Abstract
The dental basement membrane (BM) is composed of collagen types IV, VI, VII, and XVII, fibronectin, and laminin and plays an inductive role in epithelial-mesenchymal interactions during tooth development. The BM is degraded and removed during later-stage tooth morphogenesis; however, its original position defines the location of the dentin-enamel junction (DEJ) in mature teeth. We recently demonstrated that type VII collagen is a novel component of the inner enamel organic matrix layer contiguous with the DEJ. Since it is frequently co-expressed with and forms functional complexes with type VII collagen, we hypothesized that type IV collagen should also be localized to the DEJ in mature human teeth. To identify collagen IV, we first evaluated defect-free erupted teeth from various donors. To investigate a possible stabilizing role, we also evaluated extracted teeth exposed to high-dose radiotherapy – teeth that manifest post-radiotherapy DEJ instability. We now show that type IV collagen is a component within the morphological DEJ of posterior and anterior teeth from individuals aged 18 to 80 yr. Confocal microscopy revealed that immunostained type IV collagen was restricted to the 5- to 10-µm-wide optical DEJ, while collagenase treatment or previous in vivo tooth-level exposure to > 60 Gray irradiation severely reduced immunoreactivity. This assignment was confirmed by Western blotting with whole-tooth crown and enamel extracts. Without reduction, type IV collagen contained macromolecular α-chains of 225 and 250 kDa. Compositionally, our results identify type IV collagen as the first macromolecular biomarker of the morphological DEJ of mature teeth. Given its network structure and propensity to stabilize the dermal-epidermal junction, we propose that a collagen-IV-enriched DEJ may, in part, explain its well-known fracture toughness, crack propagation resistance, and stability. In contrast, loss of type IV collagen may represent a biochemical rationale for the DEJ instability observed following oral cancer radiotherapy.
Keywords: enamel organic matrix, dentin-enamel junction, confocal immunofluorescent microscopy, radiation effects, basement membrane, oral cancer
Introduction
Teeth develop through inductive and reciprocal interactions mediated through the dental basement membrane (BM), which interfaces with the dental epithelium and papilla mesenchyme (Nagai et al., 2001). The functional importance of the dental BM is reinforced by the defective ameloblast differentiation and enamel malformation observed in mice deficient in individual BM components, e.g., type VII (Umemoto et al., 2012) and type XVII collagen (Asaka et al., 2009), and laminin-332 (Ryan et al., 1999). However, the temporal fate of the dental basement membrane is unclear, since some authors have demonstrated the disappearance of type IV collagen (Thesleff et al., 1991; Nagai et al., 2001) at the onset of dentin and enamel mineralization, while others have shown that another BM marker, laminin (Slavkin et al., 1983), is retained during subsequent development. In this way, the developmental origins of the dentin-enamel junction (DEJ), the 5- to 10-µm-wide, morphologically identifiable, interfacial region separating the enamel and dentin of mature teeth, remain unclear.
Despite a lack of structural and compositional information about the DEJ and interfacial region (Marshall et al., 2003), its mechanical importance to the stability of teeth has been amply demonstrated. Specifically, the DEJ and associated inner enamel are known to inhibit crack propagation, exhibit higher fracture toughness (Imbeni et al., 2005), and rarely undergo catastrophic mechanical failure despite a lifetime of masticatory and parafunctional loading (Paine et al., 2001). However, oral cancer patients exposed to radiotherapy demonstrate post-radiation dental lesions that initiate with enamel shear fracture that can result in partial to total enamel delamination, suggesting DEJ instability (Jongebloed et al., 1988; Pioch et al., 1992; Jansma et al., 1993). While post-radiotherapy dentition breakdown has been linked to radiation-induced salivary gland damage (Vissink et al., 2003a; Kielbassa et al., 2006; Silva et al., 2009), our group completed a clinical study and reported a significant correlation between pathologic enamel loss and individual tooth-level radiation dose in oral cancer patients (Walker et al., 2011). Three tiers of tooth response were observed: at < 30 Gray (Gy), minimal tooth damage occurred; between 30 and 60 Gy, there was a 2 to 3x increased risk of tooth breakdown (salivary gland impact); and at > 60 Gy, there was a 10x increased risk of tooth damage, indicating radiation-induced damage to the tooth in addition to salivary gland damage. These findings suggest a radiogenic effect on tooth structure with increasing radiation dose to the tooth. To better understand radiotherapy-induced DEJ instability leading to dentition breakdown, our group is focused on characterizing the structure, properties, and biochemical composition (Dusevich et al., 2012; Xu et al., 2012; McGuire et al., 2014b) of the DEJ, as well as post-radiation changes (McGuire et al., 2014a).
Based on a possible developmental relationship with the dental BM, we recently identified that type VII collagen surrounds the enamel rods as a component of the enamel organic matrix (McGuire et al., 2014b). Since types VII and collagen IV are generally co-localized in basement membranes and form functional complexes (Timpl, 1989), and since the DEJ represents the original position of the dental BM (Imbeni et al., 2005), we asked whether type IV collagen was a component of this interfacial region in mature teeth, and whether it was affected in teeth exposed to high-dose radiotherapy.
