Abstract
Resveratrol is a natural phytoestrogen with antiproliferative properties present in red wine, grapes, and berries. Published reports on the effects of resveratrol in human endometrial function are limited. The objective of this study was to investigate the expression of estrogen receptor α (ESR1), Ki-67 (a proliferative marker), aryl hydrocarbon receptor (AhR), and members of the cytochrome P450 superfamily of enzymes (CYP1A1 and CYP1B1) in an in vitro and vivo assay. Alkaline phosphatase assay of estrogenicity was used to compare estrogen activity of different concentrations of resveratrol to estradiol (E2) and diethylstilbestrol (DES), using Ishikawa cell culture. Immunohistochemical expression of ESR1 and Ki67, and reverse transcriptase polymerase chain reaction of AhR, CYP1A1, and CYP1B1 were analyzed from xenograft implants of human endometrial tissue in ovariectomized immunodeficient RAG-2-γ(c) mice, after 30 days of treatment with subcutaneous pellets of E2, E2 plus progesterone (P4), or E2 plus resveratrol (6, 30, or 60 mg) for 30 days. Compared to E2, resveratrol acted as an agonist and antagonist of estrogen in low and high concentrations, respectively, when combined with E2. Xenografts of human endometrial tissues in RAG-2 mice exhibited reduced expression of ESR1 and proliferative activity (Ki67) with 60 mg of resveratrol. This study suggests that resveratrol, at high doses, has the potential benefit to reduce proliferation of human endometrium through ESR1.
Keywords: resveratrol, Ishikawa, human endometrium, endometriosis, estrogen receptor, cell proliferation
Introduction
Resveratrol (trans-3,5,40-trihydoxystilbene) is a natural phytoestrogen synthesized by plants following ultraviolet radiation. Red wine, grapes, and berries have significant concentrations of this compound. Resveratrol has been reported to have antineoplastic,1 anti-inflammatory, and antioxidant properties.2 In addition, resveratrol has been shown to inhibit growth of cancer cell lines.3–5 Researchers have investigated the antiproliferative action in endometriosis using in vivo animal and human models.6–8 Reduction in the size and activity of endometriotic implants has been observed with the use of resveratrol although uterine wet weight and endometrial proliferation and protein kinase B phosphorylation are increased by resveratrol treatment in the rat.9 Resveratrol also enhances E-cadherin and glycodelin at sites of intercellular contact in endometrial cell lines, suggesting a possible action for resveratrol in promoting endometrial receptivity toward implantation.10
Dioxins are potentially toxic polycyclic aromatic hydrocarbons that are accumulating in our drinking water and food as a result of their production as chemical industry by-products. The long environmental and biological half-life of dioxins coupled with their hydrophobic nature lead to accumulation in human and animal adipose tissue. Dioxins bind to and activate the aryl hydrocarbon receptor (AhR) triggering a variety of biologic responses. Dioxin has been shown to have effects on estrogen action and is implicated in the pathogenesis of endometriosis and other diseases affecting women.11,12
Endometriosis is a disease that affects 176 million women worldwide13 and is present in about 60% of adolescent women with chronic pelvic pain or dysmenorrhea.14 Endometriosis is defined as the presence of endometrial glands and stroma outside the uterine cavity. This condition is characterized by increased endometrial proliferation and responsiveness to estrogen and by endometrial resistance to progesterone.15,16 Both eutopic and ectopic endometrium in women with endometriosis overexpress estrogen receptor α (ERα; ESR1)17 perhaps due to defective progesterone action or excessive estrogen production.18–20 Estrogen receptor β (ERβ; ESR2) is overexpressed in endometriosis as well.21,22
Resveratrol has been shown to be an AhR antagonist with possible protective activity against the effects of dioxins23 and to have different estrogen action (agonist or antagonist) in different tissues.24 Although resveratrol appears to have potential bioactivity in human endometrium, data on its effects in human endometrial function have been limited. To gain insight into the physiological aspects of resveratrol in human endometrium, this study will provide the basis for understanding pathophysiological conditions, such as endometriosis.
In this study, we investigated the effect of resveratrol on key components related to endometrial proliferation, that is, ESR1, Ki67, AhR, and its downstream target genes CYP1A1 and CYP1B1, using previously validated methods.25,26 These data on the effects of resveratrol on endometrial responses to estrogen will provide further insight into potential mechanisms of action of resveratrol that could help explain observed beneficial effects of this phytoestrogen.
Methods
Alkaline Phosphatase Assay
The relative estrogenicity of resveratrol was studied in vitro, using the alkaline phosphatase assay in a well-differentiated endometrial cell line (Ishikawa).26 This cell line maintains ERα and ERβ (ESR1 and ESR2), androgen receptor, and progesterone receptor and is sensitive to estrogen treatment.27 Unless otherwise stated, all reagents were obtained from Sigma (St Louis, Missouri). Increased concentrations of resveratrol alone or in combination with estradiol (E2) treatment were used to study the relative estrogen activity of both substances. Ishikawa cells were cultured in medium containing phenol red-free Dulbecco Modified Eagle Medium:Nutrient Mixture F-12 (DMEM/F-12) for 48 hours before seeding in 96-well plates (BD Bioscience, San Jose, California). The cells were washed twice with 1× phosphate-buffered saline (PBS), detached with trypsin–EDTA 0.25% then resuspended in culture medium. Approximately 2 × 104 cells were added to each well with 200 µL medium and grown for 24 hours in a humid chamber at 37°C with 5% CO2. The cells were then switched to phenol red-free DMEM/F-12 medium containing E2 (10−8 mol/L) and incubated up to 3 days.
