Abstract
After decades of decline in prevalence of complete tooth loss (edentulism), the trend continues to be misinterpreted, producing flawed projections and misdirected health goals. We investigated population trends in edentulism among U.S. adults aged ≥15 yr by creating time-series data from 5 national cross-sectional health surveys: 1957-1958 (n ≈ 100,000 adults), 1971-1975 (n = 14,655 adults), 1988-1998 (n = 18,011 adults), 1999-2002 (n = 12,336 adults), and 2009-2012 (n = 10,522 adults). Birth cohort analysis was used to isolate age and cohort effects. Geographic and sociodemographic variation in prevalence was investigated with a sixth U.S. survey of 432,519 adults conducted in 2010. Prevalence through 2050 was projected with age-cohort regression models using Monte-Carlo simulation of prediction intervals. Across the 5-decade observation period, edentulism prevalence declined from 18.9% in 1957-1958 (95% confidence limits: 18.4%, 19.4%) to 4.9% in 2009-2012 (95% confidence limits: 4.0%, 5.8%). The most influential determinant of the decline was the passing of generations born before the 1940s, whose rate of edentulism incidence (5%-6% per decade of age) far exceeded later cohorts (1%-3% per decade of age). High-income households experienced a greater relative decline, although a smaller absolute decline, than low-income households. By 2010, edentulism was a rare condition in high-income households, and it had contracted geographically to states with disproportionately high poverty. With the passing of generations born in the mid-20th century, the rate of decline in edentulism is projected to slow, reaching 2.6% (95% prediction limits: 2.1%, 3.1%) by 2050. The continuing decline will be offset only partially by population growth and population aging such that the predicted number of edentulous people in 2050 (8.6 million; 95% prediction limits: 6.8 million, 10.3 million) will be 30% lower than the 12.2 million edentulous people in 2010.
Keywords: edentulous, cross-sectional studies, dental health surveys, time factors, socioeconomic factors, National Health and Nutrition Examination Survey
Introduction
Complete tooth loss (edentulism) is considered “the dental equivalent of mortality” (Weintraub and Burt, 1985) and for good reasons. Edentulism represents the end stages of dental caries and periodontitis, and some studies have found that it predicts mortality (Polzer et al., 2012). It certainly diminishes quality of life (Emami et al., 2013). Many countries monitor its prevalence, with several reporting marked declines in recent decades (Weintraub and Burt, 1985; Steele et al., 2000; Mojon et al., 2004; Crocombe and Slade, 2007). Targets for reductions in prevalence have been nominated nationally (U.S. Department of Health and Human Services Office of Disease Prevention and Health Promotion, 2013) and globally (Hobdell et al., 2003).
It is striking, therefore, that the decline remains so poorly understood. A case in point is the U.S. Healthy People 2020 objective OH-4.2 to reduce edentulism prevalence among 65- to 74-year-olds to 21.6%, a 10% relative reduction from the baseline prevalence of 24.0% (i.e., 1999-2004; U.S. Department of Health and Human Services Office of Disease Prevention and Health Promotion, 2013). The 2020 objective was lower than the 2010 objective of 22% prevalence in 65- to 74-year-olds but higher than the 2000 objective of 20% in people aged ≥65 yr. Implicitly, the targeted 10% relative reduction is predicated on the expectation that, without policies to improve oral health, prevalence for 65- to 74-year-olds in 2020 likely would be similar to that of 65- to 74-year-olds in 2000. While intuitively appealing, this expectation ignores historical experiences of the 2 relevant birth cohorts: people born in 1926-1935, whose pattern of tooth loss dictated the baseline statistic, and people born in 1946-1955, who are the target for the 2020 objective.
There are well-established graphical (MacMahon et al., 1960) and statistical methods (Clayton and Schifflers, 1987) that isolate age- and cohort-based effects, permitting more accurate projections. Hence, the aims of this study were (1) to quantify trends in edentulism prevalence among U.S. adults over 5 decades, (2) to describe geographic and sociodemographic variation in edentulism in 2010, and (3) to project prevalence for 2050.
Materials & Methods
A time series was created from 5 national surveys, each of which selected a random sample of people to represent the U.S. civilian, noninstitutional population. Prevalence in 1957-1958 was calculated with published tables from the U.S. National Health Survey (U.S. Department of Health and Health Education and Welfare, 1960). Interviews for the survey were “conducted in approximately 36,000 households and included 115,000 persons” (greater precision is not published). Interviewers asked, “Is there anyone in the family who has lost all of his teeth?” making a separate record for each affected person. Age group– and sex-specific prevalence estimates were calculated for people aged ≥15 yr, while race- and income-specific estimates were limited to those aged ≥25 yr. Race was classified as “White” or “non-White.” For annual family income, the lowest and highest reported categories (<$2,000 and ≥$7,000, respectively) each represented approximately one quarter of the population; the 2 intermediate categories ($2,000 to <$4,000 and $3,000 to <$7,000) were combined to create one “middle”-income group, representing the remaining half of the population. Standard errors were calculated with data from the publication’s Appendix II (U.S. Department of Health and Health Education and Welfare, 1960).
Prevalence in 1971-1975 was calculated with a data set from the first National Health and Nutrition Examination Survey (NHANES; U.S. Department of Health and Human Services, 1992), in which 20,749 participants aged 15 to 74 yr were dentally examined. Two variables signifying presence or absence of permanent teeth in the upper and lower jaws were combined to create a binary indicator of edentulism.
Prevalence in 1988-1994 was calculated with a data set from the NHANES III (U.S. Department of Health and Human Services, 1996), in which 18,011 participants aged ≥15 yr were dentally examined. A single variable signified presence or absence of any permanent teeth.