Materials & Methods
Collection of Teeth and Preparation
Non-carious, erupted teeth without defects (n = 30) from individuals ≥ 18 yr old were collected from dental surgery clinics within the Kansas City area according to an approved institutional review board (IRB12-50-NHSR) protocol. Using a previously described in vitro oral cancer radiotherapy model (McGuire et al., 2014a), we exposed 3 of the collected extracted teeth to a cumulative dose of 70 Gy (2 Gy/day × 5 days/wk × 7 wks). In vitro radiation exposure was done at a cancer treatment center (Kansas City Cancer Center, Kansas City, KS, USA) using a linear accelerator 2100iX (Varian Medical Systems, Palo Alto, CA, USA) with an energy of 6 MV photons. Additionally, another group of extracted teeth (n = 5; in vivo-irradiated; IRB13-143), described previously in detail, was also collected (McGuire et al., 2014a). Briefly, the tooth-level radiation dose was > 60 Gy during treatment for oral cancer from donors > 40 yr old, and the teeth were extracted ≥ 2 yr post-radiotherapy. Tooth-level radiation doses were calculated from the radiotherapy treatment records (Walker et al., 2011). Upon receipt, specimens were debrided of any soft tissue and stored non-fixed at 4°C in 0.9% phosphate-buffered saline (PBS) containing 0.002% sodium azide (NaN3; Sigma-Aldrich, St. Louis, MO, USA) before being processed. Specimens utilized for immunoblotting were stored at −80°C. Following complete or partial root removal, crowns were processed whole or sliced mesio-distally, respectively, into 750-µm-thick sections with a water-cooled diamond saw (Isomet-1000; Buehler, Lake Bluff, IL, USA).
Confocal Immunofluorescence Studies
First, one 750-µm-thick section was created from 8 third molars, 3 premolars, and 2 canines (total n = 13) extracted from different patients (ranging in age from 18-80 yr). Likewise, an individual section from 3 in vitro-irradiated third molars (n = 3) from separate patients (ranging in age from 38-45 yr) was created. Similarly, a single section was also made from 5 extracted teeth (n = 5; 2 molars, 2 premolars, and 1 canine) that received a tooth-level dose > 60 Gy of in vivo radiotherapy, from 3 separate oral cancer patients (age range: 41-56 yr), and processed for confocal immunofluorescent staining as described below. Sections were adhered with high-vacuum silicone grease (Dow Corning, Midland, MI, USA) along the root dentin onto #1 chambered borosilicate coverglass (Nalge-Nunc, Rochester, NY, USA). For exposure of epitopes, sections were completely demineralized for 14 days in 10% ethylenediaminetetraacetic acid (EDTA) (pH 7.4) containing 0.002% NaN3.
Sections were then washed with Buffer A [PBS containing 0.1% bovine serum albumin (BSA, catalog #001-000-162, JacksonImmuno, West Grove, PA, USA) and 0.05% Tween-20; pH 7.4] and immediately blocked for 2 hr in Buffer B [PBS containing 1% BSA and 0.05% Tween-20; pH 7.4] and avidin D blocking solution (Vector Labs, Burlingame, CA, USA). Sections were then washed with Buffer A containing biotin blocking solution (Vector Labs), and incubated overnight at room temperature with biotin-conjugated goat polyclonal anti-α1α2α1collagen (IV) antibody (1:100, Southern Biotech, Birmingham, AL, USA), diluted in Buffer B. Unpaired control sections were treated similarly with the same concentration of purified non-immune goat IgG (Sigma-Aldrich).
To visualize primary antibody binding, we washed specimens with Buffer A and then incubated them with streptavidin labeled with AlexaFluor594® (1 μg/mL, catalog #016-580-084, JacksonImmuno) in Buffer B. Control sections incubated with unlabeled goat IgG were subsequently incubated for 1 hr with biotin-conjugated protein A (1:10,000, EMDMillipore, Billerica, MA, USA) in Buffer B prior to incubation with AlexaFluor594®-labeled streptavidin.
After specimens were washed, immunofluorescence was visualized on a confocal laser scanning microscope (Leica TCS SP5 II, Wetzlar, Germany). Serial z-focal plane images were captured at 405 and 594 nm, approximately every 5 µm through each section, with the resonant scanner at a resolution of 1024 × 1024 pixels. Image J software was used to create images from the acquired data. We determined specific labeling by subtracting background signal from the 594 and 405 channels using the same software.
Collagenase Treatment
Demineralized molar tooth sections (n = 4) (prepared as described above) were treated for 6 hr at 37°C with purified bacterial collagenase (0.075 mg/mL, Sigma-Aldrich) diluted in 50 mM TES buffer (pH 7.4) containing 0.36 mM CaCl (Sigma-Aldrich), and then immunolabeled and imaged as described above. Adjacent sections, sequentially obtained from the same teeth, served as controls and were treated similarly but only with buffer.
Protein Extraction
Extraction of proteins from third molar tooth crowns (n = 4) from different patients was accomplished by modification of a protocol originally developed for bone (Gorski et al., 1997). Briefly, each crown was flash-frozen in liquid nitrogen, completely pulverized, and mixed with an extraction buffer cocktail containing 4 M guanidine hydrochloride, 0.5 M EDTA, and a mixture of protease and phosphatase inhibitors at pH 7.5 for 72 hr at 4°C. Samples were then centrifuged, and the supernatant fraction was dialyzed at 4°C against 3 changes of water, and then once against 5% acetic acid (Sigma-Aldrich) with 6-8 kDa molecular-weight cut-off dialysis tubing (Spectrum Laboratories, Rancho Dominguez, CA, USA). Extracts were then lyophilized to dryness and stored at −80°C. Protein amounts were determined colorimetrically by means of the Non-Interfering™ Protein Assay kit (G-Biosciences, St. Louis, MO, USA).
