Abstract
Infertility is a devastating medical condition that adversely affects emotional health and well-being of couples who desire pregnancy and parenthood. The overall demographic data suggest that the indication for more than one-third of assisted reproductive technology cycles performed in the United States includes male factor infertility. There is increasing recognition of the role that peptides present in seminal plasma have in determining sperm motility. Several recent studies suggest that peptidases, such as neutral endopeptidase (NEP) and aminopeptidase N (APN), impose significant adverse effects on sperm motility. Interestingly, several recent studies demonstrate that there is an endogenous NEP/APN inhibitor peptide called opiorphin in human seminal plasma. Our pilot studies suggest opiorphin promotes sperm motility and may positively influence sperm motility parameters in some cases of males infertility characterized by asthenozoospermia.
Keywords: male infertility, seminal peptides, sperm motility
Introduction
Infertility is a devastating medical condition that adversely affects emotional health and well-being of couples who desire pregnancy and parenthood. According to data from the Society for Assisted Reproductive Technology in 2011, 17% and 18% of patients who required assisted reproductive technology (ART) in the United States were diagnosed with male factor infertility and combined female and male factors, respectively.1 In fact, approximately one-third of in vitro fertilization and intracytoplasmic sperm injection cycles performed in the United States in 2011 resulted from male factor infertility.1
The etiology for male factor infertility is multifactorial. Most studies focus on the sperm morphology and motility.2–8 However, ejaculated semen can be divided into 2 major components with differential centrifugation: sperm cells (spermatozoa) and the aqueous phase, which is also known as seminal plasma. Seminal plasma reflects secretions from the testis, epididymis, seminal vesicles, prostate, and bulbourethral glands. It contains proteasomes, proteins and peptides, amino acids, enzymes, fructose and other carbohydrates, lipids, minerals, and trace elements that are essential for the survival of spermatozoa and their successful journey through the female reproductive tract.9
Immotile Sperm are an Important Factor in Male Infertility
A characteristic aspect of sperm is that they are motile. Although sperm morphology, motility, and total concentration are hypothesized to have the strongest correlation with successful pregnancy,5–8 sperm motility may be the most reliable predictor of male factor infertility.10–12 Consistent with this hypothesis, a prospective study designed to evaluate sperm characteristics and the likelihood of pregnancy in infertile couples reported sperm motility and the mean number of morphological abnormalities observed per abnormal spermatozoa (multiple anomalies index) were the best prognostic factors in couples diagnosed with primary infertility. In contrast, age of the male partner and percentage of normal sperm were the best prognostic factors for pregnancy in groups with secondary infertility.6 A univariate analysis by Larsen et al suggested that sperm concentration, total sperm count, and motility were the most significant predictors for fertility. A subsequent multivariate analysis by Larsen et al suggested that total motile spermatozoa was the most clinically relevant semen parameter and was the best predictor of fertility in the general male population.7 A function of seminal plasma is the regulation of sperm motility. Indeed, activation of sperm occurs upon release from the male genital tract and is modified while spermatozoa move through the female reproductive tract. Once deposited inside the female reproductive tract, spermatozoa acquire progressive motility, defined as moving actively, either linearly or in a large circle, regardless of speed,13 which culminates with hyperactive motility of spermatozoa upon arrival to the oviduct.12,14
Seminal Peptides and Male Fertility
Several studies have identified and investigated peptides present in seminal plasma that are involved in sperm motility and thereby fertility.2–4,10,15–20 Although it is recognized that seminal peptides affect sperm motility, the precise role of individual peptides is minimally understood. The rapidly growing number of peptides identified in the seminal plasma peptidome introduces an additional level of challenge for researchers interested in characterizing the role of individual seminal proteins in male fertility.9,21
Seminal plasma contains peptides and proteins that are unique to seminal plasma and others that are also observed in blood plasma. A study in 2004 by Fung et al21 identified more than 100 proteins and peptides in pooled seminal fluid from 5 individuals via mass spectrometry. A subsequent study using the same methodology9 identified 923 proteins and peptides in seminal fluid from a single individual.