Prevalence in 1999-2002 was calculated with a data set from the 1999-2002 NHANES (Centers for Disease Control and Prevention, 2000, 2002), in which 12,336 participants aged ≥15 yr were dentally examined. A single variable signified presence or absence of any permanent teeth.
Prevalence in 2009-2012 was calculated with NHANES 2009-2012 data (Centers for Disease Control and Prevention, 2010b, 2012), in which 14,970 participants aged ≥15 were dentally examined. Variables signifying the status of each permanent tooth were used to classify participants as edentulous if all 32 teeth were missing or replaced by an implant.
When the 4 NHANES data sets were analyzed, age, sex, and race were categorized for consistency with the 1957-1958 survey. For income, quartile-based distributions were created to be as close as possible to those of the 1957-1958 survey. All available data from adults in the NHANES data sets were analyzed separately with SAS survey estimation procedures to account for strata, clusters, and weights that were unique to the design of each survey. Prevalence estimates were therefore generalizable to the U.S. adult population in the respective survey period.
We additionally investigated historical influences on prevalence by creating age cohort plots (MacMahon et al., 1960). Prevalence was recalculated according to study participants’ year of birth. Five categories were dictated by the 1957-1958 survey publication: 1894-1903, 1904-1913, 1914-1923, 1924-1933, and 1934-1943. Later surveys included people born in subsequent decades: 1944-1953, 1954-1963, 1964-1973. For each birth cohort, edentulism was plotted against age, which was computed as the midpoint of the cohort’s age range at the midpoint of the survey period. Age cohort plots mimic a longitudinal study by depicting the age-associated gradient in prevalence as a cohort ages (MacMahon et al., 1960). Because edentulism is irreversible, the gradient therefore approximates edentulism incidence measured in a prospective cohort study.
Age cohort regression models (Clayton and Schifflers, 1987) were created to quantify trends and make projections. A weighted least squares linear regression model comprised the 24 data points in the age cohort plot of those born since 1914 (Appendix Table 1). Prevalence was the dependent variable, and predictor variables were cohort (modeled with dummy variables), age in years (rescaled in decades to represent the effect of 10 yr of aging), and the interaction of cohort and age. Potential curvilinear effects of age were investigated via age-squared. Weights were the inverse of the square of the standard error of the prevalence estimate; greater weight was therefore assigned to prevalence point estimates that had greater precision.
For the second aim, interview data were analyzed from the 2010 Behavioral Risk Factor Surveillance System for all 50 states and the District of Columbia. Unlike NHANES, the Behavioral Risk Factor Surveillance System includes the geographic variable of state, permitting spatial description of prevalence. The publicly available data set (Centers for Disease Control and Prevention, 2010a) was analyzed to compute edentulism prevalence and associated standard errors for 420,307 participants aged ≥25 yr. They were asked, “How many of your permanent teeth have been removed because of tooth decay or gum disease?” Participants who answered “all” were classified as edentulous. Prevalence estimates were calculated for 10-year age categories and cross-classified according to sex (male or female), race (White, Black, Asian, or other), ethnicity (Hispanic or non-Hispanic), annual household income (<$25,000, $25,000 to <$75,000, or ≥$75,000), and education (<high school, high school, or >high school). Age-standardized prevalence estimates were calculated by the direct method of standardization. The reference population was the U.S. Census 2010 distribution of 5-year age categories from 25 to ≥85 yr. Age-standardized prevalence estimates for states were mapped.
The age cohort regression model from the third aim was used to make projections of prevalence. The goal was to extrapolate prevalence data for the 6 birth cohorts that would survive to 2020 and for the 3 birth cohorts that would survive to 2050. Cohorts born after 1973 were assumed to have the age-related incidence observed in the 1954-1963 and 1964-1973 birth cohorts. Because the model used prevalence estimates of varying precision, Monte Carlo sensitivity analysis (Rothman et al., 2008) was used to calculate predicted prevalence and 95% prediction intervals from 1,000 simulation data sets. For each simulation, a prevalence value was generated at random for each of the 24 data points in the age cohort plot. The random-number generator used a normal distribution with a mean equal to the prevalence estimate and a standard deviation equal to its standard error. The set of randomly generated prevalence values was then used to create a weighted least squares linear regression model with age, cohort, and their interaction as predictor variables. Predicted prevalence was calculated by extrapolation to the midpoint of 5-year age categories in each index year. Predicted prevalence was multiplied by the predicted number of people in the population (U.S. Census Bureau Population Division, 2012) to predict overall prevalence and numbers of edentulous people in each index year. This process was repeated for 1,000 simulations; the median represented the predicted prevalence and the 2.5th and 97.5th percentiles were 95% prediction intervals.
This article is structured according to STROBE guidelines for cross-sectional studies.
Results
Edentulism prevalence declined from 18.9% in 1957-1958 to 4.9% in 2009-2012, a relative reduction of 78% (Table 1). Prevalence more than halved for each sociodemographic group, with greater relative reductions for younger than older groups and for high-income than low-income groups. In absolute terms, the greatest reduction in prevalence (25%) was among the low-income quartile. Among 65- to 74-year-olds in the 2009-2012 survey, the prevalence estimate of 13.7 (95% confidence limits: 10.5, 16.9) was substantially lower than the Healthy People 2020 target of 21.4% nominated for that age group.
Table 1.