Extraction of enamel matrix proteins from additional third-molar tooth crowns (n = 6) was accomplished by complete decalcification of each crown, enamel surface up, for 14 days in 40 mL of 10% EDTA (7.4 pH) containing 0.002% NaN3 at 20°C (McGuire et al., 2014b). The enamel organic matrix layer was then recovered by gentle wiping of the dentin surface with a ballpoint-applicator microbrush (Kerr Totalcare, Orange, CA, USA). The enamel matrix protein fraction was then dialyzed against water, lyophilized, and stored, and protein amounts were determined as described above.
SDS-PAGE and Immunoblotting
Protein samples from whole crowns (n = 4) or the extracted enamel matrix (n = 6), from different patients (ranging in age from 18 to 73 yr), were subjected to electrophoresis under non-reducing and reducing conditions as previously described, with 4% to 20% linear gradient or 7.5% gels (Gorski et al., 2011). Gels were electroblotted onto PVDF membranes, which were probed with a mouse anti-type IV collagen monoclonal antibody (M3F7, 1:500, Developmental Studies Hybridoma Bank, University of Iowa, Iowa City, IA, USA) that recognized the helical domains of α1(IV) and α2(IV) chains (Foellmer et al., 1983) or a rabbit anti-type IV collagen polyclonal antibody (T-15, 1:500, Santa Cruz Biotechnology, Santa Cruz, CA, USA) specific to the α2-chain, and then developed with species-specific horseradish-peroxidase-conjugated secondary antibodies (1:10,000, BioRad, Irvine, CA, USA). Jurkat whole-cell lysate (sc-2204, Santa Cruz Biotechnology) was used as a positive control (not shown). Chemiluminescent digital images were captured with a Fuji LAS-4000 imager (GE, Piscataway, NJ, USA). Western blotting results are representative of a minimum of n = 3 replicate gels for each tooth extract and each different antibody used.
Results
Confocal Immunofluorescent Staining Shows Type IV Collagen as a Biomarker of the DEJ in Mature Teeth
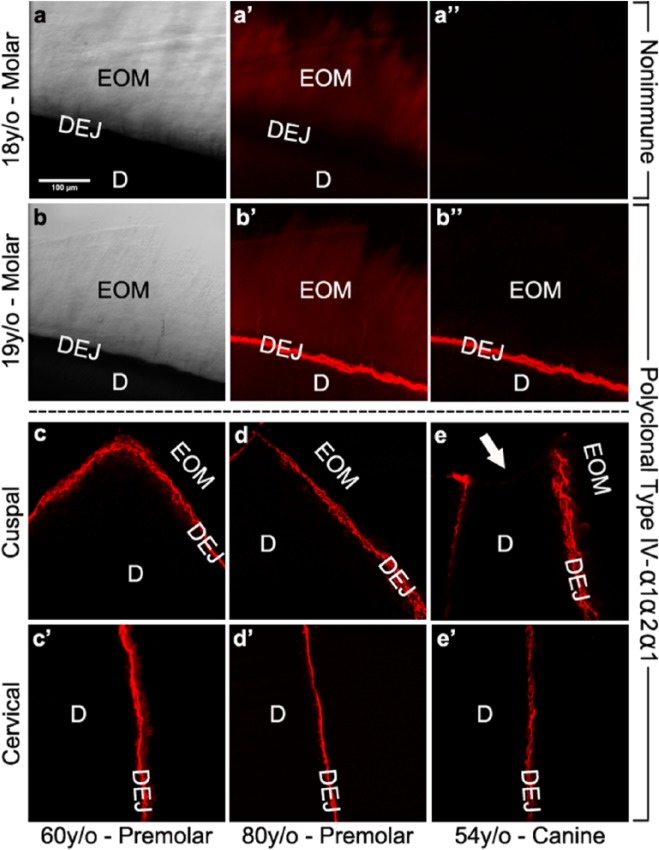
To determine the distribution of type IV collagen in mature teeth, we carried out immunofluorescent labeling studies on tooth sections containing the enamel-dentin complex. When compared with control sections and brightfield images (Figs. 1a-1a′′), anti-type IV collagen antibodies produced crisp intense fluorescent staining in a distinct linear pattern along the scalloped dentinal surface (Figs. 1b-1b′′). Interestingly, staining was localized along the DEJ regardless of whether occlusal (Figs. 1c-1e) to cervical (Figs. 1c′-1e′) tooth surfaces were examined. In contrast, no specific staining of the coronal enamel organic matrix or dentin phases also present on these sections was observed. Importantly, the staining pattern of type IV collagen was not dependent on tooth type, arch position, or patient age.
Figure 1.
Immunolocalization of type IV collagen along the dentin surface at the DEJ in patients of different ages and with different tooth types. Immunofluorescently stained images were obtained by confocal microscopy as described in ‘Methods’. Results shown are representative of 13 individual teeth. (a) Respective bright-field image of the microscopic field in a′-a′′, demonstrating the position of the enamel organic matrix attached to the dentin in a molar from an 18-year-old patient after complete demineralization. (a′) Control section stained with non-immune goat IgG. (a′′) Control section stained with non-immune IgG after background subtraction. (b) Bright-field image of a molar from a 19-year-old patient. (b′) Corresponding occlusal section stained with monospecific polyclonal goat anti-type IV antibodies. (b′′) Corresponding occlusal section stained with polyclonal anti-type IV antibodies after background subtraction. Background-subtracted images of corresponding cuspal (c-e) and cervical (c′-e′) regions of patients of different ages and different tooth types. (c-c′) Maxillary premolar from a 60-year-old patient. (d-d′) Mandibular premolar from an 80-year-old patient. (e-e′) Maxillary canine from a 54-year-old patient. Note the absence of type IV collagen staining at the cusp tip in (e), resulted from occlusal wear through the DEJ (arrow) at this site. EOM, enamel organic matrix; DEJ, dentin-enamel junction; D, dentin. Scale bar = 100 μm, applicable to all images.