Alkaline phosphatase assay was measured at 24, 48, and 72 hours. Estradiol or diethylstilbestrol (DES) at 10−8 mol/L, with or without resveratrol (10−4-10−12 mol/L), or the antiestrogen Imperial Chemical Industries (ICI 182780—10−6 mol/L, Zeneca Pharmaceuticals, Wilmington, Delaware) were incubated for 72 hours. The medium was changed daily and all experiments were performed in quadruplicates. After 72 hours of incubation, the alkaline phosphatase assay was performed. Cells were washed twice with PBS. Next, plates were frozen at −80°C for 10 minutes and then thawed to lyze the cell membranes. Cold soluble substrate of 20 µL consisting of SigmaFast p-nitrophenyl phosphate tablets dissolved in deionized H2O was added to each well on ice. The substrate solution produced a yellow color when alkaline phosphatase was present. After 1 to 3 hours of incubation at room temperature, the absorbance was read in a plate-reader at 405 nm (Biorad, Hercules, California). Each experiment was repeated 3 times with a minimum of 3 wells per each replicate assay.
Animal Protocols and Hormone Treatments
This study was approved by the Greenville Health System (GHS) Institutional Animal use and Care Committee protocol. The RAG-2γ(c) knockout mice (C57BL/6J × C57BL/10SgSnAi[KO]γc[KO]Rag-2—n = 20) were purchased from Taconic (Taconic Germantown, Philadelphia) and then housed under barrier and standard husbandry conditions. Female RAG mice were ovariectomized using sterile technique and allowed to recover for a least 1 week prior further surgery. The 20 animals were divided into 5 treatment groups (n = 4 in each group): (1) E2 alone, (2) E2 plus progesterone (P), (3) E2 plus 6 mg of resveratrol, (4) E2 plus 30 mg of resveratrol, and (5) E2 plus 60 mg of resveratrol. Resveratrol and the hormones were administered via implanted subcutaneous time-release hormone pellets (E2: 100 μg/pellet; P: 7.5 mg/pellet—Innovative Research of America, Sarasota, Florida) that were placed along the dorsal paravertebral regions bilaterally as described.28 The hormone pellets were designed to deliver a steady concentration daily dose of E2 and progesterone of 1.6 μg/d for up to 60 days and 357 μg/d for 21 days, respectively. Likewise, resveratrol pellets were designed to release a steady concentration of 6, 30, or 60 mg/d.
Human Xenografts
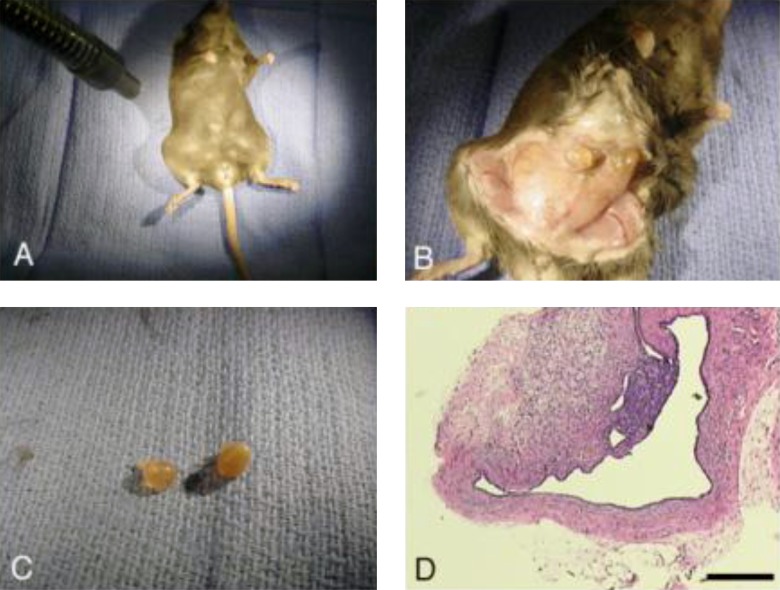
Human endometrial biopsies were obtained from cycling women at the time of laparoscopy using a pipelle device and the tissue was placed into Hams F12 medium for transport to the laboratory. Collection of human tissues was performed according to institutional review board-approved protocols at GHS, and all patients signed a written consent to allow the use of their tissue for research. In the laboratory, uniform samples (2 mm3) were dissected and placed into 2 separate positions in subcutaneous pockets on the abdomen of the ovariectomized, anesthetized RAG-2 mice. The incisions containing the xenograft implants were closed with 5-0 Vicryl (Ethicon, Piscataway, New Jersey) interrupted suture. At the end of 4 weeks, the mice were killed and the xenograft implants which were visible under the skin and easily identified were collected and analyzed (Figure 1).
Figure 1.
Composite showing the RAG2-γ(c) knockout mouse model for endometriosis. Subcutaneous implants of human endometrium were stimulated by treatment with estrogen pellets for 4 weeks. The implants are visible under the skin (A), visible endometrial xenografts (B) are harvested (C) and the histologic appearance is shown in D (scale bar = 100 µm). In a typical experiment, 2 implants would be placed and the mice treated with E2 plus 1 of 3 doses of resveratrol prior to tissue collection after 30 days.