Edentulism Prevalence among Adults in 5 U.S. Population Surveys
| Edentulism Prevalence, % (95% Confidence Limits) |
Decline: 1957-1958 to 2009-2012 |
||||||
|---|---|---|---|---|---|---|---|
| 1957-1958 | 1971-1975 | 1988-1994 | 1999-2002 | 2009-2012 | Absolute | Relative, % | |
| All people | 18.9 (18.4, 19.4) | 13.4 (12.3, 14.5) | 9.1 (8.1, 10.2) | 6.5 (5.6, 7.4) | 4.9 (4.0, 5.8) | 14.0 | 78 |
| Age at survey, yr | |||||||
| 15-24 | 0.9 (0.7, 1.2) | 0.4 (0.2, 0.7) | 0.0 (0, <0.1) | 0.0 (0, <0.1) | 0.0 (0, <0.1) | 0.9 | 100 |
| 25-34 | 3.6 (3.0, 4.1) | 3.2 (2.4, 4.0) | 0.7 (0.4, 1.1) | 0.4 (0.0, 0.9) | 0.2 (0.0, 0.3) | 3.4 | 94 |
| 35-44 | 9.6 (8.7, 10.5) | 9.2 (7.7, 10.6) | 2.7 (1.8, 3.6) | 1.4 (0.7, 2.0) | 1.1 (0.4, 1.8) | 8.5 | 89 |
| 45-54 | 22.4 (20.7, 24.0) | 16.1 (14.0, 18.1) | 9.1 (7.0, 11.3) | 4.8 (3.2, 6.4) | 3.1 (1.9, 4.2) | 19.3 | 86 |
| 55-64 | 38.1 (35.5, 40.6) | 33.2 (29.6, 36.7) | 20.1 (17.4, 22.7) | 12.7 (10.0, 15.3) | 6.6 (4.9, 8.4) | 31.5 | 83 |
| 65-74 | 55.4 (51.2, 59.5) | 46.1 (43.5, 48.6) | 28.6 (24.7, 32.5) | 22.6 (19.2, 26.0) | 13.7 (10.5, 16.9) | 41.7 | 75 |
| ≥75 | 67.3 (60.7, 73.9) | —c | 40.3 (35.6, 44.9) | 28.7 (25.0, 32.5) | 24.1 (20.1, 28.1) | 43.2 | 64 |
| Sex | |||||||
| Male | 17.6 (17.4, 17.9) | 14.6 (13.5, 15.7) | 9.7 (8.5, 11.0) | 7.3 (6.1, 8.5) | 5.4 (4.4, 6.4) | 12.2 | 69 |
| Female | 20.0 (19.8, 20.2) | 12.1 (10.7, 13.6) | 8.5 (7.5, 9.5) | 5.6 (4.7, 6.6) | 4.4 (3.4, 5.4) | 15.6 | 78 |
| Racea | |||||||
| Non-Hispanic White | 24.0 (23.3, 24.7) | 18.8 (17.4, 20.3) | 12.4 (10.9, 13.9) | 8.5 (7.1, 9.9) | 6.4 (5.1, 7.6) | 17.6 | 73 |
| Other | 12.1 (10.4, 13.8) | 11.1 (9.0, 13.2) | 7.4 (6.5, 8.4) | 6.4 (4.9, 8.0) | 4.5 (3.6, 5.5) | 7.6 | 63 |
| Incomea,b | |||||||
| Lower quartile | 37.8 (35.3, 40.3) | 23.5 (21.3, 25.7) | 16.5 (14.3, 18.7) | 17.5 (15.2, 19.9) | 12.3 (9.6, 14.9) | 25.8 | 68 |
| Middle 2 quartiles | 21.0 (20.1, 21.9) | 11.5 (10.5, 12.5) | 8.1 (7.1, 9.1) | 6.1 (5.0, 7.2) | 5.2 (4.3, 6.1) | 15.8 | 75 |
| Upper quartile | 14.8 13.6, 16.1) | 6.7 (5.3, 8.1) | 2.4 (1.8, 3.1) | 3.3 (2.1, 4.5) | 0.6 (0.2, 1.0) | 14.2 | 96 |
Data are for people aged ≥25 years.
For the 1957-1958 survey: lower, <$2,000/yr; middle half, $2,000 to <$7,000/yr; upper, ≥$7,000/yr. For the 1971-1975 survey: lower, <$6,000/yr; middle half, $6,000 to <$15,000/yr; highest, ≥$15,000/yr. For the 1999-2002 survey: lower, <$20,000/yr; middle half, $20,000 to <$75,000/yr; highest, ≥$75,000/yr. For the 2009-2012 survey: lower, <$25,000/yr; middle half, $25,000 to <$100,000/yr; highest, ≥$100,000/yr.
Not applicable: in the 1971-1975 survey, dental examinations were restricted to people aged <75 yr.
The age cohort plot revealed steep age gradients of edentulism incidence in the 4 cohorts born before 1934 (Fig. 1). The effects appeared to plateau when those cohort members reached their seventies, although the interpretation was obscured by large standard errors at older ages. For the 1934-1943 and 1944-1953 birth cohorts, age gradients were flatter. Neither of those cohorts was observed beyond their midsixties, making it impossible to judge if the age gradient flattened in older age. Age gradients were again flatter for the 2 most recent birth cohorts, born 1954-1963 and 1964-1973. Furthermore, age gradients in the 2 most recent birth cohorts overlapped completely. This was in marked contrast to each preceding cohort, where there were successively steeper age gradients for each preceding birth cohort.
Figure 1.