Type IV Collagen at the DEJ is Susceptible to Bacterial Collagenase
The collagenous character of DEJ-collagen IV staining was confirmed by degradation with purified bacterial collagenase, which is well-regarded to cleave peptide bonds adjacent to interrupted Glycine-X-Y repeats of type IV collagen (Timpl, 1989). As shown in Figs. 2b and 2b′, collagenase-treated sections exhibited a greatly diminished staining pattern, whereas immunostaining for digestion controls was unchanged (Figs. 2a-2a′).
Figure 2.
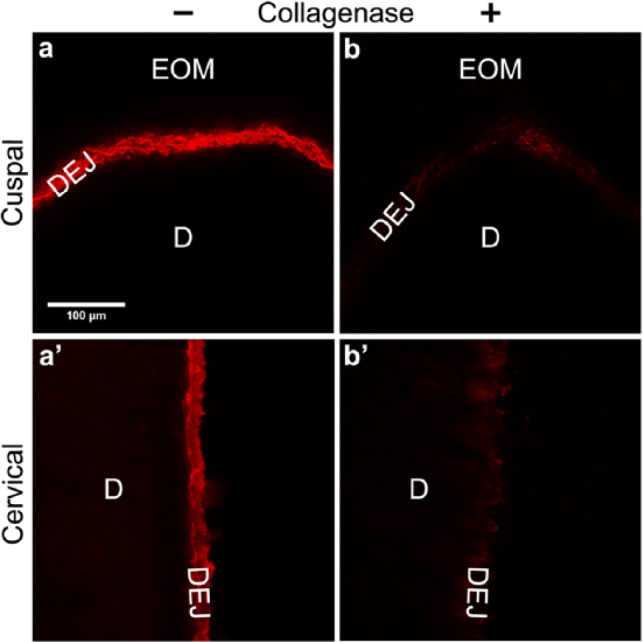
Immunostained type IV collagen at the DEJ is susceptible to bacterial collagenase treatment. Immunofluorescently stained images were obtained by confocal microscopy as described in ‘Methods’. Results shown are representative of 4 experiments. (a-a′) Immunostained appearance of the cuspal and cervical region of a control section without collagenase. (b-b′) Immunoreactivity of type IV collagen was greatly diminished in both the cuspal and cervical regions after 6 hr of digestion with purified bacterial collagenase. Note control and collagenase-treated sections were from the same tooth. EOM, enamel organic matrix; DEJ, dentin-enamel junction; D, dentin. Scale bar = 100 μm, applicable to all images.
Western Blotting Confirms Macromolecular Type IV Collagen within Enamel and Crown Extracts
To establish the molecular character of type IV collagen, we analyzed whole-crown and enamel organic matrix extracts by SDS-PAGE (Fig. 3). Whole-crown and enamel extract lanes were loaded with 15 and 30 µg of protein, respectively. In the absence of chemical reduction, macromolecular type IV α2-chains in crown extracts migrated as monomeric bands at 225 and 250 kDa (Fig. 3a). After reduction, additional smaller bands were also detected prominently at 25 to 30 kDa. Based on their detection with both a monoclonal and a polyclonal antibody, we believe that these smaller bands represent internal fragments of type IV collagen (Figs. 3a, 3c). Comparison of patterns in crown and enamel extracts reveals some differences (Figs. 3a-3d). In particular, only the monomeric 225-kDa type IV α2-chain was evident in enamel extracts in the absence of reduction. After reduction, the 225-kDa band in both the enamel and crown extracts migrated more slowly, suggesting a conformational unfolding (Figs. 3a, 3b). Second, smaller-fragment bands were less enriched in enamel extracts (Fig. 3d) than in crown extracts (Fig. 3c), since twice as much protein needed to be applied in the latter case to achieve similar band intensities. Finally, similar-sized immunoreactive bands were also detected in the Jurkat cell lysate positive control (not shown).
Figure 3.
