Abstract
Despite the concerted efforts of research and professional and advocacy stakeholders, recent evidence suggests that improvements in the oral health of young children in the United States has not followed the prevailing trend of oral health improvement in other age groups. In fact, oral health disparities in the youngest children may be widening, yet efforts to translate advances in science and technology into meaningful improvements in populations’ health have had limited success. Nevertheless, the great strides in genomics, biological, behavioral, social, and health services research in the past decade have strengthened the evidence base available to support initiatives and translational efforts. Concerted actions to accelerate this translation and implementation process are warranted; at the same time, policies that can help tackle the upstream determinants of oral health disparities are imperative. This article summarizes the proceedings from the symposium on the interdisciplinary continuum of pediatric oral health that was held during the 43rd annual meeting of the American Association for Dental Research, Charlotte, North Carolina, USA. This report showcases the latest contributions across the interdisciplinary continuum of pediatric oral health research and provides insights into future research priorities and necessary intersectoral synergies. Issues are discussed as related to the overwhelming dominance of social determinants on oral disease and the difficulty of translating science into action.
Keywords: research agenda, health disparities, pediatric dentistry, dental research, health policy, translational research
Introduction
Despite the concerted efforts of research and professional and advocacy stakeholders, recent evidence suggests that improvements in the oral health of young children in the United States has not followed the prevailing trend of oral health improvement in other age groups (Tomar and Reeves, 2009). In fact, oral health disparities in the youngest children may be widening. Arguably, the translation of advances in science and technology into meaningful improvements in the population’s health has fallen short of goals articulated in Healthy People 2020 and the U.S. Surgeon General’s report on oral health in America (Milgrom et al., 2009). The accelerating rate of emerging “new science” (Iacopino, 2007) relevant to oral health is expected to continue, rendering this translation gap a critical issue.
Conceptual models describing influences on children’s oral health are complex and include a constellation of proximal and distal determinants that range from genetic and biological to cultural, behavioral, social, and administrative (Patrick et al., 2006; Fisher-Owens et al., 2007; Chi, 2013; Lee and Divaris, 2014). High-impact research is being conducted at all these levels. However, it is unclear if and how disciplines inform and synergize with one another, and strategies are needed to streamline the translation of evidence to policy and practice. To this end, a symposium titled “An Interdisciplinary Framework for Pediatric Oral Health Research” was organized within the context of the 43rd annual meeting of the American Association for Dental Research, Charlotte, North Carolina, USA. The goal of the symposium was to summarize the latest scientific advances across the broad spectrum of pediatric oral health and outline an interdisciplinary research framework providing insights into priority areas for future basic and translational research. Here we present synopses of the panelists’ talks and a summary of discussions and issues that emerged from the symposium. The 4 presentations with synchronized audio recordings are available free of charge for International Association for Dental Research members at the association’s Knowledge Community (http://www.iadr.org/i4a/pages/index.cfm?pageid=3951).
Advances in Genetics and Pediatric Oral Health Applications
The successful completion of the Human Genome Project (Sachidanandam et al., 2001; Venter et al., 2001) in 2000 led to the development of new tools for human genetic studies. These tools have been widely applied to many human traits of complex etiology, including orofacial clefts (OFC) and dental caries, which are both of interest for pediatric oral health. One particular tool—genome-wide association (GWA)—saw an explosion of studies starting in 2005, with studies of OFC first published in 2010 and caries in 2011. GWA studies of OFCs have been remarkably successful compared with other human complex traits, with about 13 genes/regions showing statistically significant association. Five of those (ARHGAP29, IRF6, 8q24, VAX1, and NOG) have been extensively replicated (Leslie and Marazita, 2013) and are estimated to account for about 55% of OFC cases (Marazita, 2012). Notably, it is also known that coding variants in IRF6 and NOG lead to syndromes but that other variants, primarily in regulatory regions, are associated with nonsyndromic forms of OFC.
GWA studies are only a first step, as they represent population-level statistical results but cannot reveal which genes/loci are etiologic in any particular individual or family. To begin to approach individual-level genetic prediction, detailed studies of phenotypes are necessary, both to refine disease definition and to assess additional measures in affected individuals and their relatives. For example, the University of Pittsburgh OFC study has recruited a large number of families worldwide and has assessed a comprehensive battery of phenotypes that, though within normal ranges, are increased in the unaffected relatives of individuals with OFC as compared with controls (e.g., discontinuities in the orbicularis oris muscle segregate in OFC families and are associated with BMP4).