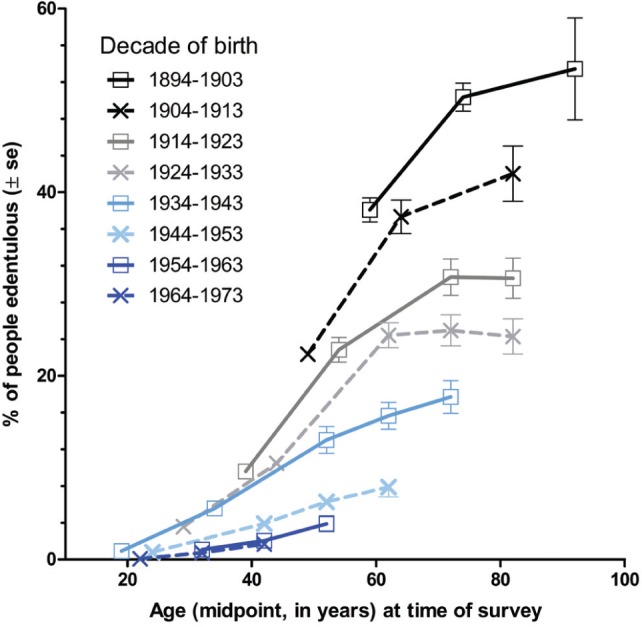
Age cohort plot of edentulism prevalence in 8 birth cohorts of U.S. adults in 5 national surveys, 1957-1958 to 2009-2012.
The patterns observed qualitatively in Figure 1 generally were confirmed by age cohort regression models (Table 2). Virtually all variance (R2 = 0.985) was explained by the model that had dummy variables for all 6 birth cohorts (Table 2, Model 1). Of note, the age gradient (β = 0.49) for the 1954-1963 birth cohort did not differ significantly from the age gradient for the reference cohort, born in 1964-1973 (p = .474). Furthermore, when the 2 most recent birth cohorts were combined (model 2), the overall fit of the model was virtually identical (R2 = 0.984; p = .34, comparing fit of models 1 and 2). In the reduced model 2, edentulism incidence was 0.92% per decade in the reference cohort, born between 1954 and 1973. For earlier cohorts, incidence increased successively by approximately 1% per decade compared with the reference cohort and was greatest in the 1914-1923 cohort (5.02 + 0.92 = 5.94% per decade). Although not shown in Table 2, a third model that included quadratic effects of age did not significantly improve the fit of model 1 (R2 = 0.994, p = .68, comparing fit of quadratic model with model 1).
Table 2.
Weighted Least Squares Regression Models of Age Cohort Effects of Edentulism Prevalence in 6 Cohorts
| Parameter | β | SE | p Value |
|---|---|---|---|
| Model 1a | |||
| Intercept | −15.70 | 6.26 | .028 |
| Age (per decade) in reference birth cohortb | 0.75 | 0.27 | .016 |
| Birth cohortc | |||
| 1914-1923 | −11.68 | 3.09 | .003 |
| 1924-1933 | −9.05 | 1.51 | <.001 |
| 1934-1943 | −3.83 | 0.96 | .002 |
| 1944-1953 | −2.10 | 1.08 | .075 |
| 1954-1963 | −1.35 | 2.20 | .551 |
| 1964-1973 | Reference | ||
| Age × cohort (per decade of age)d | |||
| 1914-1923 | 5.19 | 0.72 | <.001 |
| 1924-1933 | 4.16 | 0.47 | <.001 |
| 1934-1943 | 2.57 | 0.43 | <.001 |
| 1944-1953 | 1.10 | 0.41 | .020 |
| 1954-1963 | 0.49 | 0.66 | .474 |
| 1964-1973 | Reference | ||
| Model 2e | |||
| Intercept | −19.41 | 4.48 | .001 |
| Age (per decade) in reference birth cohortf | 0.92 | 0.18 | .000 |
| Birth cohortg | −11.31 | 2.94 | .002 |
| 1914-1923 | |||
| 1924-1933 | −8.68 | 1.39 | <.001 |
| 1934-1943 | −3.46 | 0.83 | .001 |
| 1944-1953 | −1.73 | 0.95 | .091 |
| 1954-1973 | Reference | ||
| Age × cohorth | |||
| 1914-1923 | 5.02 | 0.67 | <.001 |
| 1924-1933 | 3.99 | 0.42 | <.001 |
| 1934-1943 | 2.41 | 0.37 | <.001 |
| 1944-1953 | 0.94 | 0.35 | .018 |
| 1954-1973 | Reference | ||
Six 10-year cohorts, R2 = 0.985.
Analysis of variance (ANOVA): df = 1, F = 463.7, p < .001.
ANOVA: df = 5, F = 44.2, p < .001.
ANOVA: df = 5, F = 24.3, p < .001.
Four 10-year cohorts + one 20-year cohort, R2 = 0.984.
ANOVA: df = 1, F = 504.8, p < .001.
ANOVA: df = 4, F = 55.5, p < .001.
ANOVA: df = 4, F = 37.5, p < .001.
Cross-sectional analysis of the 2010 Behavioral Risk Factor Surveillance System data for ≥25-year-olds (Table 3) expanded on the sociodemographic patterns seen in Table 1. Specifically, Asians had markedly lower prevalence than any other racial group, and there was a large inverse association between educational attainment and edentulism prevalence (Table 3). The latter difference was similar in magnitude to the income-related difference. For both socioeconomic indicators, the magnitude of disparity did not change appreciably with age-standardized prevalence. However, age standardization attenuated differences between Hispanics and non-Hispanics.
Table 3.