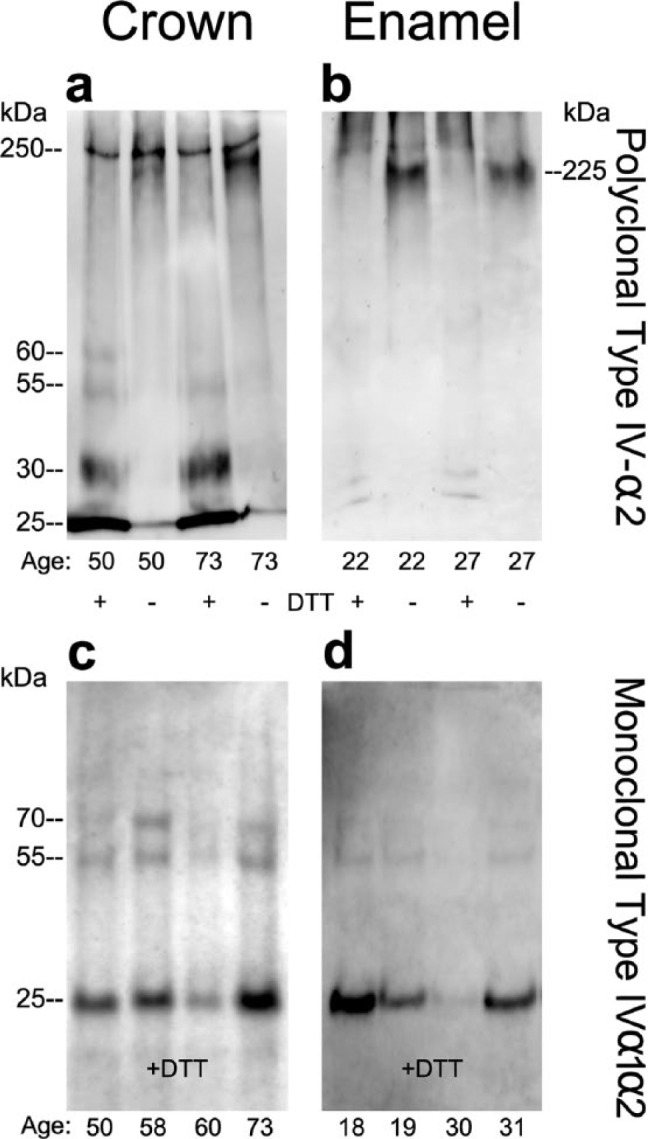
Immunoblotting of whole-crown and enamel organic matrix extracts identified intact type IV collagen α2-chains and associated fragments. Whole-crown extracts and enamel extracts (see ‘Methods’), from donors of different ages, were subjected to electrophoresis under either reducing or non-reducing conditions with 7.5% gels (a, b) or 4% to 20% linear gradient gels (c, d) and electroblotted onto polyvinylidene difluoride membranes. Blots were then probed with polyclonal (a-b) or monoclonal (c-d) anti-type IV collagen antibodies. (a-b) Under non-reducing conditions, intact type IV collagen α2-chains migrated as characteristic monomeric bands at 250 and 225 kDa. After reduction, smaller (30-25 kDa) fragments were detected more prominently in the crown extracts compared with enamel organic matrix extracts. (c-d) Both whole-crown extracts and enamel organic matrix extracts contained fragments of 25 kDa under reducing conditions when probed with monospecific anti-type IV collagen antibodies. Protein amounts loaded onto gel lanes for whole-crown and enamel organic matrix extracts were 15 μg and 30 μg, respectively. Antibodies used: polyclonal anti-type IV collagen antibody specific to the α2(IV) chain and monoclonal anti-type IV collagen antibody, which recognizes the helical domain of α1(IV) and α2(IV) chains. Molecular-weight estimates were based on globular standards co-electrophoresed on the same gel.
In vivo Oral Cancer Radiotherapy Reduces Immunoreactive Type IV Collagen Content at the DEJ
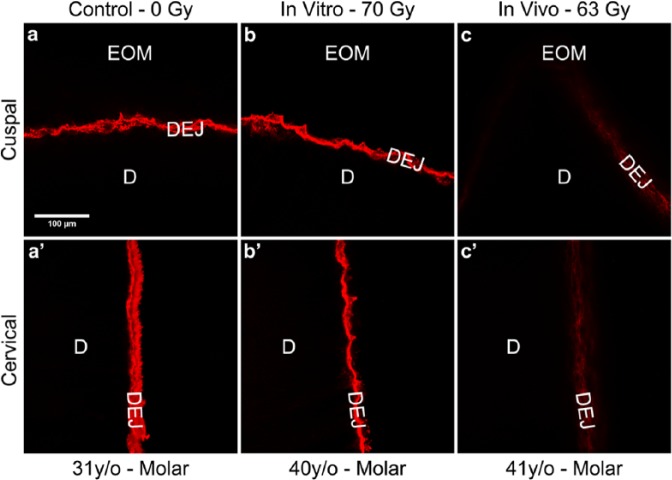
To determine if radiotherapy affects type IV collagen content at the DEJ, we carried out similar immunofluorescent labeling studies on extracted teeth exposed to in vitro simulated radiotherapy or previously exposed to > 60 Gy at the tooth-level during oral cancer radiotherapy (in vivo radiation). Only tooth regions with intact enamel-to-dentin (no enamel loss or visible decay) were evaluated. When compared with the reproducible, robust immunostaining of age-related non-irradiated (within experiment) control (Figs. 4a-4a′) or in vitro-irradiated teeth (Figs. 4b-4b′), in vivo-irradiated tooth sections (Figs. 4c-4c′) demonstrated a dramatically reduced staining pattern, indicating an in vivo radiation-induced effect on DEJ-collagen IV. Reduced immunoreactivity was consistently observed in 5 in vivo-irradiated teeth from 3 separate oral cancer patients. Interestingly, the reduction in immunostaining observed in teeth previously exposed to > 60 Gy (Figs. 4c-4c′) was similar to that observed after bacterial collagenase treatment, which degrades type IV collagen (Figs. 2b-2b′).
Figure 4.
Immunoreactivity for type IV collagen along the DEJ was reduced after in vivo high-dose radiotherapy. Immunofluorescently stained images were obtained by confocal microscopy as described in ‘Methods’. Results depicted are representative of 13 control (a, a′), 3 in vitro (b, b′), and 5 in vivo (c, c′) irradiated teeth, respectively. The immunostained appearance of the cuspal (a-b) and cervical (a′-b′) regions of control tooth sections (0 Gy) and in vitro-irradiated tooth sections (70 Gy) was consistently more robust than that for cuspal (c) and cervical (c′) regions from age-related in vivo-irradiated (> 60 Gy) teeth. EOM, enamel organic matrix; DEJ, dentin-enamel junction; D, dentin. Scale bar = 100 μm, applicable to all images.