Immunohistochemistry
Each endometrial sample collected from the xenograft tissue was analyzed by immunocytochemistry for ERα (NCL-L-ER-6F11—dilution 1:250, Leica Biosystems, Newcastle, United Kingdom) and Ki67 (MKI67—dilution 1:75—Biogenex, San Ramon, California), a nuclear marker of cell proliferation, according to previous publication.29 For this purpose, the collected xenograft tissue was fixed in 10% buffered formalin solution and then paraffin embedded. Sections of 5-µm thick were obtained and immunostained for ESR1 and Ki67. Semiquantitative HSCORE was assigned in a blinded manner by 2 investigators (BAL and SCA) and the results were compared between groups. The use of HSCORE has been previously validated as a semiquantitative assay for immunohistochemical staining.30
Reverse Transcriptase Polymerase Chain Reaction
Tissue samples were homogenized in 600 µL of buffer RLT (Qiagen RNeasy Mini) using a Tissue Tearor (BioSpec Products, Bartlesville, Oklahoma) at 4°C. The Qiagen RNeasy Mini Kit (Valencia, California) was then used for the isolation of RNA from the lysate according to the manufacturer’s instructions. RNA was quantified using the Quant-iT RiboGreen RNA Assay Kit (Invitrogen Molecular Probes, Eugene, Oregon) and a CytoFluor Multi-Well Plate Reader Series 4000 (Perseptive Biosystems, Framingham, Massachusetts) according to the manufacturers’ instructions.
The production and amplification of complementary DNA were measured in a single reaction in a DNA Opticon 2 System (MJ Research, Inc, Waltham, Massachusetts) using 2× SYBR Green Master Mix and Multiscribe Reverse Transcriptase (Applied Biosystems, Inc, Foster City, California). The primer sequences were: AhR, 5′-GTGCACAGCTCTGCTTCAGT-3′ and 5′-CTACTCCACTTCAGCCACCA-3′; CYP1A1, 5′-CGGCCCCGGCTCTCT-3′ and 5′-GTGTCGGAAGGTCTCCCAGGAT-3′; and CYP1B1, 5′-ATACAAGGCAGACGGTCCCT-3′ and 5′-CTGCTCCTCCTCTTCACCAG-3′ The reverse transcriptase polymerase chain reaction (RT-PCR) used the following cycles: 48 C for 30 minutes, 95°C for 10 minutes, and 40 cycles of 58°C for 1 minute and 95°C for 10 seconds. The RT-PCR data were performed using the 2−(Sample Ct − GAPDH Ct] method, as described previously.31
The cytosolic AhR was analyzed because of its crosstalk with estrogen receptor and regulation of the expression of several isoforms of cytochrome P450, including CYP1A1 and CYP1B1. CYP1A1, and CYP1B1 are responsible for E2 hydroxylation.32 The AhR has been linked as an antiestrogenic and is a ligand to resveratrol.33
Statistical Analysis
Comparison of results was made on Prism Software 6.0 (GraphPad, Inc, San Diego, CA) using analysis of variance with Dunn corrections for multiple comparisons. Statistical significance was assigned for P < .05.
Results
In Vitro Assay
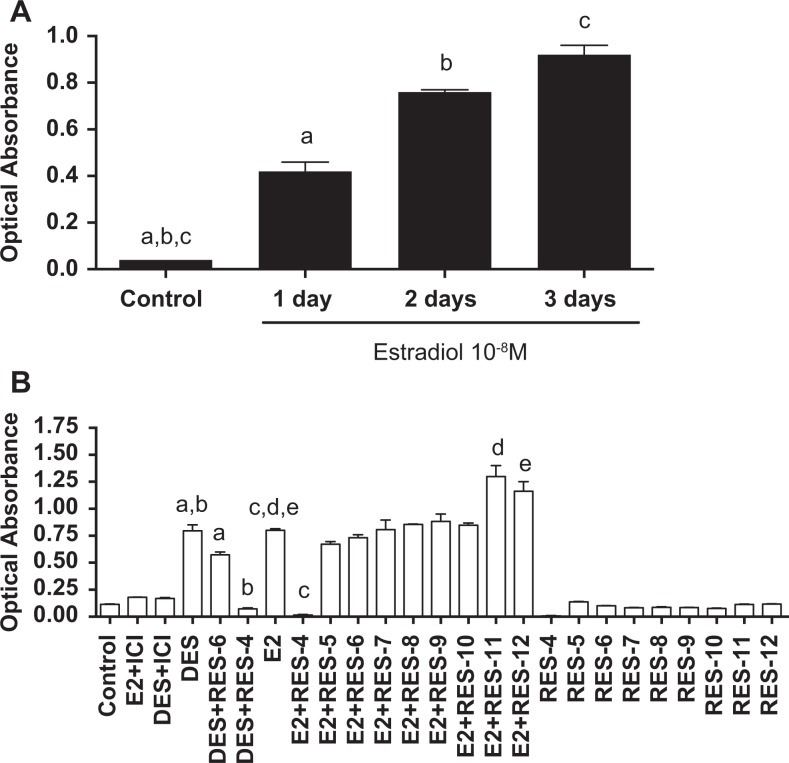
Three days of incubation of Ishikawa cells with E2 at 10−8 mol/L resulted in the highest estrogen activity according to the alkaline phosphatase assay (Figure 2A). Based on these results, subsequent assays were done with 3 days of treatment. The comparison of different doses of resveratrol with E2 revealed that resveratrol at 10−4 mol/L had little estrogenic activity but combined with E2 it acted as an estrogen antagonist. In contrast, low doses of resveratrol (10−11 and 10−12 mol/L) with E2 displayed an agonistic effect (Figure 2B).
Figure 2.
A, Estrogenicity in Ishikawa cells after treatment of estradiol during different time periods. Alkaline phosphatase assay of Littlefield provided a means to compare the estrogenicity. ANOVA P < .0001, n = 4 in each group. B, Estrogenicity obtained from Ishikawa cells treated for 3 days with different concentrations of resveratrol in the presence or absence of estradiol or DES at 10−8 mol/L, or with DES + ICI (an antiestrogen). Bars represent mean (±SEM); post hoc test (pairing letters), all P < .05. ANOVA indicates analysis of variance; DES, diethylstilbestrol; ICI, Imperial Chemical Industries; SEM, standard error of the mean.