The seminal plasma peptidome is comprised of proteins and peptides that originate from the blood plasma by exudation through the lumen of the male genital tract as well as those synthesized and secreted by the testis, epididymis, vas deferens, prostate, seminal vesicle, and bulbourethral glands. Seminal plasma proteins that arise from blood include prealbumin, albumin, globulin, transferrin, α-antitrypsin, β-lipoprotein, β-glycoprotein, orsomucoid, kininogen, peptide hormones, immunoglobulin (Ig) G, IgA, and IgM. These proteins mediate diverse functions in the seminal plasma which include regulation of osmotic pressure and pH and the transport of ions, lipids, and hormones from the testicular vascular structures.22 Peripheral testosterone regulates the biosynthesis and secretion of several proteins in seminal plasma.22 A limited number of studies (Table 1) have linked specific seminal proteins and peptides with sperm motility and physiology.
Table 1.
Major Components of the Seminal Plasma Peptidome.
| Seminal Peptide | Reproductive Affect | Relevant Species |
|---|---|---|
| Bovine seminal plasma proteins32 | Sperm membrane stabilization (decapacitation) and destabilization (capacitation) | Bovine |
| Calmodulin-binding proteins32 | Sperm membrane stabilization (decapacitation) and destabilization (capacitation) | Bovine |
| Spermadhesins33,34 | Binds heparin and involved in capacitation | Boar |
| Seminal plasmin22 | Regulation of calcium transport and positive modulation of the zona pellucida-induced acrosome reaction | Bovine |
| Clusterin35 | Improper spermatogenesis or irregular epididymal maturation | Human |
| Heparin-binding proteins36 | Increases the frequency of zona pellucida-induced acrosome reactions by epididymal sperm incubated with heparin and also improved progressive motility | Buffalo, bovine |
| Fertilization-associated antigen22,37 | Increases pregnancy rates by 15% in females inseminated with FAA-positive sperm compared to FAA-negative sperm | Human |
| Heat shock proteins38 | Downregulated in ejaculated sperm from infertile men with oligoteratozoospermia | Human |
| Osteopontin39,40 | Affects sperm adhesion to oocyte and blocks polyspermy | Bovine |
| Immobilin22 | Inhibits sperm motility in the cauda epididymis by mechanically creating a highly viscous and elastic fluid used to store spermatozoa | Rat |
| Fibronectin, laminin, and vitronectin22 | Extracellular matrix proteins associated with sperm to oocyte binding | Human, murine |
| Inhibin22 | Decreases basal FSH secretion | Human |
| Macrophage migration inhibitory factor22 | Modulates Sertoli cell inhibin production and assists with motility and maturation of sperm flagellum during epididymal transit | Human |
| Cysteine-rich secretory proteins41 | Involved in the late stages of sperm maturation and accounts for approximately 15% of the protein content in epididymal fluid | Murine |
Abbreviations: FAA, fertilization-associated antigen; FSH, follicle-stimulating hormone.
Seminal Peptides and Semen Parameters
Some peptides in seminal fluid are hypothesized to affect sperm parameters. A study in 2007 by Marinoni et al discovered a novel vasoactive peptide, adrenomedullin. Andrenomedullin is a vasorelaxant found in nonvascular tissues, including human ovaries, endometrium, testis, prostate, and seminal fluid.3 Interestingly, adrenomedullin is reported to be higher in infertile men with oligozoospermia compared to normozoospermic men (see definitions in Table 2).3 Dysregulation of macrophage migration inhibitory factor (MIF), a ubiquitous cytokine, adversely affects fertility through effects on human sperm maturation and motility.2 Aljabari et al reported a correlation between MIF levels in human seminal fluid and fertility status. They found an abnormal biphasic profile of MIF in the seminal fluid of patients with impaired sperm parameters. Although high levels of MIF were found in azoospermic semen and low levels of MIF in asthenozoospermic semen, normozoospermic semen had intermediate MIF levels.2 Zenzmaier et al reported human chorionic gonadotropin (hCG) and its free subunits (hCGα and hCGβ) are produced in the male reproductive tract and found in high concentrations in seminal fluid, especially hCGα. Human chorionic gonadotropin-α levels were significantly lower in men with impaired semen quality. Moreover, oligozoospermia was characterized by reduced seminal plasma hCG levels compared with normozoospermic men.4
Table 2.
Medical Definitions for Semen Samples Per WHO 2010.13
| Asthenozoospermia | Percentage of progressively motile spermatozoa <32% (fifth percentile relative to the average fertile male) |
| Azoospermia | Absence of spermatozoa in the ejaculate |
| Necrozoospermia | Absence of live or motile spermatozoa in ejaculate |
| Normozoospermia | Total number of spermatozoa >39 million per ejaculate or concentration >15 million/mL, and percentages of progressively motile (PR) >32% and morphologically normal spermatozoa >4% |
| Oligozoospermia | Total number of spermatozoa <39 million per ejaculate or concentration <15 million/mL |
Abbreviation: WHO, World Health Organization.