Discussion
At present, the molecular composition of the dentin-enamel junction of mature teeth is poorly characterized. Type IV collagen is initially expressed strongly in the dental basement membrane during tooth germ development (Kjoelby et al., 1994; Heikinheimo and Salo, 1995; Nagai et al., 2001). However, as tooth morphogenesis progresses, the dental BM disappears coincident with terminal differentiation of ameloblasts and initial enamel deposition (Silva and Kailis, 1972; Kallenbach and Piesco, 1978). Disappearance of the dental BM is believed to permit cell-cell contacts between the pre-ameloblasts and odontoblasts needed to induce ameloblast differentiation (Slavkin and Bringas, 1976), and the dental BM represents the position of the optical DEJ (Imbeni et al., 2005). Since we focused on mature teeth, the type IV collagen immunolocalized to the DEJ could be from cells of mesenchymal (Heikinheimo and Salo, 1995) and/or epithelial origin (Regauer et al., 1990). However, the temporal origination of DEJ-collagen IV shown here cannot be explained at present. Collectively, localization of type IV collagen as a DEJ component is demonstrated by confocal immunofluorescent staining and by Western blot analyses with separate mono- and poly-specific antibodies, by its susceptibility to purified bacterial collagenase digestion, and by its resistance to pepsin digestion (not shown). Furthermore, the reduction of DEJ-collagen IV immunostaining, in in vivo- irradiated teeth – teeth that manifest DEJ instability – suggests that it may contribute functionally to uniting enamel to dentin. Notably, the immunostaining pattern in in vivo-irradiated teeth mimicked the reduction in collagen IV staining observed after collagenase digestion.
Evaluation of tooth-crown- and enamel-matrix-derived immunoblots revealed several interesting comparisons with tissue-derived type IV collagen. First, the 225- to 250-kDa immunoreactive α2-chains observed in mature tooth extracts closely match the hydrodynamic size of intact type IV collagen α-chains (Werle et al., 1997). Second, similar to type IV collagen, the 250-kDa band was resistant to reduction with dithiothreitol (Reddy et al., 1993; Werle et al., 1997). Third, under reducing conditions, both the total-crown and enamel matrix extracts gave rise to small immunoreactive fragments of Mr = 25-30 kDa when probed with either polyclonal or monoclonal anti-type collagen IV antibodies. We believe that these arise from high-molecular-weight disulfide-bonded complexes that do not enter the running gel in the absence of reduction (Foellmer et al., 1983). Taken together, these Western blotting results demonstrate the presence of macromolecular type IV collagen, and confocal immunostaining localizes type IV collagen specifically to the optical DEJ.
The focus of our biochemical studies of irradiated teeth has been the DEJ. It can be destabilized following oral cancer radiotherapy, resulting in pathologic enamel loss followed by decay of the exposed dentin (Jongebloed et al., 1988; Jansma et al., 1993). Although radiation ‘caries’ is typically explained by radiation-induced xerostomia (Vissink et al., 2003a), tooth-level radiation dose (above the salivary gland threshold) has also been linked to increasing severity of dentition breakdown (Walker et al., 2011). While previous in vitro radiation studies of extracted teeth reported decreased stability of the DEJ (Pioch et al., 1992) as well as changes in enamel properties speculated to be the result of radiation effects on unidentified enamel organic components (Soares et al., 2010), the current investigation is the first to evaluate extracted teeth from oral cancer patients post-radiotherapy. To date, a molecular mechanism for radiotherapy-induced enamel delamination has remained elusive.
DEJ stability, i.e., fracture- and crack-deflecting characteristics, is well-documented (Imbeni et al., 2005) and is likely dependent upon organic constituents in the DEJ region. We hypothesize that localization of both types IV and VII collagen (McGuire et al., 2014b) to this region could help explain these previously reported properties of the DEJ. By analogy with the mechanical stability of the dermal-epidermal junction in skin (Timpl, 1989; Regauer et al., 1990), types IV and VII collagen could form multimeric complexes with dentinal type I collagen fibrils (Lin et al., 1993) to stabilize the dentin-enamel junction. The reproducible loss of DEJ-collagen IV immunostaining in in vivo-irradiated teeth could be due to (1) direct effects of radiation on the structure of the collagen protein that reduce immunoreactivity, (2) indirect effects mediated by odontoblasts and/or other dental pulp cells, and (3) indirect effects mediated by changes in the extracellular matrix at the DEJ. It is interesting that the type IV collagen immunostaining pattern was unchanged immediately subsequent to simulated high-dose radiotherapy. These data exclude the first possibility of direct effects on immunoreactivity. Instead, the comparison of in vivo and in vitro irradiation implies that live cells – within and surrounding a tooth – in combination with time elapsed are factors that reduce DEJ-collagen IV immunoreactivity. However, endodontically treated teeth are not resistant to dentition breakdown post-radiotherapy. Therefore, native proteases within the mature tooth may also play a role. Clinical observations have noted a lag period of approximately 1 yr for initial enamel loss of in vivo-irradiated teeth (Vissink et al., 2003b). In our clinical study, besides reporting the significant tooth-level radiation dose effect, we also reported that elapsed time post-radiotherapy was a significant factor related to the severity of dentition breakdown, i.e., with increasing time, the risk for breakdown increased (Walker et al., 2011). We hypothesize that in vivo high-dose radiotherapy causes induction and activation of enzymes that degrade collagens over a period of months/years. In support of this mechanism, radiotherapy increases the expression and activation of matrix metalloproteinases (MMPs) in various tissues (Araya et al., 2001; Strup-Perrot et al., 2006). To this end, we have shown that MMP-20, a type IV collagenase (Väänänen et al., 2001), is localized to the DEJ and that its processing is altered in teeth post-radiotherapy (McGuire et al., 2014a).