In Vivo Assay
A mouse model using human endometrial xenograft implants demonstrated that resveratrol exerted biologic activity on human endometrial tissues in vivo. Overall weight of the recovered tissues after a month of treatment was not significantly different among treatment groups (data not shown).
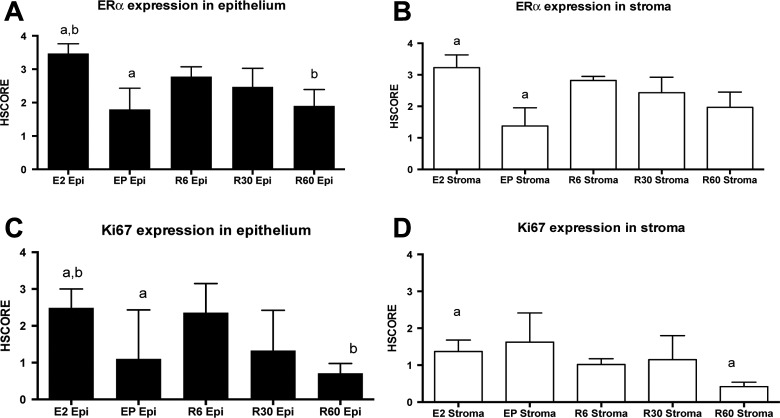
Immunostaining for Ki67 and ESR1 revealed a different immunostaining expression among different forms of treatment (Figures 3 and 4). Mice treated with E2 plus P or E2 plus resveratrol showed decreased cell proliferation (Figure 3C) as well as reduced ESR1 (Figure 3A). The reduction in ESR1 was not as profound with resveratrol as with E2 plus P (Figure 4), but at the highest dose (60 mg) resveratrol exhibited a reduction in both ESR1 and Ki-67 in endometrial epithelial cells (Figure 3A and C). In stromal cells, ESR1 levels were reduced by E2 plus P, but not by resveratrol (Figure 3B), and Ki-67 expression was reduced specifically by resveratrol in human endometrial stroma in the presence of 60 mg/d of resveratrol (Figure 3D).
Figure 3.
Immunohistochemical appearance of estrogen receptor α (ERα) and the proliferation marker Ki67 in human eutopic endometrium used as a xenografts tissue recovered from the mouse model (mean ± SEM). A, ERα expression in epithelial endometrium is reduced in the presence of treatment with estradiol (1.6 μg/d) and progesterone (357 μg/d) and with estradiol (1.6 μg + resveratrol 60 mg/d. B, ERα expression is reduced in stroma cells, compared to those treated with estradiol only. Ki-67 expression is reduced in endometrial stromal cells treated with estradiol plus progesterone (C and D). All ANOVA—Dunnett post hoc test (pairing letters)—All P ≤ .01. Epi indicates epithelial endometrium; SEM, standard error of the mean.
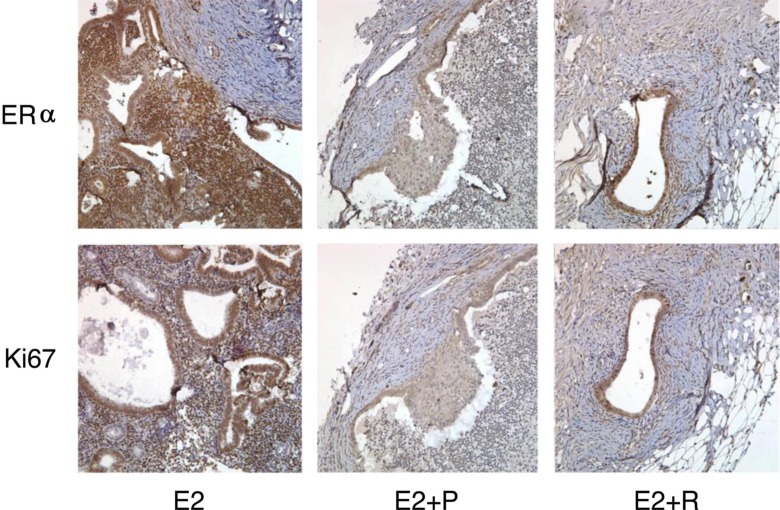
Figure 4.
Photomicrograph of human eutopic endometrium used a xerograph in RAG mouse. Estrogen treated implants contained more estrogen receptors and Ki67, a marker of proliferation, than the implants from mice treated with E2 plus progesterone (E2 + P). In contrast to the E2-treated animals, mice treated with 60 mg of resveratrol (E2 + R) had reduced Ki67 and ERα immunostaining. Magnification ×400. E2 indicates estradiol; P, progesterone; R, resveratrol; ERα, estrogen receptor α.