Unadjusted and Age-Standardized Prevalence of Edentulism among U.S. Adults Aged ≥25 Yr at Time of Survey: BRFSS 2010a
| Unadjusted Prevalence of Edentulism by Age, % (SE) |
Prevalence, % (SE) |
||||||||
|---|---|---|---|---|---|---|---|---|---|
| 25-34 | 35-44 | 45-54 | 55-64 | 65-74 | 75-84 | ≥85 | All Ages | Age Standardizedb | |
| Sex | |||||||||
| Male | 0.5 (<0.1) | 1.1 (0.1) | 3.1 (0.2) | 7.2 (0.2) | 14.0 (0.3) | 17.1 (0.5) | 17.7 (0.9) | 4.9 (<0.1) | 5.3 (2.7) |
| Female | 0.8 (<0.1) | 1.2 (<0.1) | 3.4 (0.1) | 7.1 (0.2) | 14.8 (0.3) | 20.1 (0.4) | 19.8 (0.6) | 6.0 (<0.1) | 5.8 (3.0) |
| Race | |||||||||
| White | 0.7 (<0.1) | 1.2 (<0.1) | 3.1 (0.1) | 6.8 (0.1) | 13.7 (0.2) | 18.1 (0.3) | 18.0 (0.5) | 5.5 (<0.1) | 9.8 (5.0) |
| Black | 0.5 (0.1) | 1.3 (0.3) | 4.4 (0.4) | 10.8 (0.5) | 21.8 (1.0) | 27.0 (1.3) | 35.5 (2.9) | 6.7 (0.2) | 10.9 (5.6) |
| Asian | 0.1 (<0.1) | 0.3 (0.2) | 1.4 (0.4) | 2.7 (0.7) | 4.2 (1.1) | 11.5 (2.6) | 16.2 (6.9) | 1.4 (0.2) | 3.4 (1.8) |
| Otherc | 1.2 (0.4) | 1.5 (0.6) | 4.2 (0.6) | 8.9 (0.8) | 19.9 (1.6) | 29.6 (3.3) | 27.1 (5.5) | 5.3 (0.3) | 8.4 (4.3) |
| Ethnicity | |||||||||
| Hispanic | 0.3 (0.1) | 0.3 (<0.1) | 1.7 (0.3) | 5.3 (0.6) | 12.0 (1.2) | 21.3 (2.2) | 21.2 (3.7) | 2.6 (0.1) | 5.0 (2.6) |
| Non-Hispanic | 0.7 (<0.1) | 1.3 (<0.1) | 3.5 (0.1) | 7.3 (0.1) | 14.6 (0.2) | 18.6 (0.3) | 18.8 (0.5) | 5.8 (<0.1) | 5.7 (2.9) |
| Income | |||||||||
| <$25,000 | 1.0 (0.1) | 3.3 (0.3) | 9.3 (0.4) | 16.4 (0.4) | 27.3 (0.5) | 28.8 (0.6) | 26.7 (0.9) | 12.1 (0.2) | 11.1 (5.7) |
| $25,000-<$75,000 | 0.6 (0.1) | 0.9 (0.1) | 2.8 (0.1) | 6.4 (0.2) | 10.9 (0.3) | 13.3 (0.4) | 12.0 (0.8) | 4.4 (<0.1) | 4.4 (2.2) |
| ≥$75,000 | 0.2 (<0.1) | 0.3 (<0.1) | 0.6 (<0.1) | 1.6 (0.1) | 3.3 (0.3) | 4.8 (0.5) | 6.9 (1.3) | 0.9 (<0.1) | 1.4 (0.7) |
| Education | |||||||||
| <High school | 1.3 (0.2) | 2.7 (0.4) | 10.6 (0.6) | 21.4 (0.8) | 34.5 (0.9) | 37.9 (1.0) | 37.3 (1.6) | 15.2 (0.3) | 13.8 (7.0) |
| High school | 0.9 (0.2) | 2.6 (0.2) | 5.2 (0.2) | 12.0 (0.3) | 19.4 (0.4) | 22.5 (0.5) | 22.9 (0.9) | 8.7 (0.1) | 7.9 (4.0) |
| >High school | 0.4 (<0.1) | 0.5 (<0.1) | 1.5 (<0.1) | 3.6 (0.1) | 7.5 (0.2) | 10.7 (0.3) | 11.0 (0.6) | 2.6 (<0.1) | 2.9 (1.5) |
Behavioral Risk Factor Surveillance System for the 50 states and the District of Columbia; n = 420,307 participants aged ≥25 yr.
Age-adjusted prevalence estimates of complete edentulism standardized to the year 2000 census population distribution.
“Other” race group comprises American Indians, Alaskan Natives, and multiracial groups.
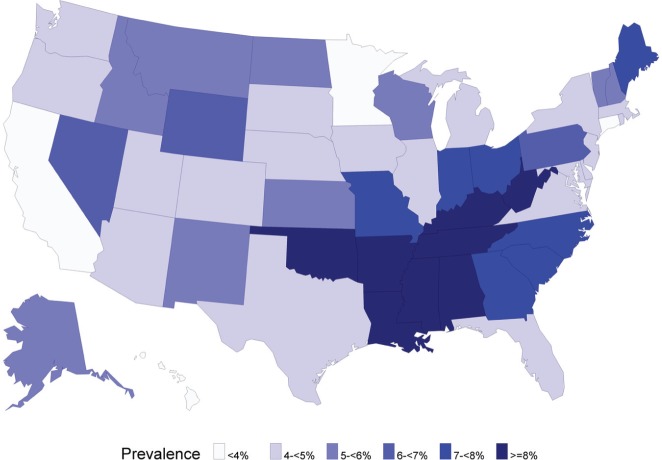
Conspicuous geographic concentration characterized edentulism prevalence across the nation in 2010 (Fig. 2). States with highest edentulism prevalence (≥8%) bordered the Appalachian mountains and Mississippi delta. By contrast, the lowest prevalence rates were widely scattered across Hawaii, California, Minnesota, and Connecticut. When aggregated to the U.S. Census Bureau’s 4 geographic regions, prevalence in the South (6.7%) was almost twice the level seen in the West (3.6%; Appendix Table 2). This differed conspicuously from the geographic pattern in the 1957-1958 survey, when prevalence varied only by a factor of 1.25, from 20.3% (Northeast) to 25.3% (North Central).