In summary, our results demonstrate that type IV collagen is a new structural component of the enamel organic matrix and a novel biomarker of the DEJ in mature human teeth. We hypothesize that collagen IV plays an important biochemical/structural role in molecular bonding of enamel and dentin. In support of this hypothesis, loss of type IV collagen in teeth following in vivo radiotherapy may represent a mechanism of post-irradiation DEJ instability observed in oral cancer patients, who experience increased incidence of enamel delamination and resultant dentition breakdown.
Acknowledgments
The authors thank Mr. Steve Wichman, radiation physicist, Kansas City Cancer Centers; Ms. Rachel Reed; Dr. Ahmad Mousa; Dr. LeAnn Tiede-Lewis; and Dr. Steven Thomas for their excellent technical assistance. In addition, we acknowledge use of the confocal microscope at the University of Missouri, Kansas City School of Dentistry Confocal Microscopy Core. This facility is supported by the UMKC Office of Research Services, UMKC Center of Excellence in Dental and Musculoskeletal Tissues, and NIH grant S10RR027668.
Footnotes
This study was supported by National Institutes of Health (NIH)/National Institute of Dental and Craniofacial Research (NIDCR) grants R01-DE021462 and F32-DE022984.
The authors declare no potential conflicts of interest with respect to the authorship and/or publication of this article.
References
- Araya J, Maruyama M, Sassa K, Fujita T, Hayashi R, Matsui S, et al. (2001). Ionizing radiation enhances matrix metalloproteinase-2 production in human lung epithelial cells. Am J Physiol Lung Cell Mol Physiol 280:L30-L8. [DOI] [PubMed] [Google Scholar]
- Asaka T, Akiyama M, Domon T, Nishie W, Natsuga K, Fujita Y, et al. (2009). Type XVII collagen is a key player in tooth enamel formation. Am J Pathol 174:91-100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dusevich V, Xu C, Wang Y, Walker MP, Gorski JP. (2012). Identification of a protein-containing enamel matrix layer which bridges with the dentine–enamel junction of adult human teeth. Arch Oral Biol 57:1585-1594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foellmer HG, Madri JA, Furthmayr H. (1983). Methods in laboratory investigation. Monoclonal antibodies to type IV collagen: probes for the study and structure and function of basement membranes. Lab Invest 48:639-649. [PubMed] [Google Scholar]
- Gorski JP, Kremer EA, Chen Y, Ryan S, Fullenkamp C, Delviscio J, et al. (1997). Bone acidic glycoprotein-75 self-associates to form macromolecular complexes in vitro and in vivo with the potential to sequester phosphate ions. J Cell Biochem 64:547-564. [PubMed] [Google Scholar]
- Gorski JP, Huffman NT, Chittur S, Midura RJ, Black C, Oxford J, et al. (2011). Inhibition of proprotein convertase SKI-1 blocks transcription of key extracellular matrix genes regulating osteoblastic mineralization. J Biol Chem 286:1836-1849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heikinheimo K, Salo T. (1995). Expression of basement membrane type IV collagen and type IV collagenases (MMP-2 and MMP-9) in human fetal teeth. J Dent Res 74:1226-1234. [DOI] [PubMed] [Google Scholar]
- Imbeni V, Kruzic JJ, Marshall GW, Marshall SJ, Ritchie RO. (2005). The dentin-enamel junction and the fracture of human teeth. Nat Mater 4:229-232. [DOI] [PubMed] [Google Scholar]
- Jansma J, Vissink A, Jongebloed WL, Retief DH, ’s-Gravenmade EJ. (1993). Natural and induced radiation caries: a SEM study. Am J Dent 6:130-136. [PubMed] [Google Scholar]
- Jongebloed WL, ’s-Gravenmade EJ, Retief DH. (1988). Radiation caries. A review and SEM study. Am J Dent 1:139-146. [PubMed] [Google Scholar]
- Kallenbach E, Piesco N. (1978). The changing morphology of the epithelium-mesenchyme interface in the differentiation zone of growing teeth of selected vertebrates and its relationship to possible mechanisms of differentiation. J Biol Buccale 6:229-240. [PubMed] [Google Scholar]
- Kielbassa AM, Hinkelbein W, Hellwig E, Meyer-Lückel H. (2006). Radiation-related damage to dentition. Lancet Oncol 7:326-335. [DOI] [PubMed] [Google Scholar]
- Kjoelby M, Thesleff I, Sahlberg C, Fejerskov O, Josephsen K. (1994). Degradation of the dental basement membrane during mouse tooth development in vitro. Int J Dev Biol 38:455-462. [PubMed] [Google Scholar]
- Lin CP, Douglas WH, Erlandsen SL. (1993). Scanning electron microscopy of type I collagen at the dentin-enamel junction of human teeth. J Histochem Cytochem 41:381-388. [DOI] [PubMed] [Google Scholar]
- Marshall SJ, Balooch M, Habelitz S, Balooch G, Gallagher R, Marshall GW. (2003). The dentin-enamel junction - a natural, multilevel interface. J Eur Ceram Soc 23:2897-2904. [Google Scholar]