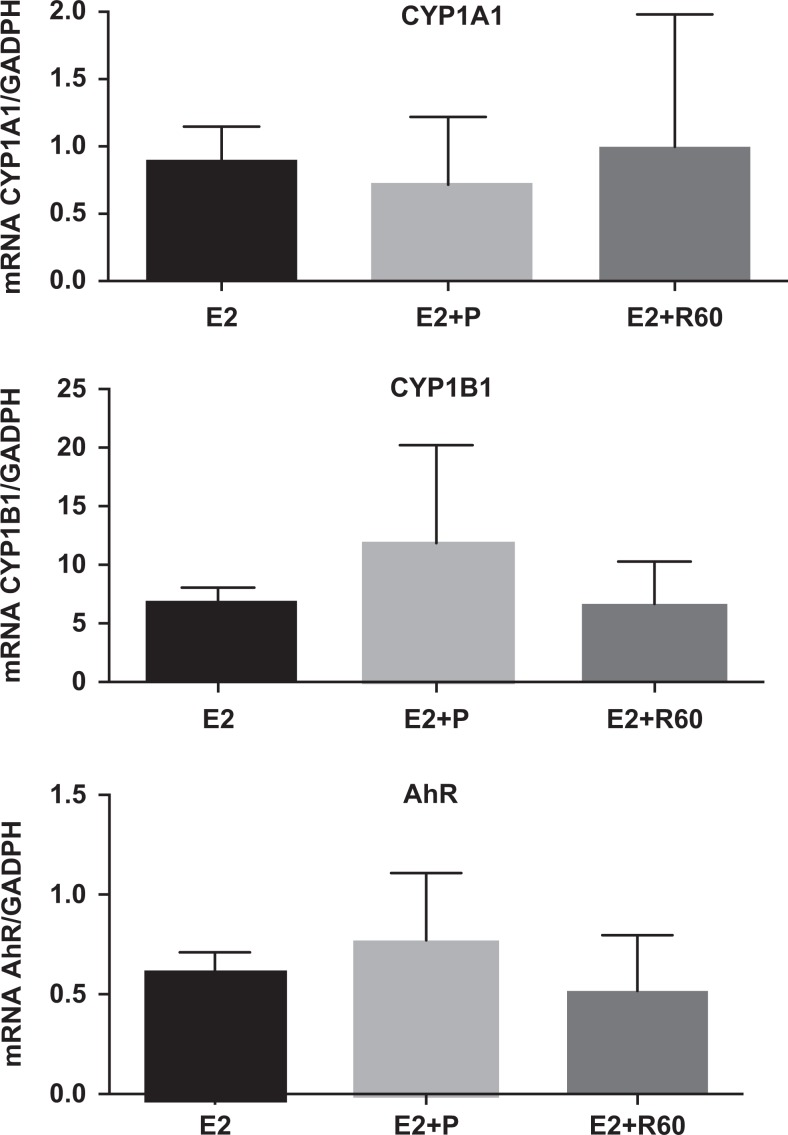
To further investigate the possibility that resveratrol was acting through AhR, we examined AhR itself, and 2 downstream target proteins of AhR, CYP1A1, and CYP1B1. The RT-PCR was performed on messenger RNA (mRNA) isolated from the implants recovered from the RAG mice treated with E2, E2 plus P, and E2 plus 60 mg of resveratrol. The reduction of AhR with resveratrol was not significant among any of the treatment groups (Figure 5). Likewise, mRNA expression of CYP1A1 and CYP1B1 was not significantly different compared to E2 or E2 plus progesterone (Figure 5).
Figure 5.
Comparisons are shown for AhR, Cyp1A1, and Cyp1B1 mRNA by RT-PCR (mean ± SEM) in human xenografts endometrial tissue from RAG-2 mice treated with estradiol (E2), E2 plus progesterone (P), or E2 plus 60 mg of resveratrol. No significant difference was found among groups. AhR indicates aryl hydrocarbon receptor; mRNA, messenger RNA; RT-PCR, reverse transcriptase polymerase chain reaction; SEM, standard error of the mean.
Discussion
There is increasing interest in the dietary components of food and the possible benefits of specific compounds on health and disease. In the present study, we used both in vivo and in vitro techniques to investigate the role of an active component of red wine in endometrial activity. Resveratrol alone appears to have weak estrogen activity in Ishikawa cells. In the presence of estrogen, however, it acts as both an estrogen agonist and an estrogen antagonist at low and high concentrations, respectively (Figure 2B). These findings are in accordance with those published by Bowes et al whose work provided more serial dilutions with resveratrol in a cell line transfected with ERα and ERβ.34 The mechanism for the antagonistic effect at higher concentrations does not appear to be related to estrogen metabolism, since the effect is seen with the nonmetabolizable synthetic estrogen, DES, (Figure 1B). Increased cellular toxicity of high-dose resveratrol was not ruled out in this study but has not been shown by others.35,36
We demonstrate that resveratrol slows endometrial proliferation of xenografts using the immunocompromised mouse model. Others have observed this reduction in proliferation.36 Although the mechanism for this effect on blocking proliferation has been proposed to be antagonism of estrogen through AhR,37 we find no difference in AhR activity in resveratrol-treated and untreated xenograft samples. We also did not find evidence for direct effects of resveratrol on ESR1 levels that paralleled proliferation data (Ki67 immunostaining). Immunohistochemical analysis of eutopic human implants in RAG-2γ(c) knockout mice (Figure 3) suggests that the antiestrogenic, antiproliferative activity of resveratrol may be related to reduction of ESR1 specifically in endometrial epithelium. These results are in accordance with those published by Kang et al.36 Furthermore, resveratrol has been shown to act through nongenomic action of alternative estrogen receptors such as GPR3038 and might be acting through alternative estrogen receptors such as ESR2.24
Animal models have proven useful for the study of endometriosis. Although primate models have become the most applicable in vivo model to date,21,39 rodent models of endometriosis are more accessible and less expensive.40,41 Using the nude mouse model, other researchers were able to study xenografts of human endometrial tissue more effectively.42 The use of the RAG-2 knockout mice has been previously characterized and demonstrated to be an eloquent model for studying hormonal responses of human endometriotic tissues.25 Our studies have used this particular mouse model for the study of the effect of herbal treatments on endometriosis.29
Resveratrol appears to be a more potent in vivo antiestrogen at higher doses tested (60 mg). More recently, Chen et al reported that resveratrol inhibits the expression of CYP1A1 and CYP1B1 induced by dioxin (2,3,7,8-tetrachlrodibenzeno-p-dioxin—TCDD) binding to AhR.33 Likewise, Casper et al found that resveratrol competes with AhR in the presence of an AhR ligand, such as TCCD, and inhibits CYP1A1 activity.23 With our sample size, we were not able to find a difference bigger than 50% among the mean expression of CYP1A1, CYP1B1, or AhR when xenograft implants were treated with E2 plus 60 mg of resveratrol (Figure 4). Possible explanations for this discrepancy might be related to the different dosages used in each study and the use of an active AhR agonist, dioxin or TCDD, in the studies by Chen et al. Indeed, the use 50 μmol/L of resveratrol alone did not elicit the expression of CYP1A1 or CYP1B1 but 50 μmol/L of resveratrol with 2 nmol/L of TCDD inhibits CYP1A1 and CYP1B1 expression at the mRNA and protein level.33 Despite differences in the resveratrol doses used between these studies, the low concentration of resveratrol in combination with E2, used in our in vitro study, yielded a higher estrogenic activity, consistent with the results of Bhat and Pezzuto.24