Figure 2:
Age-standardized edentulism prevalence among adults aged ≥25 yr, U.S. states, 2010.
Source: 2010 Behavioral Risk Factor Surveillance System for the 50 states and the District of Columbia; n = 420,307 participants aged ≥25 yr (Centers for Disease Control and Prevention, 2010a). Age-standardized prevalence in the District of Columbia was 3.0%.
Based on the second age cohort model (Table 3), the prevalence of edentulism in 2020 is predicted to reduce to 4.6% (95% prediction limits: 4.2%, 4.9%; Appendix Table 3). By 2050, prevalence is predicted to reach 2.6% (95% prediction limits: 2.1%, 3.1%). This net reduction of 2.3% predicted during the next 4 decades is only marginally greater than the net reduction of 1.6% observed in the single decade between the 2 most recent NHANES surveys (Table 1). Reduction in prevalence through 2050 will be partly offset by population growth and population aging, with the consequence that the predicted 8.6 million edentulous people in 2050 (Appendix Table 3) will be only 30% less than the estimated 12.2 million edentulous people observed in the 2009-2012 survey.
Discussion
During the half century spanning these surveys, prevalence of edentulism in U.S. adults declined from 18.9% to 4.9%. The initial rapid decline slowed conspicuously after the passing of cohorts born before the 1930s. The rate of decline thereafter was dictated by later birth cohorts who experienced successively lower age-related incidence of edentulism. However, in the 2 most recent birth cohorts of 1954-1963 and 1965-1973, incidence rates were equivalent at about 1% per decade. In these 5 decades, socioeconomic disparities became more pronounced such that edentulism is now virtually unknown in the highest quartile of the income distribution. Instead, edentulism is concentrated in low-income households located in states with a long history of poverty. With the aging of remaining birth cohorts from the 20th century, population prevalence is projected to decline only slowly in coming decades.
The validity of these findings is supported by each survey’s methodological rigor. Adults’ survey participation rates have been constant at approximately 70% since 1971-1974, and sampling weights adjust statistically for nonresponse. These 5 surveys offer a more comprehensive picture than that of previous studies of U.S. trends that used 3 of these surveys (Cunha-Cruz et al., 2007) or 2 (Weintraub and Burt, 1985; Brown, 1994; Beltran-Aguilar et al., 2005). Yet despite the prolonged time series, age cohort analyses were restricted to 24 data points. An apparent plateau in edentulism incidence seen around the age of 70 years in early birth cohorts was therefore not observable in cohorts born since the 1930s, which may have accounted for the statistically nonsignificant quadratic effect of age. Projections instead assumed that incidence would be linear through the life course and that it would be identical (0.94% per decade) in cohorts born after 1973. If edentulism incidence truly flattens in older age or if it declines further in new cohorts, these projections will overestimate edentulism. Conversely, these projections might underestimate prevalence if there are profound changes in dental disease or its management, such as a ban on mercury-amalgam fillings (Beazoglou et al., 2007).
The 74% relative reduction in edentulism prevalence was comparable with relative reductions reported in other countries, albeit during shorter intervals. Between 1968 and 2009, there was an 84% relative reduction in the United Kingdom (Steele et al., 2012), and during 2-decade intervals, relative reductions were 57% in Finland (Suominen-Taipale et al., 1999), 61% in Australia (Sanders et al., 2004), and 84% in Sweden (Osterberg et al., 2000). Our projection that prevalence will continue to decline, albeit more slowly than in past decades, is also consistent with projections for the United Kingdom (Steele et al., 2000). There is less evidence about trends in socioeconomic disparities in edentulism, despite impassioned calls for policies to narrow the oral health gap between rich and poor (Sheiham et al., 2011). The most thorough investigation to date used 3 NHANES surveys (1971-1974, 1988-1994, and 1999-2002) to describe absolute differences between low and high socioeconomic groups, with the authors concluding that socioeconomic disparities remained unchanged (Cunha-Cruz et al., 2007). Based on absolute trends, our own findings over 5 decades actually show a greater decline in prevalence within the low-income group (25.8%) than the high-income group (14.2%). However, we instead give emphasis to relative declines of 68% and 96%, respectively, to conclude that income disparities have widened. In doing so, we acknowledge that “determining whether an inequality is increasing or declining is a normative as well as a mathematical exercise” that depends on the choice of relative versus absolute measures (Harper et al., 2010). We justify emphasis on relative trends on the grounds that edentulism effectively was “cured” for high-income adults by 2009-2012. In practical terms, resources to further reduce prevalence in high-income groups would be wasted, representing a misdirected health policy that is unfair to low-income groups.
As the legacy of the edentulism epidemic recedes into history, we comment on its origin. In the late 19th century, dentistry was evolving from mechanical trade to profession. It was the period during which dental education became organized, state regulation was established, and the first dental journal—American Journal of Dental Science—emerged. The fledgling profession was receptive to scientific theory. So when Billings declared in 1912 that “ill-fitted crowns on teeth and much bridge-work may harbor septic infection in the mouth and produce systemic disease” (p. 486), focal infection theory was propelled to prominence. When treatment by dental extraction was advocated, it ushered in “a crusade of tooth extraction” (Billings, 1930, p. 773) lasting 3 to 4 decades. By the 1940s, realization that tooth extraction had no impact on diseases attributed to oral sepsis led to the theory being widely discredited and to the cessation of wholesale extraction of teeth by the 1950s (Easlick, 1951). Although focal infection theory was a major determinant of the epidemic, it was not the sole factor. High rates of dental caries in the absence of community water fluoridation programs or preventive philosophies contributed. Indeed, the normality of complete tooth loss ensured its freedom from social stigma. Yet, unlike that in the United Kingdom, a shortage of dentists was not a contributing factor. At the height of the U.S. edentulism epidemic in 1930, the dentist:population ratio of 1:1,732 was the greatest supply of dentists in the nation’s history (Burt, 1978; Mertz and O’Neil, 2002).