- McGuire JD, Mousa AA, Zhang BJ, Todoki LS, Huffman NT, Chandrababu KB, et al. (2014a). Extracts of irradiated mature human tooth crowns contain MMP-20 protein and activity. J Dent 42:626-636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGuire JD, Walker MP, Mousa A, Wang Y, Gorski JP. (2014b). Type VII collagen is enriched in the enamel organic matrix associated with the dentin-enamel junction of mature human teeth. Bone 63:29-35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagai N, Nakano K, Sado Y, Naito I, Gunduz M, Tsujigiwa H, et al. (2001). Localization of type IV collagen alpha 1 to alpha 6 chains in basement membrane during mouse molar germ development. Int J Dev Biol 45:827-831. [PubMed] [Google Scholar]
- Paine ML, White SN, Luo W, Fong H, Sarikaya M, Snead M. (2001). Regulated gene expression dictates enamel structure and tooth function. Matrix Biol 20:273-292. [DOI] [PubMed] [Google Scholar]
- Pioch T, Golfels D, Staehle HJ. (1992). An experimental study of the stability of irradiated teeth in the region of the dentinoenamel junction. Endod Dent Traumatol 8:241-244. [DOI] [PubMed] [Google Scholar]
- Reddy GK, Hudson BG, Bailey AJ, Noelken ME. (1993). Reductive cleavage of the disulfide bonds of the collagen IV noncollagenous domain in aqueous sodium dodecyl sulfate: absence of intermolecular nondisulfide cross-links. Biochem Biophys Res Commun 190:277-282. [DOI] [PubMed] [Google Scholar]
- Regauer S, Seiler GR, Barrandon Y, Easley KW, Compton CC. (1990). Epithelial origin of cutaneous anchoring fibrils. J Cell Biol 111(5 Pt 1):2109-2115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryan MC, Lee K, Miyashita Y, Carter WG. (1999). Targeted disruption of the LAMA3 gene in mice reveals abnormalities in survival and late stage differentiation of epithelial cells. J Cell Biol 145:1309-1323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silva AR, Alves FA, Antunes A, Goes MF, Lopes M. (2009). Patterns of demineralization and dentin reactions in radiation-related caries. Caries Res 43:43-49. [DOI] [PubMed] [Google Scholar]
- Silva DG, Kailis DG. (1972). Ultrastructural studies on the cervical loop and the development of the amelo-dentinal junction in the cat. Arch Oral Biol 17:279-286. [DOI] [PubMed] [Google Scholar]
- Slavkin HC, Bringas P., Jr (1976). Epithelial-mesenchyme interactions during odontogenesis. IV. Morphological evidence for direct heterotypic cell-cell contacts. Dev Biol 50:428-442. [DOI] [PubMed] [Google Scholar]
- Slavkin HC, Brownell AG, Bringas P, MacDougall M, Bessem C. (1983). Basal lamina persistence during epithelial-mesenchymal interactions in murine tooth development in vitro. J Craniofac Genet Dev Biol 3:387-407. [PubMed] [Google Scholar]
- Soares CJ, Castro CG, Neiva NA, Soares PV, Santos-Filho PC, Naves LZ, et al. (2010). Effect of gamma irradiation on ultimate tensile strength of enamel and dentin. J Dent Res 89:159-164. [DOI] [PubMed] [Google Scholar]
- Strup-Perrot C, Vozenin-Brotons MC, Vandame M, Benderitter M, Mathe D. (2006). Expression and activation of MMP-2, -3, -9, -14 are induced in rat colon after abdominal X-irradiation. Scand J Gastroenterol 41:60-70. [DOI] [PubMed] [Google Scholar]
- Thesleff I, Partanen AM, Vainio S. (1991). Epithelial-mesenchymal interactions in tooth morphogenesis: the roles of extracellular matrix, growth factors, and cell surface receptors. J Craniofac Genet Dev Biol 11:229-237. [PubMed] [Google Scholar]
- Timpl R. (1989). Structure and biological activity of basement membrane proteins. Eur J Biochem 180:487-502. [DOI] [PubMed] [Google Scholar]
- Umemoto H, Akiyama M, Domon T, Nomura T, Shinkuma S, Ito K, et al. (2012). Type VII collagen deficiency causes defective tooth enamel formation due to poor differentiation of ameloblasts. Am J Pathol 181:1659-1671. [DOI] [PubMed] [Google Scholar]
- Väänänen A, Srinivas R, Parikka M, Palosaari H, Bartlett JD, Iwata K, et al. (2001). Expression and regulation of MMP-20 in human tongue carcinoma cells. J Dent Res 80:1884-1889. [DOI] [PubMed] [Google Scholar]
- Vissink A, Jansma J, Spijkervet FK, Burlage FR, Coppes RP. (2003a). Oral sequelae of head and neck radiotherapy. Crit Rev Oral Biol Med 14:199-212. [DOI] [PubMed] [Google Scholar]
- Vissink A, Burlage FR, Spijkervet FK, Jansma J, Coppes RP. (2003b). Prevention and treatment of the consequences of head and neck radiotherapy. Crit Rev Oral Biol Med 14:213-225. [DOI] [PubMed] [Google Scholar]
- Walker MP, Wichman B, Cheng AL, Coster J, Williams KB. (2011). Impact of radiotherapy dose on dentition breakdown in head and neck cancer patients. Pract Radiat Oncol 1:142-148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Werle E, Hao L, Hasslacher C, Fiehn W. (1997). Western blotting of NC1 type IV collagen fragments in human plasma. Eur J Clin Invest 27:579-588. [DOI] [PubMed] [Google Scholar]
- Xu C, Reed R, Gorski JP, Wang Y, Walker MP. (2012). The distribution of carbonate in enamel and its correlation with structure and mechanical properties. J Mater Sci 47:8035-8043. [DOI] [PMC free article] [PubMed] [Google Scholar]