Dental caries is the most common disease of childhood and is due to a combination of genetic and environmental/behavioral factors (Selwitz et al., 2007). Early evidence of a genetic component comes from animal models and human studies, with particularly strong evidence from studies of twins reared apart. The caries phenotype is traditionally expressed as the number of decayed, missing, or filled surfaces or teeth in the mouth. The Marazita group has undertaken GWA studies of this traditional caries definition starting in 2011 (Shaffer et al., 2011), but more recent studies have revolved around refinement of the caries phenotype (Zeng et al., 2014). Hierarchical clustering was used to define clusters of tooth surfaces with similar caries experience in subjects from the Center for Oral Health Research in Appalachia, with replication in subjects of the National Health and Nutrition Examination Survey (Shaffer et al., 2013a). Five clusters were defined in the center’s subjects: 3 with significant evidence of heritability (anterior mandibular, posterior nonpit/fissure, middentition) and 2 with no significance of evidence of heritability (pit/fissure, maxillary incisors). The same clusters were independently identified in subjects of the National Health and Nutrition Examination Survey. GWA studies were done with each cluster—statistically significant association was seen in the clusters with significant heritability—for example, association with LYZL2 (a bacteriolytic factor involved in host defense) for the anterior mandibular cluster yet no association in the clusters with little evidence of heritability (Shaffer et al., 2013b).
In summary, the tools of the Human Genome Project combined with detailed phenotype studies are being applied to disorders of pediatric oral health impact. The results from combining these complementary types of studies hold great promise in bringing etiologic clarity to OFCs and caries, thereby leading to improved translational avenues.
Improving Children’s Oral Health via Behavioral and Social Science Research
Achieving meaningful population improvements in oral health is a complex process that requires scientifically rigorous and well-executed research plans through behavioral and social science. The development of conceptual models is the basis for behavioral intervention and for much of social science research. Investigators use such models to guide their inquiries. For example, a recent model introduced by Lee and Divaris (2014) illustrates a framework for addressing oral health disparities. This framework includes macroenvironment, population/community, and personal factors that affect health outcomes. Each of these domains can influence health disparities, which are “differences in health outcomes and their determinants between segments of the population, as defined by social, demographic, environmental, and geographic attributes,” according to the Centers for Disease Control and Prevention. At the core of reducing health disparities should be actions and policies informed by well-designed behavioral and social science research.
Health literacy has recently emerged as an important determinant of oral health outcomes and health disparities. Healthy People 2010 defines health literacy as “the degree to which individuals have the capacity to obtain, process, understand and act on basic health information and services needed to make appropriate health decisions.” The Carolina Oral Health Literacy study provided the first population-based data on oral health literacy and its correlates, indicating that oral health literacy disparities among racial groups may persist after controlling for education and sociodemographic characteristics (Lee et al., 2011). This line of research also found oral health literacy associations with oral health status, oral health-related behaviors, knowledge, and quality of life. Importantly, it highlighted that low health literacy among caregivers was associated with children having worse oral health status, deleterious oral health behaviors (e.g., no daily brushing/cleaning and nighttime bottle use), suboptimal use of dental services, and interruptions in children’s public insurance coverage (Vann et al., 2010; Lee et al., 2012; Divaris et al., 2014). Self-efficacy and dental neglect are 2 behavioral constructs that have emerged as important mediators between literacy and oral health outcomes (Lee et al., 2012). There exist several behavioral pathways by which distal determinants—including individuals’ interface with the health system—may affect children’s oral health (Gao et al., 2010), and health literacy is part of several of these important pathways.
The current state of knowledge has set the stage for interventions to address or circumvent the negative sequelae of behavioral determinants and other barriers, including low oral health literacy, and consequently help reduce health disparities. While several behaviors are desirable (e.g., twice-daily tooth brushing with fluoridated dentifrice, control of the amount and frequency of dietary fermentable carbohydrates, and early preventive oral health-related visits), there is no consensus with regard to the “most promising” modifiable behavioral targets. Future research should address this knowledge gap and test interventions that may address modifiable factors or circumvent nonmodifiable ones, including socioeconomic or cultural barriers. These could include social cognitive approaches, such as tailoring and targeting oral health promotion messages to vulnerable parts of the population. Successful interventions should be acceptable, culturally and linguistically sensitive, and easily adopted, and they should have good fidelity. For these interventions to be scaled up and become sustainable, it is essential that they be accompanied with enabling policy and system changes.