Neutral Endopeptidase and Sperm Motility
Seminal plasma neutral endopeptidase (NEP/CD10) and aminopeptidase N (APN/CD13) are hypothesized to adversely affect sperm motility.10 Both APN and NEP modulate motility of capacitated spermatozoa. However, each enzyme appears to control different aspects of sperm motility.10 For example, APN activity is increased in men with asthenozoospermia or necrozoospermia.17 Subiran et al recently reported that both APN and NEP adversely affect motility as well as the trajectory of human capacitated spermatozoa.20 Consistent with the hypothesis that APN and NEP adversely affect sperm motility, Subiran et al recently reported inhibition of APN and NEP with synthetic inhibitors improved sperm motility10; spermatozoa incubated with leuhistin, an APN-specific inhibitor, exhibited asymmetrical movement indicative of the hyperactivation,10 and thiorphan or phosphoramidon, NEP inhibitors, improved human sperm progressive motility. The clinical relevance of these motility semen parameter changes relate to evidence that suggests hyperactivation is necessary for fertilization, and progressive motility is necessary for movement through the female reproductive tract.10,18
Another group of peptides found in seminal plasma that may affect sperm motility include kinins, mainly bradykinin (BK), and other components of the kallikrein-kinin system. Siems et al reported that BK significantly increased sperm motility when used at subnanomolar concentrations.19 This effect was stabilized and even increased by inhibiting BK hydrolysis in semen samples.
The degradation of BK in semen and on washed sperm cells of various species (human, pig, cattle, and sheep) is mainly controlled by 2 peptidases, the angiotensin-converting enzyme (ACE/KII) and NEP. Both NEP and ACE/KII are primarily found in seminal plasma and to a lesser extent in sperm cells.15 Additionally, in human samples, low activity of kininase I (carboxypeptidase N) is also detected and may contribute to BK degradation. It is worth noting that there is considerable interspecies variation in the levels of BK-degrading peptidases, which should be considered in any approach where nonhuman models are used to investigate the potential development of inhibitors designed to maintain the stimulating effect of BK on sperm motility.16 As sperm membrane-bound ACE/KII and NEP are the primary routes of BK hydrolysis, a cocktail of enzyme inhibitors (phosphoramidon-blocking NEP activity and lisinopril-blocking ACE activity) has been suggested to enhance sperm motility in breeding bulls and rams.19
Several recent reports have demonstrated that there is an endogenous NEP/APN inhibitor peptide called opiorphin in seminal plasma.23–27 In rats, sialorphin, the opiorphin homologue, is primarily expressed in the corpus cavernosum, prostate, and the submandibular gland and its expression is under androgenic regulation.23,28 The mean concentration of opiorphin in human semen is 12.9 ± 1.8 ng/mL (mean ± standard error of the mean).29 Of particular interest is the finding that opiorphin is significantly reduced (4.7 ± 1.0 ng/mL, n = 4) in semen of patients with congenital bilateral absence of the vas deferens (CBAVD), a medical condition associated with anomalies of the vas deferens and seminal vesicle.29 This study suggests more than 60% of opiorphin in semen may originate from tissue upstream of the vas deferens and most likely the prostate.29 As one might surmise, CBAVD is characterized by infertility with intact testicular spermatogenesis. However, the percentage of motile sperm is significantly reduced in patients with CBAVD thereby raising the possibility that reduced opiorphin may contribute to this unanticipated clinical finding.30 Several conditions associated with infertility, such as diabetes and erectile dysfunction in aging men, demonstrated reduced opiorphin expression in seminal plasma.25–27 However, the exact role of opiorphins in subfertility as well as infertility has not been investigated.