The findings of our study, including the reduction in endometrial ESR1, specifically in endometrial epithelium in response to resveratrol, suggest that this compound may provide a novel approach to the treatment of endometriosis. Bulun has suggested that ESR2 acts as an antiestrogen in endometriosis43 and ESR2–ESR1 ratios are higher in endometriosis.44 Resveratrol acts through both estrogen receptors45 but has different actions in each estrogen receptor type.46 Future studies will examine the possibility that resveratrol is acting to induce ESR2 as a mechanism for these noted effects. The dose of 60 mg/d of resveratrol used in our animal model corresponds to an unusual high dose of resveratrol for humans. However, a recent study7 using a 30-mg pill per day in 12 patients with endometriosis provided an important clinical ground to pursuit a larger clinical trial. Oral micronized resveratrol (SRT501) can reach up to 185 µmol/L in human plasma and it is well tolerated.47 Together with our results, and the knowledge accumulated on the pharmacokinetics, bioavailability of oral resveratrol,48 different formulations,47,49 and enhancers for bioavailability,50 the path for larger and well-designed clinical trials is open.
In summary, using both in vivo and in vitro models we demonstrated that resveratrol acts as an antiestrogen in vivo and in vitro at high concentrations in Ishikawa cells and in human endometrium. The immunocompromised mouse model demonstrated that cellular proliferation in human endometrial xenografts declines in parallel with decreasing epithelial ERα levels. The mechanism of this reduced proliferation remains to be determined. Since overexpression or overactivity of estrogen receptor has been proposed as a central defect leading to infertility in women with endometriosis, and critical biomarkers of endometrial receptivity have been shown to be inhibited by overexpression of ESR1,17 it is possible that resveratrol may have other potential benefits for the treatment of infertility. Given the compounding effects of alcohol in reproductive-aged women and the impracticality and expense involved in achieving therapeutic doses, it seems unlikely that red wine would be a convenient source for resveratrol treatment by itself. Purified resveratrol compounds or better delivery systems for such compounds may someday prove beneficial.
Footnotes
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship and/or publication of this article: This work was supported by NICHD/NIH through cooperative agreement U54 HD-30476 (BAL and SLY) as part of the Specialized Cooperative Centers Program in Reproduction Research and Infertility, by NICHD/NIH R01 HD067721 (SLY and BAL), 5 P20 RR-016461 and 8 P20 GM103499 (NIH SC-INBRE) and EPS-0903795 (NSF SC-EPSCOR) (EH), and by Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq) 240239/2012-1 (RFS).
References
- 1. Kinghorn AD, Su BN, Jang DS, et al. Natural inhibitors of carcinogenesis. Planta Med. 2004;70(8):691–705 [DOI] [PubMed] [Google Scholar]
- 2. Jiang H, Shang X, Wu H, et al. Resveratrol downregulates PI3K/Akt/mTOR signaling pathways in human U251 glioma cells. J Exp Ther Oncol. 2009;8(1):25–33 [PMC free article] [PubMed] [Google Scholar]
- 3. Sun C, Hu Y, Liu X, et al. Resveratrol downregulates the constitutional activation of nuclear factor-kappaB in multiple myeloma cells, leading to suppression of proliferation and invasion, arrest of cell cycle, and induction of apoptosis. Cancer Genet Cytogenet. 2006;165(1):9–19 [DOI] [PubMed] [Google Scholar]
- 4. Hwang JT, Kwak DW, Lin SK, Kim HM, Kim YM, Park OJ. Resveratrol induces apoptosis in chemoresistant cancer cells via modulation of AMPK signaling pathway. Ann N Y Acad Sci. 2007;1095:441–448 [DOI] [PubMed] [Google Scholar]
- 5. Vanamala J, Radhakrishnan S, Reddivari L, Bhat VB, Ptitsyn A. Resveratrol suppresses human colon cancer cell proliferation and induces apoptosis via targeting the pentose phosphate and the talin-FAK signaling pathways-A proteomic approach. Proteome Sci. 2011;9(1):49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Bruner-Tran KL, Osteen KG, Taylor HS, Sokalska A, Haines K, Duleba AJ. Resveratrol inhibits development of experimental endometriosis in vivo and reduces endometrial stromal cell invasiveness in vitro. Biol Reprod. 2011;84(1):106–112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Maia HJ, Haddad C, Pinheiro N, Casoy J. Advantages of the association of resveratrol with oral contraceptives for management of endometriosis-related pain. Int J Womens Health. 2012;4:543–549 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Ricci AG, Olivares CN, Bilotas MA, et al. Natural therapies assessment for the treatment of endometriosis. Hum Reprod. 2013;28(1):178–188 [DOI] [PubMed] [Google Scholar]