The Intersection of Science and Policy
Definitions of health policy tend to be dispassionate and factual; they frequently fall short from dealing with vulnerability, temporality, and compassion because they come with no real sense of philosophy or attention to the human condition. This dimension is critical and particularly relevant to children’s oral health, where multiple layers of vulnerability may be at play. Arguably, to be effective over a reasonable time frame, policy should include both a science foundation and a clear orientation toward “doing what is right for children.” It also seems appropriate that policy should aim to achieve meaningful endpoints—for example, setting a goal of 100% of children having a dental home versus reducing the percentage of four-year-olds with early childhood caries from 45% to 35% or some partial success as articulated in Healthy People goals. Moreover, it seems appropriate to argue for the importance of oral health issues relative to other health issues. The Table presents a summary of the desired properties of policy to be effective in improving children’s oral health.
Table.
Desired Properties of Policy to be Effective in Improving Children’s Oral Health
| Recognize the overwhelming dominance of social determinants of health |
| Explicitly acknowledge vulnerability |
| Entail compassion for those in need |
| Be guided by social justice and human rights principles |
| Consider temporality of problems, priorities, and goals |
| Include meaningful and measurable endpoints |
| Validate the importance of oral health relative to other health issues |
| Synergize with science avoiding the conjuring, overinterpreting, or misinterpreting of findings |
Arguably, we have a long way to go in determining where our scientific dollar should be invested. The Fisher-Owens model (Fisher-Owens et al., 2007) speaks to the often overwhelming dominance of social determinants on dental caries, yet we do not invest enough in using these relationships to effect change. They too often remain sociological curiosities that the scientific community has difficulty translating into action. This is where policy can be effective. The American Academy of Pediatric Dentistry Policy Center brings together several elements of the academy, including councils involved with scientific issues, advocacy, clinical services, and evidence-based care; it works closely with the American Dental Association on child oral health; and it conducts research into policy and scientific issues. One of the first policy center briefs dealt with case management. Until a realistic biological track to a cure is discovered and vetted, this remains the best avenue to stopping dental disease. Even in the face of a biological cure, addressing health literacy and ethos would be required to curb dental disease.
A host of oral health issues—some based in science, some not—percolate within the discourse on pediatric oral health. Some examples of the establishment’s use of science to influence policy are the following: workforce needs, the health care system’s capacity, and the Health Resources and Services Administration’s Health Professional Shortage Area metric. These have dominated the access to care issue for almost 50 yr; how relevant are they today? We probably should direct our concern elsewhere. How much hope have we placed in the Affordable Care Act to eliminate disparities? Key obstacles to access in the act persist. Another popular discussion revolves around the “holy grails” of sensitivity and specificity for caries risk assessment and its meaningful use. It is likely that all these issues will continue to elude us. It appears that we are still in a period of siloing singular solutions to solve pediatric oral health care issues. Another example is the model of physician-delivered oral health services, which is now closing in on its second decade of funding and emphasis, largely to no avail: fluoride varnish, referral to a dentist, and oral health education have largely been ineffective as a national strategy, although marginally effective in some places.
We have entered challenging territory in policy related to pediatric oral health. The dangerous ground is the agendizing of science. Too often, we see a search for a simple solution to the complex problems around pediatric oral health. Evidence-based dentistry presents a double-edged sword when it comes to science and its interface with policy. Few could argue with the ultimate goals of evidence-based dentistry, but it will remain a third leg—and a shorter one at that—in the care of children. An intersection between science and policy is obvious and important (Figure), and a number of important roles exist for legitimate policy makers in the area of pediatric oral health. The first is to cull science for accuracy. The second is to guide or direct scientific endeavor. The satellite view of funding agencies often differs dramatically from that of boots on the ground. Third is promotion of good science over bad. Finally, policy may be needed to clarify and explain scientific findings.
Figure.
