] [Google Scholar]
- Koziel M, Peters M. Viral hepatitis in HIV infection. N Engl J Med. 2007;356:1445–1454. doi: 10.1056/NEJMra065142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Litwin A, Harris KJ, Nahvi S, Zamor P, Soloway I, Tenore P, Kaswan D, Gourevitch M, Arnsten J. Successful treatment of chronic hepatitis C with pegylated interferon in combination with ribavirin in a methadone maintenance treatment program. J Subst Abuse Treat. 2009;37:32–40. doi: 10.1016/j.jsat.2008.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mauss S, Berger F, Goelz J, Jacob B, Schmutz G. A prospective controlled study of interferon-based therapy of chronic hepatitis C in patients on methadone maintenance. Hepatology. 2004;40:120–124. doi: 10.1002/hep.20279. [DOI] [PubMed] [Google Scholar]
- McCarthy J, Flynn N. Hepatitis C in methadone maintenance patients: prevalence and public policy implications. J Addict Dis. 2001;20:19–31. doi: 10.1300/J069v20n01_03. [DOI] [PubMed] [Google Scholar]
- Mehta SH, Genberg BL, Astemborski J, Kavasery R, Kirk GD, Vlahov D, Strathdee SA, Thomas DL. Limited uptake of hepatitis C treatment among injection drug users. J Community Health. 2008;33:126–133. doi: 10.1007/s10900-007-9083-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mehta SH, Lucas GM, Mirel LB, Torbenson M, Higgins Y, Moore RD, Thomas DL, Sulkowski MS. Limited effectiveness of antiviral treatment for hepatitis C in an urban HIV clinic. AIDS. 2006;20:2361–2369. doi: 10.1097/QAD.0b013e32801086da. [DOI] [PubMed] [Google Scholar]
- National Institute of Health. NIH concenus statement on management of hepatitis C: 2002. NIH Consens State Sci Statements. 2002;19 (3):1–46. [PubMed] [Google Scholar]
- Novick DM, Kreek MJ. Critical issues in the treatment of hepatitis C virus infection in methadone maintenance patients. Addiction. 2008;103:905–918. doi: 10.1111/j.1360-0443.2008.02188.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piccolo P, Borg L, Lin A, Melia D, Ho A, Kreek M. Hepatitis C virus and human immunodeficiency virus-1 co-infection in former heroin addicts in methadone maintenance treatment. J Addict Dis. 2002;21:55–66. doi: 10.1300/J069v21n04_06. [DOI] [PubMed] [Google Scholar]
- Rachline A, Palmer P, Simon F, Molina JM. Case report: Cure of chronic infection with hepatitis C virus after 6 weeks of peg-interferon and ribavirin in a patient co-infected with HIV. J Med Virol. 2010;82:1150–1151. doi: 10.1002/jmv.21781. [DOI] [PubMed] [Google Scholar]
- Rauch A, Egger M, Reichen J, Furrer H. Chronic hepatitis C in HIV-infected patients: low eligibility and applicability of therapy with pegylated interferon-alpha plus ribavirin. J Acquir Immune Defic Syndr. 2005;38:238–240. doi: 10.1097/01.qai.0000148535.97081.72. [DOI] [PubMed] [Google Scholar]
- Schaefer M, Schmidt F, Folwaczny C, Lorenz R, Martin G, Schindlbeck N, Heldwein W, Soyka M, Grunze H, Koenig A, Loeschke K. Adherence and mental side effects during hepatitis C treatment with interferon alfa and ribavirin in psychiatric risk groups. Hepatology. 2003;37:443–451. doi: 10.1053/jhep.2003.50031. [DOI] [PubMed] [Google Scholar]
- Sherman K, Rouster S, Chung R, Rajicic N. Hepatitis C Virus prevalence among patients infected with Human Immunodeficiency Virus: a cross-sectional analysis of the US adult AIDS Clinical Trials Group. Clin Infect Dis. 2002;34:831–837. doi: 10.1086/339042. [DOI] [PubMed] [Google Scholar]
- Soriano V, Miralles C, Berdun MA, Losada E, Aguirrebengoa K, Ocampo A, Arazo P, Cervantes M, de los Santos I, San Joaquin I, Echeverria S, Galindo MJ, Asensi V, Barreiro P, Sola J, Hernandez-Burruezo JJ, Guardiola J, Blanco F, Martin-Carbonero L, Garcia-Samaniego J, Nunez M. Premature treatment discontinuation in HIV/HCV-coinfected patients receiving pegylated interferon plus weight-based ribavirin. Antivir Ther. 2007;12:469–476. [PubMed] [Google Scholar]
- Strader D, Wright T, Thomas D, Seeff L. Diagnosis, management, and treatment of hepatitis C. Hepatology. 2004;39:1147–1171. doi: 10.1002/hep.20119. [DOI] [PubMed] [Google Scholar]
- Sulkowski M, Thomas D. Hepatitis C in the HIV-Infected Person. Ann Intern Med. 2003;138:197–207. doi: 10.7326/0003-4819-138-3-200302040-00012. [DOI] [PubMed] [Google Scholar]
- Sylvestre D, Clements B. Adherence to hepatitis C treatment in recovering heroin users maintained on methadone. Eur J Gastroenterol Hepatol. 2007;19:741–747. doi: 10.1097/MEG.0b013e3281bcb8d8. [DOI] [PubMed] [Google Scholar]
- Sylvestre D, Loftis J, Hauser P, Genser S, Cesari H, Borek N, Kresina T, Seeff L, Francis H. Co-occurring Hepatitis C, substance use, and psychiatric illness: treatment issues and developing integrated models of care. J Urban Health. 2004;81:719–734. doi: 10.1093/jurban/jth153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sylvestre D, Zweben J. Integrating HCV services for drug users: a model to improve engagement and outcomes. Int J Drug Policy. 2007;18:406–410. doi: 10.1016/j.drugpo.2007.01.010. [DOI] [PubMed] [Google Scholar]
- Taylor LE, Costello T, Alt E, Yates G, Tashima K. Psychiatric illness and illicit drugs as barriers to hepatitis C treatment among HIV/hepatitis C virus co-infected individuals. AIDS. 2002;16:1700–1701. doi: 10.1097/00002030-200208160-00024. [DOI] [PubMed] [Google Scholar]
- Torriani F, Rodriguez-Torres M, Rockstroh J, Lissen E, Gonzalez-García J, Lazzarin A, Carosi G, Sasadeusz J, Katlama C, Montaner J, Sette HJ, Passe S, De Pamphilis J, Duff F, Schrenk U, Dieterich D. Peginterferon Alfa-2a plus ribavirin for chronic hepatitis C virus infection in HIV-infected patients. N Engl J Med. 2004;351:438–450. doi: 10.1056/NEJMoa040842. [DOI] [PubMed] [Google Scholar]
- Wagner G, Ryan G, Osilla K, Bhatti L, Goetz M, Witt M. Treat early or wait and monitor? A qualitative analysis of provider hepatitis C virus treatment decision-making in the context of HIV coinfection. AIDS Patient Care STDS. 2009;23:715–725. doi: 10.1089/apc.2009.0049. [DOI] [PMC free article] [PubMed] [Google Scholar]