- 9. Singh M, Parent S, Leblanc V, Asselin E. Resveratrol modulates the expression of PTGS2 and cellular proliferation in the normal rat endometrium in an AKT-dependent manner. Biol Reprod. 2011;84(5):1045–1052 [DOI] [PubMed] [Google Scholar]
- 10. Shirane A, Wada-Hiraike O, Tanikawa M, et al. Regulation of SIRT1 determines initial step of endometrial receptivity by controlling E-cadherin expression. Biochem Biophys Res Commun. 2012;424(3):604–610 [DOI] [PubMed] [Google Scholar]
- 11. Rier S, Foster WG. Environmental dioxins and endometriosis. Toxicol Sci. 2002;70(2):161–170 [DOI] [PubMed] [Google Scholar]
- 12. Schug TT, Janesick A, Blumberg B, Heindel JJ. Endocrine disrupting chemicals and disease susceptibility. J Steroid Biochem Mol Biol. 2011;127(3-5):204–215 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Johnson NP, Hummelshoj L. Consensus on current management of endometriosis. Hum Reprod. 2013;28(11):1552–1568 [DOI] [PubMed] [Google Scholar]
- 14. Janssen EB, Rijkers AC, Hoppenbrouwers K, Meuleman C, D'Hooghe TM. Prevalence of endometriosis diagnosed by laparoscopy in adolescents with dysmenorrhea or chronic pelvic pain: a systematic review. Hum Reprod Update. 2013;19(5):570–582 [DOI] [PubMed] [Google Scholar]
- 15. Burney RO, Talbi S, Hamilton AE, et al. Gene expression analysis of endometrium reveals progesterone resistance and candidate susceptibility genes in women with endometriosis. Endocrinology. 2007;148(8):3814–3826 [DOI] [PubMed] [Google Scholar]
- 16. Young SL, Lessey BA. Progesterone function in human endometrium: clinical perspectives. Semin Reprod Med. 2010;28(1):5–16 [DOI] [PubMed] [Google Scholar]
- 17. Lessey BA, Palomino WA, Apparao KB, Young SL, Lininger RA. Estrogen receptor-alpha (ER-alpha) and defects in uterine receptivity in women. Reprod Biol Endocrinol. 2006;4(suppl 1):S9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Bulun SE, Yang S, Fang Z, Gurates B, Tamura M, Sebastian S. Estrogen production and metabolism in endometriosis. Ann N Y Acad Sci. 2002;955:75–85 [DOI] [PubMed] [Google Scholar]
- 19. Igarashi TM, Bruner-Tran KL, Yeaman GR, et al. Reduced expression of progesterone receptor-B in the endometrium of women with endometriosis and in cocultures of endometrial cells exposed to 2,3,7,8-tetrachlorodibenzo-p-dioxin. Fertil Steril. 2005;84(1):67–74 [DOI] [PubMed] [Google Scholar]
- 20. Bulun SE, Cheng YH, Yin P, et al. Progesterone resistance in endometriosis: link to failure to metabolize estradiol. Mol Cell Endocrinol. 2006;248(1-2):94–103 [DOI] [PubMed] [Google Scholar]
- 21. Fazleabas AT, Brudney A, Chai D, Langoi D, Bulun SE. Steroid receptor and aromatase expression in baboon endometriotic lesions. Fertil Steril. 2003;80(suppl 2):820–827 [DOI] [PubMed] [Google Scholar]
- 22. Bulun SE, Cheng YH, Pavone ME, et al. Estrogen receptor-beta, estrogen receptor-alpha, and progesterone resistance in endometriosis. Semin Reprod Med. 2010;28(1):36–43 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Casper RF, Quesne M, Rogers IM, et al. Resveratrol has antagonist activity on the aryl hydrocarbon receptor: implications for prevention of dioxin toxicity. Mol Pharmacol. 1999;56(4):784–790 [PubMed] [Google Scholar]
- 24. Bhat KP, Pezzuto JM. Resveratrol exhibits cytostatic and antiestrogenic properties with human endometrial adenocarcinoma (Ishikawa) cells. Cancer Res. 2001;61(16):6137–6144 [PubMed] [Google Scholar]
- 25. Greenberg LH, Slayden OD. Human endometriotic xenografts in immunodeficient RAG-2/gamma(c)KO mice. Am J Obstet Gynecol. 2004;190(6):1788–1795 [DOI] [PubMed] [Google Scholar]
- 26. Littlefield BA, Gurpide E, Markiewicz L, McKinley B, Hochberg RB. A simple and sensitive microtiter plate estrogen bioassay based on stimulation of alkaline phosphatase in Ishikawa cells: estrogenic action of delta 5 adrenal steroids. Endocrinology. 1990;127(6):2757–2762 [DOI] [PubMed] [Google Scholar]
- 27. Mo B, Vendrov AE, Palomino WA, DuPont BR, Apparao KB, Lessey BA. ECC-1 cells: a well-differentiated steroid-responsive endometrial cell line with characteristics of luminal epithelium. Biol Reprod. 2006;75(3):387–394 [DOI] [PubMed] [Google Scholar]
- 28. Lovely LP, Appa Rao KB, Gui Y, Lessey BA. Characterization of androgen receptors in a well-differentiated endometrial adenocarcinoma cell line (Ishikawa). J Steroid Biochem Mol Biol. 2000;74(4):235–241 [DOI] [PubMed] [Google Scholar]
- 29. Collins NH, Lessey EC, DuSell CD, et al. Characterization of antiestrogenic activity of the Chinese herb, prunella vulgaris, using in vitro and in vivo (Mouse Xenograft) models. Biol Reprod. 2009;80(2):375–383 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Budwit-Novotny DA, McCarty KS, Cox EB, et al. Immunohistochemical analyses of estrogen receptor in endometrial adenocarcinoma using a monoclonal antibody. Cancer Res. 1986;46(10):5419–5425 [PubMed] [Google Scholar]