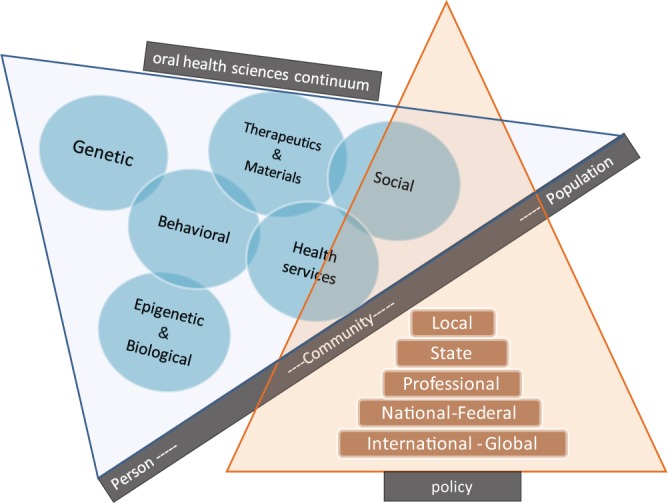
Conceptual illustration of the intersection between science and policy in pediatric oral health. The science base underlying children’s oral health covers a wide spectrum, ranging from genetic/genomics research and behavioral sciences to dental materials, health services, and social sciences. It is underscored that social determinants of health and other upstream factors are dominant influences on populations’ health. Policies that are informed by science but also “sensitive to human condition” have the potential to affect these upstream determinants and lead to meaningful improvements in children’s oral health.
Translation of Advances in Science and Technology for the Prevention of Dental Caries in Children
The ultimate endpoint of efforts in translating research into practice should be to fully address oral health disparities and achieve Healthy People 2020 goals for all parts of our society. Two sample studies were presented from the Northwest Center to Reduce Oral Health Disparities at the University of Washington:
Baby Smiles: a randomized community-based trial of motivational interviewing to promote maternal and age 1 dental visits among Medicaid recipients (Milgrom et al., 2013)
Everybody Brush: a randomized controlled trial of free distribution of fluoride toothpaste and toothbrushes, plus telephone and mail reminders with a focus on increasing use of fluoride toothpaste among children <3 yr old.
Baby Smiles used counselors from Oregon-based Women, Infant, and Children centers and health departments, who also functioned as patient navigators to promote prenatal dental utilization by mothers enrolled in Medicaid and age 1 visits for their children. The goal on maternal visits was based on literature demonstrating the effect of basic dental care on reducing the transmission of Mutans streptococci from mother to child and subsequent dental caries in the child (Köhler and Andréen, 1994) and evidence that mothers who have a usual source of dental care are more likely to take their children to the dentist (Grembowski et al., 2008). Preliminary results were encouraging and showed high rates of maternal utilization and caries reductions among their children (Milgrom et al., 2008, Milgrom et al., 2010): >90% of participating mothers had perinatal visits and >50% of their children had an age 1 dental visit. This study was unique because of its community partnership and extensive participation by dental managed care organizations.
Another example of a recently initiated intervention based in a managed dental care organization is the Everybody Brush study in central Oregon, involving more than 11,000 families. Its science base relies on robust evidence for the effectiveness of fluoride toothpaste (Marinho et al., 2003) and 2 studies from the United Kingdom demonstrating that free home distribution of toothpaste resulted in reductions in childhood caries (Davies et al., 2002; Davies et al., 2005). Study outcomes include self-reported tooth brushing, use of toothpaste, as well as administrative data and clinically determined caries levels. Another unique feature of the study is its funding source, because it is primarily supported by a managed care firm and a small award from the government.
In terms of next steps, this translational work is being well documented and widely disseminated to enable implementation in other settings. Future efforts will involve translational research of other caries management modalities with a strong science base: diamine silver fluoride and varnishes that contain both fluoride and iodine (Milgrom et al., 2009).
Summary
As illustrated in the Figure, the science base underlying pediatric oral health covers a wide spectrum, ranging from genetic/genomics research and behavioral sciences to dental materials, health services, and social sciences. The social determinants of health and other upstream factors are dominant influences on populations’ health. Policies that are informed by science and are also “sensitive to human condition” have the potential to affect these upstream determinants and lead to meaningful improvements in children’s oral health.