The lowered expression of opiorphin-like genes in rat models of disease associated with subfertility, the presence of opiorphin in semen, and the ability of synthetic NEP inhibitors to improve sperm motility in humans prompted us to hypothesize that reduced seminal opiorphin contributes to reduced sperm motility in infertile couples. To test this hypothesis, we determined whether opiorphin supplementation would improve sperm motility parameters in male patients seeking fertility assistance. Briefly, we evaluated the sperm concentration, progression, and motility in a group of 10 men presenting to our fertility center for an initial semen analysis as part of a typical infertility evaluation (Table 3). Semen samples were collected and evaluated after 2 to 4 days of sexual abstention. After collection, each semen sample was allowed to liquefy at 37°C for at least 30 minutes. The time from semen production to semen analysis varied from 2 to 6 hours. We subsequently used 2010 World Health Organization guidelines to separate sperm into 3 motility groups: progressive, nonprogressive, and immotile. Spermatozoa concentration and motility were counted in triplicate before and immediately after the addition of NEP inhibitors (opiorphin, phosphoramidon, and thiorphan). Mean values are reported with standard deviations. Although conducted in a small patient population, preliminary results (Figure 1 and Table 4) suggest that opiorphin inhibits seminal NEP and may improve sperm motility parameters. In half of the patients studied, opiorphin increased sperm motility by as much as 87% above baseline values. Of the men who responded to exogenous opiorphins, we observed up to 120% improvement in forward progression. Interestingly, 60% of the men who responded positively to opiorphin were obese, thereby raising the possibility that abnormal opiorphin expression, in part, contributes to obesity-related male subfertility. This would be consistent with androgen regulation of opiorphin and the relative androgen deficiency seen in many obese men.31 Synthetic NEP inhibitors, phosphoramidon and thiorphan, tended to mediate a similar but less robust effect on semen parameters. Overall these preliminary data suggest that the endogenous NEP inhibitor, opiorphin, may offer potential benefit for the treatment of idiopathic male infertility characterized by asthenozoospermia.
Table 3.
Basic Infertility Assessment at Initial Visit Subsequent to History and Physical Examination.
| Female | Male |
|---|---|
| Hysterosalpingogram and/or saline infusion sonogram | Semen analysis |
| Cycle day 2-3 follicle-stimulating hormone, estradiol, and antral follicle count | Genetic assessment as indicated |
| Genetic assessment, thyroid-stimulating hormone, and prolactin as indicated | Gonadal steroids, gonadotropins, prolactin, and inhibin as indicated |
Figure 1.
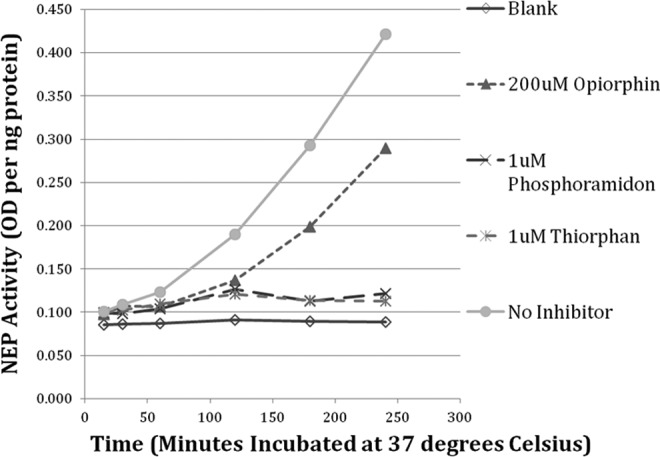
Colorimetric assay for neutral endopeptidase (NEP) activity in seminal plasma over 6 time points (15, 30, 60, 120, 180, and 240 minutes incubated at 37°C) at baseline and with addition of NEP inhibitors.
Table 4.