Edentulism has contracted geographically to states near the Appalachian Mountains and Mississippi Delta, consistent with findings for trends in tooth loss (Gorsuch et al., 2014). The likely reason for this spatial concentration is intractable rural poverty, endemic since the Great Depression, when mechanization of agriculture reduced demand for tenant labor. Displaced tenants migrated northward to employment opportunities offered by urban industrialization (Fligstein, 1983). By 1965, 1 in 3 Appalachians lived below the poverty threshold. Structural economic changes in the 1980s fueled negative population growth and exacerbated the loss of jobs in farming, forestry, and manufacturing. Today unemployment rates are higher than the national average.
These trends affect the provision of dental care because tooth retention is such a strong predictor of dental attendance (Macek et al., 2004). The projected slow decline in number of edentulous people through 2050 refutes a premise of a consensus statement (Feine et al., 2002) on implant-retained overdentures asserting that the number will increase. These considerations have flow-on effects for dental education. One report (Waldman et al., 2007) provocatively titled “Should the Teaching of Full Denture Prosthetics Be Maintained in Schools of Dentistry?” was equivocal in answering the question. We hope the current findings reduce some of the uncertainty.
Supplementary Material
Footnotes
A supplemental appendix to this article is published electronically only at http://jdr.sagepub.com/supplemental.
A. A. Akinkugbe was supported by National Institutes of Health NRSA T90 Training Grant NIH/National Institute of Dental and Craniofacial Research (NIDCR) 5T90DE021986-03.
The authors declare no potential conflicts of interest with respect to the authorship and/or publication of this article.
References
- Beazoglou T, Eklund S, Heffley D, Meiers J, Brown LJ, Bailit H. (2007). Economic impact of regulating the use of amalgam restorations. Public Health Rep 122:657-663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beltran-Aguilar ED, Barker LK, Canto MT, Dye BA, Gooch BF, Griffin SO, et al. (2005). Surveillance for dental caries, dental sealants, tooth retention, edentulism, and enamel fluorosis—United States, 1988-1994 and 1999-2002. MMWR Surveill Summ 54:1-43. [PubMed] [Google Scholar]
- Billings F. (1912). Chronic focal infections and their etiologic relations to arthritis and nephritis. Arch Intern Med (Chic) 9:484-498. [Google Scholar]
- Billings F. (1930). Focal infection as the cause of general disease. Bull N Y Acad Med 6:759-773. [PMC free article] [PubMed] [Google Scholar]
- Brown LJ. (1994). Trends in tooth loss among U.S. employed adults from 1971 to 1985. J Am Dent Assoc 125:533-540. [DOI] [PubMed] [Google Scholar]
- Burt BA. (1978). Influences for change in the dental health status of populations: an historical perspective. J Public Health Dent 38:272-288. [DOI] [PubMed] [Google Scholar]
- Centers for Disease Control and Prevention (2000). National Health and Nutrition Examination Survey Data, 1999-2000. URL accessed on 7/15/2014 at: http://wwwn.cdc.gov/nchs/nhanes/search/nhanes99_00.aspx.
- Centers for Disease Control and Prevention (2002). National Health and Nutrition Examination Survey Data, 2001-2002. URL accessed on 7/15/2014 at: http://wwwn.cdc.gov/nchs/nhanes/search/nhanes01_02.aspx.
- Centers for Disease Control and Prevention (2010a). Behavioral Risk Factor Surveillance System. URL accessed on 7/15/2014 at: http://www.cdc.gov/brfss/annual_data/annual_2010.htm.
- Centers for Disease Control and Prevention (2010b). National Health and Nutrition Examination Survey Data, 2009-2010. URL accessed on 7/15/2014 at: http://wwwn.cdc.gov/nchs/nhanes/search/nhanes09_10.aspx.
- Centers for Disease Control and Prevention (2012). National Health and Nutrition Examination Survey Data, 2011-2012. URL accessed on 7/15/2014 at: http://wwwn.cdc.gov/nchs/nhanes/search/nhanes11_12.aspx.