Ongoing activities and advances in genetics; biological, material, and behavioral sciences; and translational research have laid the groundwork for reducing the burden of oral disease. Evidently, a remarkable amount of high-impact research relevant to pediatric oral health is being conducted, from the nanolevel to the macrolevel. Advances in genome sciences are exponential and have a relatively straightforward impact on mechanistic discovery and personalized health care; translation of these advances to population health benefits is envisaged (Collins et al., 2003), but progress to this end has been slow (Burke et al., 2010). Similarly, an expanding evidence base exists for a number of chemotherapeutic, behavioral, and workforce-related oral health interventions, but translational applications so far have been limited. Oral health policy needs to be informed by science but must also be pragmatic and entail a social justice and humanistic safety net. To be most effective, policy and translational efforts should tackle upstream, disparities-creating determinants; to be sustainable, these actions must be community oriented, as well as culturally and linguistically appropriate.
Optimal use of the available and emerging knowledge base to bring about population-based equitable oral health improvements will require concerted efforts of multiple parties—academic, professional, and administrative. To be effective, it is imperative that these efforts not only be revitalized via a new wave of synergy involving dental education, funding institutions, and advocacy stakeholders but also reach out to sectors outside dentistry.
Acknowledgments
The authors acknowledge the contribution of Dr. William V. Giannobile, Najjar Professor of Dentistry and Biomedical Engineering and Chair of the Department of Periodontics and Oral Medicine at the School of Dentistry, University of Michigan, for serving as the moderator of the symposium.
Footnotes
This work was supported by the following National Institute of Dental and Craniofacial Research grants: R01-DE018045 (JYL), R01-DE016148 (MLM), R01-DE014899 (MLM), 1U54-DE0 19346 (PM), and K08-DE020856 (DLC).
The authors declare no potential conflicts of interest with respect to the authorship and/or publication of this article.
References
- Burke W, Burton H, Hall AE, Karmali M, Khoury MJ, Knoppers B, et al. ; Ickworth Group (2010). Extending the reach of public health genomics: what should be the agenda for public health in an era of genome-based and “personalized” medicine? Genet Med 12:785-791. [DOI] [PubMed] [Google Scholar]
- Chi DL. (2013). Reducing Alaska Native paediatric oral health disparities: a systematic review of oral health interventions and a case study on multilevel strategies to reduce sugar-sweetened beverage intake. Int J Circumpolar Health 72:21066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collins FS, Green ED, Guttmacher AE, Guyer MS; US National Human Genome Research Institute (2003). A vision for the future of genomics research. Nature 422:835-847. [DOI] [PubMed] [Google Scholar]
- Davies GM, Worthington HV, Ellwood RP, Bentley EM, Blinkhorn AS, Taylor GO, et al. (2002). A randomised controlled trial of the effectiveness of providing free fluoride toothpaste from the age of 12 months on reducing caries in 5-6 year old children. Community Dent Health 19:131-136. [PubMed] [Google Scholar]
- Davies GM, Duxbury JT, Boothman NJ, Davies RM, Blinkhorn AS. (2005). A staged intervention dental health promotion programme to reduce early childhood caries. Community Dent Health 22:118-122. [PubMed] [Google Scholar]
- Divaris K, Lee JY, Baker AD, Gizlice Z, Rozier RG, Dewalt DA, et al. (2014). Influence of caregivers and children’s entry into the dental care system. Pediatrics 133:e1268-e1276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fisher-Owens SA, Gansky SA, Platt LJ, Weintraub JA, Soobader MJ, Bramlett MD, et al. (2007). Influences on children’s oral health: a conceptual model. Pediatrics 120:e510-e520. [DOI] [PubMed] [Google Scholar]
- Gao XL, Hsu CY, Xu YC, Loh T, Koh D, Hwarng HB. (2010). Behavioral pathways explaining oral health disparity in children. J Dent Res 89:985-990. [DOI] [PubMed] [Google Scholar]
- Grembowski D, Spiekerman C, Milgrom P. (2008). Linking mother and child access to dental care. Pediatrics 122:e805-814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iacopino AM. (2007). The influence of “new science” on dental education: current concepts, trends, and models for the future. J Dent Educ 71:450-462. [PubMed] [Google Scholar]