Preliminary Data From the Initial Semen Specimens Collected From 10 Male Patients Undergoing a Basic Infertility Evaluation.a
| Samples | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 |
|---|---|---|---|---|---|---|---|---|---|---|
| Age, years | 31 | 39 | 28 | 32 | 55 | 38 | 27 | 45 | 41 | 39 |
| BMI, kg/m2 | 25.1 | 23.7 | 37.5 | 28.7 | n/a | 33.9 | 24.8 | 33.4 | 25.8 | 34.9 |
| No inhibitor | ||||||||||
| Concentration, million/mL | 8.9 ± 0.3 | 183.3 ± 15.3 | 82.7 ± 14.0 | 59.3 ± 2.1 | 85.0 ± 9.2 | 13.0 ± 2.6 | 6.3 ± 1.5 | 20.7 ± 3.1 | 64.0 ± 14.9 | 147.3± 9.0 |
| Motility, % | 11.3 ± 3.9 | 40.1 ± 3.1 | 25.0 ± 6.3 | 25.3 ± 0.9 | 53.1 ± 8.3 | 58.0 ± 12.5 | 39.4 ± 18.3 | 51.2 ± 12.3 | 24.5 ± 6.2 | 28.7 ± 5.1 |
| Forward Progression, % | 6.8 ± 0.2 | 29.0 ± 1.8 | 6.5 ± 0.6 | 16.3 ± 0.5 | 45.7 ± 9.5 | 45.0 ± 13.6 | 29.7 ± 26.3 | 18.9 ± 5.4 | 10.3 ± 3.2 | 16.1 ± 3.1 |
| 200 µmol/L opiorphin | ||||||||||
| Motility (fold change from baseline) | 0.74 | 0.99 | 1.43 | 1.06 | 0.86 | 1.33 | 1.27 | 1.12 | 1.38 | 1.87 |
| Forward progression (fold change from baseline) | 0.00 | 1.00 | 1.43 | 1.06 | 0.84 | 1.51 | 0.84 | 2.19 | 2.12 | 2.20 |
| 1 µmol/L phosphoramidon | ||||||||||
| Motility (fold change from baseline) | 0.15 | 1.00 | 1.12 | 0.83 | 1.00 | 0.78 | 0.87 | 1.16 | 0.85 | 1.60 |
| Forward Progression (fold change from baseline) | 0.00 | 1.04 | 1.71 | 0.67 | 0.97 | 0.75 | 0.88 | 2.49 | 0.72 | 1.88 |
| 1 µmol/L thiorphan | ||||||||||
| Motility (fold change from baseline) | 0.18 | 0.85 | 1.12 | 0.77 | 0.95 | 1.33 | 1.04 | 1.31 | 1.58 | 1.72 |
| Forward Progression (fold change from baseline) | 0.00 | 0.88 | 2.03 | 0.31 | 0.94 | 1.50 | 0.51 | 1.93 | 1.58 | 1.84 |
Abbreviations: PR, progressive motility; NP, nonprogressive motility; IM, immotile; SD, standard deviation.
a Motility = (PR + NP)/(PR + NP + IM). Forward progression = PR/(PR + NP + IM). Baseline data reported as mean ± SD. Data for inhibitors presented as fold change relative to baseline values. Increased motility and forward progression > 15% from baseline are highlighted in gray. This indicates >1 SD increase from the mean motility and forward progression.
Conclusion
A variety of peptides in seminal fluid appear to have a role in sperm motility. Nonetheless, there are significant gaps in our knowledge regarding their specific roles in male infertility. Of particular interest is the recent finding that opiorphin, an endogenous NEP inhibitor, is present in seminal plasma. We provide original preliminary data that suggests opiorphin supplementation in semen samples from a subgroup of men with asthenozoospermia improves sperm motility parameters. The importance of seminal peptides in male fertility is an area ripe for investigation. Moreover, understanding the clinical importance of various seminal peptides in male fertility may provide an opportunity to develop novel pharmacotherapy intended to enhance fertility or provide contraceptive benefit.
Footnotes
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: The work was supported in part by grant R01DK087872 from the NIH/NIDDK awarded to Kelvin P. Davies. The NIH/NIDDK grant supported research described in the paper; NIH/NIDDK has no commercial interests in potential products developed as a result of this contribution.