- Clayton D, Schifflers E. (1987). Models for temporal variation in cancer rates: I. Age-period and age-cohort models. Stat Med 6:449-467. [DOI] [PubMed] [Google Scholar]
- Crocombe LA, Slade GD. (2007). Decline of the edentulism epidemic in Australia. Aust Dent J 52:154-156. [DOI] [PubMed] [Google Scholar]
- Cunha-Cruz J, Hujoel PP, Nadanovsky P. (2007). Secular trends in socio-economic disparities in edentulism: USA, 1972-2001. J Dent Res 86:131-136. [DOI] [PubMed] [Google Scholar]
- Easlick KA. (1951). An evaluation of the effect of dental foci of infection on health. J Am Dent Assoc 42:615-697. [PubMed] [Google Scholar]
- Emami E, de Souza RF, Kabawat M, Feine JS. (2013). The impact of edentulism on oral and general health. Int J Dent 2013:498305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feine JS, Carlsson GE, Awad MA, Chehade A, Duncan WJ, Gizani S, et al. (2002). The McGill consensus statement on overdentures: mandibular two-implant overdentures as first choice standard of care for edentulous patients. Montreal, Quebec, May 24-25, 2002. Int J Oral Maxillofac Implants 17:601-602. [PubMed] [Google Scholar]
- Fligstein N. (1983). The transformation of southern agriculture and the migration of blacks and whites, 1930-1940. Int Migr Rev 17:268-290. [PubMed] [Google Scholar]
- Gorsuch MM, Sanders SG, Wu B. (2014). Tooth loss in Appalachia and the Mississippi delta relative to other regions in the United States, 1999-2010. Am J Public Health 104:e85-e91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harper S, King NB, Meersman SC, Reichman ME, Breen N, Lynch J. (2010). Implicit value judgments in the measurement of health inequalities. Milbank Q 88:4-29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hobdell M, Petersen PE, Clarkson J, Johnson N. (2003). Global goals for oral health 2020. Int Dent J 53:285-288. [DOI] [PubMed] [Google Scholar]
- Macek MD, Cohen LA, Reid BC, Manski RJ. (2004). Dental visits among older U.S. adults, 1999: the roles of dentition status and cost. J Am Dent Assoc 135:1154-1162; quiz, 1165. [DOI] [PubMed] [Google Scholar]
- MacMahon B, Pugh TF, Ipsen J. (1960). Epidemiologic Methods. Boston, MA: Little Brown and Co. [Google Scholar]
- Mertz E, O’Neil E. (2002). The growing challenge of providing oral health care services to all Americans. Health Aff (Millwood) 21:65-77. [DOI] [PubMed] [Google Scholar]
- Mojon P, Thomason JM, Walls AW. (2004). The impact of falling rates of edentulism. Int J Prosthodont 17:434-440. [PubMed] [Google Scholar]
- Osterberg T, Carlsson GE, Sundh V. (2000). Trends and prognoses of dental status in the Swedish population: analysis based on interviews in 1975 to 1997 by Statistics Sweden. Acta Odontol Scand 58:177-182. [DOI] [PubMed] [Google Scholar]
- Polzer I, Schwahn C, Volzke H, Mundt T, Biffar R. (2012). The association of tooth loss with all-cause and circulatory mortality. Is there a benefit of replaced teeth? A systematic review and meta-analysis. Clin Oral Investig 16:333-351. [DOI] [PubMed] [Google Scholar]
- Rothman KJ, Greenland S, Lash TL. (2008). Modern Epidemiology. Philadelphia, PA: Wolters Kluwer Health / Lippincott Williams & Wilkins, pp. 364-372. URL accessed on 7/15/2014 at: http://www.loc.gov/catdir/enhancements/fy0828/2007036316-t.html. [Google Scholar]
- Sanders AE, Slade GD, Carter KD, Stewart JF. (2004). Trends in prevalence of complete tooth loss among Australians, 1979-2002. Aust N Z J Public Health 28:549-554. [DOI] [PubMed] [Google Scholar]
- Sheiham A, Alexander D, Cohen L, Marinho V, Moyses S, Petersen PE, et al. (2011). Global oral health inequalities: task group—implementation and delivery of oral health strategies. Adv Dent Res 23:259-267. [DOI] [PubMed] [Google Scholar]
- Steele JG, Treasure E, Pitts NB, Morris J, Bradnock G. (2000). Total tooth loss in the United Kingdom in 1998 and implications for the future. Br Dent J 189:598-603. [DOI] [PubMed] [Google Scholar]
- Steele JG, Treasure ET, O’Sullivan I, Morris J, Murray JJ. (2012). Adult Dental Health Survey 2009: transformations in British oral health 1968-2009. Br Dent J 213:523-527. [DOI] [PubMed] [Google Scholar]
- Suominen-Taipale AL, Alanen P, Helenius H, Nordblad A, Uutela A. (1999). Edentulism among Finnish adults of working age, 1978-1997. Community Dent Oral Epidemiol 27:353-365. [DOI] [PubMed] [Google Scholar]
- U.S. Census Bureau Population Division (2012). Projections of the population by age and sex for the United States: 2015 to 2060 (NP2012-T12). URL accessed on 7/15/2014 at: http://www.census.gov/population/projections/data/national/2012/summarytables.html.
- U.S. Department of Health and Health Education and Welfare (1960). Loss of teeth. United States July 1957–June 1958. Public Health Service publication No. 584-B22. URL accessed on 7/15/2014 at: http://www.cdc.gov/nchs/data/public_health/SeriesB_22.pdf.
- U.S. Department of Health and Human Services (1992). Public use data tape documentation, dental, ages 1-74. Tape number 4235. National Health and Nutrition Examination Survey, 1971-75. URL accessed on 7/15/2014 at: http://www.cdc.gov/nchs/data/nhanes/nhanesi/4235.pdf.
- U.S. Department of Health and Human Services (1996). Third National Health and Nutrition Examination Survey (NHANES III), 1988-94. NHANES III examination data file documentation catalog number 76200. URL accessed on 7/15/2014 at: ftp://ftp.cdc.gov/pub/Health_Statistics/NCHS/nhanes/nhanes3/1A/exam.dat.
- U.S. Department of Health and Human Services, Office of Disease Prevention and Health Promotion (2013). Healthy People 2020. URL accessed on 7/15/2014 at: http://healthypeople.gov/2020/topicsobjectives2020/objectiveslist.aspx?topicId=32.
- Waldman HB, Perlman SP, Xu L. (2007). Should the teaching of full denture prosthetics be maintained in schools of dentistry? J Dent Educ 71:463-466. [PubMed] [Google Scholar]
- Weintraub JA, Burt BA. (1985). Oral health status in the United States: tooth loss and edentulism. J Dent Educ 49:368-378. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.