- Köhler B, Andréen I. (1994). Influence of caries-preventive measures in mothers on cariogenic bacteria and caries experience in their children. Arch Oral Biol 39:907-911. [DOI] [PubMed] [Google Scholar]
- Lee JY, Divaris K. (2014). The ethical imperative of addressing oral health disparities: a unifying framework. J Dent Res 93:224-230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee JY, Divaris K, Baker AD, Rozier RG, Lee SY, Vann WF., Jr (2011). Oral health literacy levels among a low-income WIC population. J Public Health Dent 71:152-160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee JY, Divaris K, Baker AD, Rozier RG, Vann WF., Jr (2012). The relationship of oral health literacy and self-efficacy with oral health status and dental neglect. Am J Public Health 102:923-929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leslie EJ, Marazita ML. (2013). Genetics of cleft lip and cleft palate. Am J Med Genet C Semin Med Genet 163C:246-258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marazita ML. (2012). The evolution of human genetic studies of cleft lip and cleft palate. Annu Rev Genomics Hum Genet 13:263-283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marinho VC, Higgins JP, Sheiham A, Logan S. (2003). Fluoride toothpastes for preventing dental caries in children and adolescents. Cochrane Database Syst Rev 1:CD002278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milgrom P, Ludwig S, Shirtcliff RM, Smolen D, Sutherland M, Gates PA, et al. (2008). Providing a dental home for pregnant women: a community program to address dental care access: a brief communication. J Public Health Dent 68:170-173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milgrom P, Zero DT, Tanzer JM. (2009). An examination of the advances in science and technology of prevention of tooth decay in young children since the Surgeon General’s Report on Oral Health. Acad Pediatr 9:404-409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milgrom P, Sutherland M, Shirtcliff RM, Ludwig S, Smolen D. (2010). Children’s tooth decay in a public health program to encourage low-income pregnant women to utilize dental care. BMC Public Health 10:76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milgrom P, Riedy CA, Weinstein P, Mancl LA, Garson G, Huebner CE, et al. (2013). Design of a community-based intergenerational oral health study: “Baby Smiles.” BMC Oral Health 13:38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patrick DL, Lee RS, Nucci M, Grembowski D, Jolles CZ, Milgrom P. (2006). Reducing oral health disparities: a focus on social and cultural determinants. BMC Oral Health 6(Suppl 1):S4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sachidanandam R, Weissman D, Schmidt SC, Kakol JM, Stein LD, Marth G, et al. (2001). A map of human genome sequence variation containing 1.42 million single nucleotide polymorphisms. Nature 409:928-933. [DOI] [PubMed] [Google Scholar]
- Selwitz RH, Ismail AI, Pitts NB. (2007). Dental caries. Lancet 369:51-59. [DOI] [PubMed] [Google Scholar]
- Shaffer JR, Wang X, Feingold E, Lee M, Begum F, Weeks DE, et al. (2011). Genome-wide association scan for childhood caries implicates novel genes. J Dent Res 90:1457-1462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaffer JR, Feingold E, Wang X, Weeks DE, Weyant RJ, Crout R, et al. (2013a). Clustering tooth surfaces into biologically informative caries outcomes. J Dent Res 92:32-37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaffer JR, Feingold E, Wang X, Lee M, Tcuenco K, Weeks DE, et al. (2013b). GWAS of dental caries patterns in the permanent dentition. J Dent Res 92:38-44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomar SL, Reeves AF. (2009). Changes in the oral health of US children and adolescents and dental public health infrastructure since the release of the Healthy People 2010 Objectives. Acad Pediatr 2009. 9:388-395. [DOI] [PubMed] [Google Scholar]
- Vann WF, Jr, Lee JY, Baker D, Divaris K. (2010). Oral health literacy among female caregivers: impact on oral health outcomes in early childhood. J Dent Res 89:1395-1400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Venter JC, Adams MD, Myers EW, Li PW, Mural RJ, Sutton GG, et al. (2001). The sequence of the human genome. Science 291:1304-1351. (Published erratum in: Science 2001 292:1838). [DOI] [PubMed] [Google Scholar]
- Zeng Z, Feingold E, Wang X, Weeks DE, Lee M, Cuenco DT, et al. (2014). Genome-wide association study of primary dentition pit-and-fissure and smooth surface caries. Caries Res 48:330-338. [DOI] [PMC free article] [PubMed] [Google Scholar]