References
- 1. SART: IVF Success Rates. https://www.sartcorsonline.com/rptCSR_PublicMultYear.aspx?ClinicPKID=0. Accessed July 8, 2013
- 2. Aljabari B, Calogero AE, Perdichizzi A, et al. Imbalance in seminal fluid MIF indicates male infertility. Mol Med. 2007;13(3-4):199–202 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Marinoni E, Vellucci O, Letizia C, Sessa M, Moscarini M, Di Iorio R. The level of adrenomedullin immunoreactivity in seminal fluid is higher in oligozoospermic subjects and correlates with semen biochemical parameters. Eur J Obstet Gynecol Reprod Biol. 2007;131(2):169–175 [DOI] [PubMed] [Google Scholar]
- 4. Zenzmaier C, Gerth R, Gruschwitz M, Lindner H, Plas E, Berger P. Decreased levels of genuine large free hCG alpha in men presenting with abnormal semen analysis. Reprod Biol Endocrinol. 2011;9:114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Jouannet P, Ducot B, Feneux D, Spira A. Male factors and the likelihood of pregnancy in infertile couples. I. Study of sperm characteristics. Int J Androl. 1988;11(5):379–394 [DOI] [PubMed] [Google Scholar]
- 6. Ducot B, Spira A, Feneux D, Jouannet P. Male factors and the likelihood of pregnancy in infertile couples. II. Study of clinical characteristics--practical consequences. Int J Androl. 1988;11:395–404 [DOI] [PubMed] [Google Scholar]
- 7. Larsen L, Scheike T, Jensen TK, et al. Computer-assisted semen analysis parameters as predictors for fertility of men from the general population. The danish first pregnancy planner study team. Hum Reprod. 2000;15(7):1562–1567 [DOI] [PubMed] [Google Scholar]
- 8. Zinaman MJ, Brown CC, Selevan SG, Clegg ED. Semen quality and human fertility: a prospective study with healthy couples. J Androl. 2000;21(1):145–153 [PubMed] [Google Scholar]
- 9. Pilch B, Mann M. Large-scale and high-confidence proteomic analysis of human seminal plasma. Genome biology. 2006;7(5):R40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Subiran N, Pinto FM, Agirregoitia E, Candenas L, Irazusta J. Control of APN/CD13 and NEP/CD10 on sperm motility. Asian J Androl. 2010;12(6):899–902 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Quill TA, Wang D, Garbers DL. Insights into sperm cell motility signaling through sNHE and the CatSpers. Mol Cellular Endocrinol. 2006;250(1-2):84–92 [DOI] [PubMed] [Google Scholar]
- 12. Yoshida M, Kawano N, Yoshida K. Control of sperm motility and fertility: diverse factors and common mechanisms. Cell Mol Life Sci. 2008;65(21):3446–3457 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. World Health Organization. WHO laboratory manual for the Examination and processingof human semen Fifth Edition. Geneva: World Health Organization; 2010 [Google Scholar]
- 14. Turner RM. Moving to the beat: a review of mammalian sperm motility regulation. Reprod Fertil Dev. 2006;18(1-2):25–38 [DOI] [PubMed] [Google Scholar]
- 15. Boettger A, Kertscher U, Steinmann C, Baeger U, Siems WE, Heder G. Degradation of bradykinin in semen of ram and boar. Biochem Pharmacol. 1993;45(10):1983–1988 [DOI] [PubMed] [Google Scholar]
- 16. Heder G, Bottger A, Siems WE, Rottmann M, Kertscher U. The enzymatic degradation of bradykinin in semen of various species. Andrologia. 1994;26(5):295–301 [DOI] [PubMed] [Google Scholar]
- 17. Irazusta J, Valdivia A, Fernandez D, Agirregoitia E, Ochoa C, Casis L. Enkephalin-degrading enzymes in normal and subfertile human semen. J Androl. 2004;25(5):733–739 [DOI] [PubMed] [Google Scholar]
- 18. Pinto FM, Ravina CG, Subiran N, et al. Autocrine regulation of human sperm motility by tachykinins. Reprod Biol Endocrinol. 2010;8:104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Siems WE, Maul B, Wiesner B, et al. Effects of kinins on mammalian spermatozoa and the impact of peptidolytic enzymes. Andrologia. 2003;35(1):44–54 [DOI] [PubMed] [Google Scholar]
- 20. Subiran N, Agirregoitia E, Valdivia A, Ochoa C, Casis L, Irazusta J. Expression of enkephalin-degrading enzymes in human semen and implications for sperm motility. Fertil Steril. 2008;89(5 suppl):1571–1577 [DOI] [PubMed] [Google Scholar]
- 21. Fung KY, Glode LM, Green S, Duncan MW. A comprehensive characterization of the peptide and protein constituents of human seminal fluid. Prostate. 2004;61(2):171–181 [DOI] [PubMed] [Google Scholar]
- 22. Seminal Plasma Proteins. http://precedings.nature.com/documents/7001/version/1. Accessed July 8, 2013