- 31. Schmittgen TD, Livak KJ. Analyzing real-time PCR data by the comparative C(T) method. Nat Protoc. 2008;3(6):1101–1108 [DOI] [PubMed] [Google Scholar]
- 32. Tsuchiya Y, Nakajima M, Yokoi T. Cytochrome P450-mediated metabolism of estrogens and its regulation in human. Cancer Lett. 2005;227(2):115–124 [DOI] [PubMed] [Google Scholar]
- 33. Chen ZH, Hurh YJ, Na HK, et al. Resveratrol inhibits TCDD-induced expression of CYP1A1 and CYP1B1 and catechol estrogen-mediated oxidative DNA damage in cultured human mammary epithelial cells. Carcinogenesis. 2004;25(10):2005–2013 [DOI] [PubMed] [Google Scholar]
- 34. Bowers JL, Tyulmenkov VV, Jernigan SC, Klinge CM. Resveratrol acts as a mixed agonist/antagonist for estrogen receptors alpha and beta. Endocrinology. 2000;141(10):3657–3667 [DOI] [PubMed] [Google Scholar]
- 35. Lu R, Serrero G. Resveratrol, a natural product derived from grape, exhibits antiestrogenic activity and inhibits the growth of human breast cancer cells. J Cell Physiol. 1999;179(3):297–304 [DOI] [PubMed] [Google Scholar]
- 36. Kang NH, Hwang KA, Lee HR, Choi DW, Choi KC. Resveratrol regulates the cell viability promoted by 17beta-estradiol or bisphenol A via down-regulation of the cross-talk between estrogen receptor alpha and insulin growth factor-1 receptor in BG-1 ovarian cancer cells. Food Chem Toxicol. 2013;59:373–379 [DOI] [PubMed] [Google Scholar]
- 37. Macpherson L, Matthews J. Inhibition of aryl hydrocarbon receptor-dependent transcription by resveratrol or kaempferol is independent of estrogen receptor alpha expression in human breast cancer cells. Cancer Lett. 2010;299(2):119–129 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Dong WH, Chen JC, He YL, Xu JJ, Mei YA. Resveratrol inhibits Kv2.2 currents through the estrogen receptor GPR30-mediated PKC pathway. Am J Physiol Cell Physiol. 2013;305(5):C547–C557 [DOI] [PubMed] [Google Scholar]
- 39. Gashaw I, Hastings JM, Jackson KS, Winterhager E, Fazleabas AT. Induced endometriosis in the baboon (Papio anubis) increases the expression of the proangiogenic factor CYR61 (CCN1) in eutopic and ectopic endometria. Biol Reprod. 2006;74(6):1060–1066 [DOI] [PubMed] [Google Scholar]
- 40. Vernon MW, Wilson EA. Studies on the surgical induction of endometriosis in the rat. Fertil Steril. 1985;44(5):684–694 [PubMed] [Google Scholar]
- 41. Isaacson KB, Xu Q, Lyttle CR. The effect of estradiol on the production and secretion of complement component 3 by the rat uterus and surgically induced endometriotic tissue. Fertil Steril. 1991;55(2):395–402 [PubMed] [Google Scholar]
- 42. Hull ML, Charnock-Jones DS, Chan CL, et al. Antiangiogenic agents are effective inhibitors of endometriosis. J Clin Endocrinol Metab. 2003;88(6):2889–2899 [DOI] [PubMed] [Google Scholar]
- 43. Bulun SE, Monsavais D, Pavone ME, et al. Role of estrogen receptor-beta in endometriosis. Semin Reprod Med. 2012;30(1):39–45 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Trukhacheva E, Lin Z, Reierstad S, Cheng YH, Milad M, Bulun SE. Estrogen receptor (ER) beta regulates ERalpha expression in stromal cells derived from ovarian endometriosis. J Clin Endocrinol Metab. 2009;94(2):615–622 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Saleh MC, Connell BJ, Saleh TM. Resveratrol induced neuroprotection is mediated via both estrogen receptor subtypes, ER(alpha) and ER(beta). Neurosci Lett. 2013;548:217–221 [DOI] [PubMed] [Google Scholar]
- 46. Robb EL, Stuart JA. Resveratrol interacts with estrogen receptor-beta to inhibit cell replicative growth and enhance stress resistance by upregulating mitochondrial superoxide dismutase. Free Radic Biol Med. 2011;50(7):821–831 [DOI] [PubMed] [Google Scholar]
- 47. Howells LM, Berry DP, Elliott PJ, et al. Phase I randomized, double-blind pilot study of micronized resveratrol (SRT501) in patients with hepatic metastases--safety, pharmacokinetics, and pharmacodynamics. Cancer Prev Res (Phila). 2011;4(9):1419–1425 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Almeida L, Vaz-da-Silva M, Falcao A, et al. Pharmacokinetic and safety profile of trans-resveratrol in a rising multiple-dose study in healthy volunteers. Mol Nutr Food Res. 2009;53(suppl 1):S7–S15 [DOI] [PubMed] [Google Scholar]
- 49. Amiot MJ, Romier B, Dao TM, et al. Optimization of trans-Resveratrol bioavailability for human therapy. Biochimie. 2013;95(6):1233–1238 [DOI] [PubMed] [Google Scholar]
- 50. Johnson JJ, Nihal M, Siddiqui IA, et al. Enhancing the bioavailability of resveratrol by combining it with piperine. Mol Nutr Food Res. 2011;55(8):1169–1176 [DOI] [PMC free article] [PubMed] [Google Scholar]