- 23. Rougeot C, Messaoudi M, Hermitte V, et al. Sialorphin, a natural inhibitor of rat membrane-bound neutral endopeptidase that displays analgesic activity. Proc Natl Acad Sci U S A. 2003;100(14):8549–8554 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Calenda G, Tong Y, Tar M, et al. Vcsa1 acts as a marker of erectile function recovery after gene therapeutic and pharmacological interventions. J Urol. 2009;181(6):2806–2815 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Tong Y, Tar M, Davelman F, Christ G, Melman A, Davies KP. Variable coding sequence protein A1 as a marker for erectile dysfunction. BJU Int. 2006;98(2):396–401 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Tong Y, Tar M, Melman A, Davies K. The opiorphin gene (ProL1) and its homologues function in erectile physiology. BJU Int. 2008;102(6):736–740 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Tong Y, Tar M, Monrose V, DiSanto M, Melman A, Davies KP. hSMR3A as a marker for patients with erectile dysfunction. J Urol. 2007;178(1):338–343 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Chua RG, Calenda G, Zhang X, et al. Testosterone regulates erectile function and Vcsa1 expression in the corpora of rats. Mol Cell Endocrinol. 2009;303(1-2):67–73 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Dufour E, Villard SS, Mellon V, et al. Opiorphin secretion pattern in healthy volunteers: gender difference and organ specificity. Biochem Anal Biochem. 2013; 2(3):2–11 [Google Scholar]
- 30. Qiu Y, Yang DT, Wang SM, Sun HQ, Jia YF. Successful pregnancy and birth after intrauterine insemination using caput epididymal sperm by percutaneous aspiration. Asian J Androl. 2003;5(1):73–75 [PubMed] [Google Scholar]
- 31. Guay AT, Traish A. Testosterone deficiency and risk factors in the metabolic syndrome: implications for erectile dysfunction. Urol Clin North Am. 2011;38(2):175–183 [DOI] [PubMed] [Google Scholar]
- 32. Srivastava N, Jerome A, Srivastava SK, Ghosh SK, Kumar A. Bovine seminal PDC-109 protein: an overview of biochemical and functional properties[published online February 22, 2013]. Animal Reprod Sci. 2013 [DOI] [PubMed] [Google Scholar]
- 33. Petrunkina AM, Harrison RA, Topfer-Petersen E. Only low levels of spermadhesin AWN are detectable on the surface of live ejaculated boar spermatozoa. Reprod Fertil Dev. 2000;12(7-8):361–371 [DOI] [PubMed] [Google Scholar]
- 34. Topfer-Petersen E, Calvete JJ. Sperm-associated protein candidates for primary zona pellucida-binding molecules: structure-function correlations of boar spermadhesins. J Reproduction Fertil Suppl. 1996;50:55–61 [PubMed] [Google Scholar]
- 35. Zalata A, El-Samanoudy AZ, Shaalan D, El-Baiomy Y, Taymour M, Mostafa T. Seminal clusterin gene expression associated with seminal variables in fertile and infertile men. J Urol. 2012;188(4):1260–1264 [DOI] [PubMed] [Google Scholar]
- 36. Kumar A, Singh LP, Harshan HM, Majumdar AC. Seminal plasma non-heparin binding proteins (NHBP) reduce the cryoinjury to buffalo cauda epididymal spermatozoa induced by heparin binding proteins (HBP). Animal Reprod Sci. 2008;104(2-4):220–226 [DOI] [PubMed] [Google Scholar]
- 37. Tsujimura A, Shida K, Kitamura M, et al. Molecular cloning of a murine homologue of membrane cofactor protein (CD46): preferential expression in testicular germ cells. Biochem J. 1998;330(pt 1):163–168 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Cedenho AP, Lima SB, Cenedeze MA, Spaine DM, Ortiz V, Oehninger S. Oligozoospermia and heat-shock protein expression in ejaculated spermatozoa. Hum Reprod. 2006;21(7):1791–1794 [DOI] [PubMed] [Google Scholar]
- 39. Goncalves RF, Wolinetz CD, Killian GJ. Influence of arginine-glycine-aspartic acid (RGD), integrins (alphaV and alpha5) and osteopontin on bovine sperm-egg binding, and fertilization in vitro. Theriogenology. 2007;67(3):468–474 [DOI] [PubMed] [Google Scholar]
- 40. Erikson DW, Way AL, Chapman DA, Killian GJ. Detection of osteopontin on Holstein bull spermatozoa, in cauda epididymal fluid and testis homogenates, and its potential role in bovine fertilization. Reproduction (Cambridge, England). 2007;133(5):909–917 [DOI] [PubMed] [Google Scholar]
- 41. Turunen HT, Sipila P, Krutskikh A, et al. Loss of cysteine-rich secretory protein 4 (Crisp4) leads to deficiency in sperm-zona pellucida interaction in mice. Biol Reprod. 2012;86(1):1–8 [DOI] [PubMed] [Google Scholar]


