Abstract
This randomized double-blind study, with 24-week treatment and 24-week posttreatment periods, evaluated the effects of elagolix (150 mg every day, 75 mg twice a day) versus subcutaneous depot medroxyprogesterone acetate (DMPA-SC) on bone mineral density (BMD), in women with endometriosis-associated pain (n = 252). All treatments induced minimal mean changes from baseline in BMD at week 24 (elagolix 150 mg: −0.11%/−0.47%, elagolix 75 mg: −1.29%/−1.2%, and DMPA-SC: 0.99%/−1.29% in the spine and total hip, respectively), with similar or less changes at week 48 (posttreatment). Elagolix was associated with improvements in endometriosis-associated pain, assessed with composite pelvic signs and symptoms score (CPSSS) and visual analogue scale, including statistical noninferiority to DMPA-SC in dysmenorrhea and nonmenstrual pelvic pain components of the CPSSS. The most common adverse events (AEs) in elagolix groups were headache, nausea, and nasopharyngitis, whereas the most common AEs in the DMPA-SC group were headache, nausea, upper respiratory tract infection, and mood swings. This study showed that similar to DMPA-SC, elagolix treatment had minimal impact on BMD over a 24-week period and demonstrated similar efficacy on endometriosis-associated pain.
Keywords: elagolix, GnRH antagonists, endometriosis, pelvic pain
Introduction
Endometriosis is an estrogen-dependent disease that affects 5% to 10% of women of reproductive age.1 Available treatment options include nonsteroidal anti-inflammatory drugs, oral contraceptives, and high-dose progestins including subcutaneous depot medroxyprogesterone acetate (DMPA-SC) and gonadotropin-releasing hormone (GnRH) agonists. Both GnRH agonists and progestins have proven efficacy for the treatment of endometriosis. However, patients receiving progestin therapy may experience an increase in bothersome symptoms including irregular breakthrough bleeding, mood changes, and breast tenderness,2–4 and add-back therapy is recommended with the use of GnRH agonists to avoid unacceptable hypoestrogenic side effects, including progressive bone loss.5–7
Elagolix is a short-acting, nonpeptide, GnRH antagonist, administered orally, that unlike injectable depot GnRH agonists and antagonists, produces a dose-dependent suppression of ovarian estrogen production, that is, from partial suppression at lower doses to full suppression at higher doses.8,9 This attribute may provide reduction in endometriosis-associated pain, while minimizing the hypoestrogenic side effects that limit long-term treatment with GnRH agonists. In addition, oral administration and a short half-life (˜6 hours) allows for rapid elimination of elagolix from the body, if treatment needs to be discontinued for any reason.
The primary objective of the study was to assess the effects of elagolix versus DMPA-SC on bone mineral density (BMD) during treatment for 24 weeks with a subsequent 24-week posttreatment period. The reason DMPA-SC was selected as an active comparator was 2-fold: (1) due to its established efficacy as a treatment for endometriosis-associated pain, which is comparable to that of leuprolide acetate2,3 and (2) its well-characterized effects on BMD. The DMPA-SC use in premenopausal women results in a modest, but progressive, decrease in BMD that is reversible after treatment is stopped, and thus it was also used as a positive control to guarantee assay sensitivity to detect even subtle bone changes on- and off-treatment.10–15
In phase 1 studies, elagolix was shown to suppress estradiol (E2) production in a dose-dependent manner.8,9 Two doses of elagolix were administered in the current study. The 150 mg every day dose was selected because in preliminary studies, it demonstrated efficacy on endometriosis-associated pain versus placebo (Neurocrine Bioscience data on file) at partially suppressed E2 levels. The 75 mg twice a day dose was also evaluated because preliminary studies suggested that twice a day dosing might be more effective at reducing estradiol than every day dosing,9 which could potentially translate to higher efficacy.
Materials and Methods
Study Design
This was a phase 2, randomized, multicenter, double-blind, and active-controlled study that was conducted from December 2006 to November 2008 at 78 US centers. The study consisted of a 24-week double-blind treatment period and a subsequent 24-week posttreatment follow-up period. During the treatment period, patients were randomized 1:1:1 to elagolix 150 mg every day, elagolix 75 mg twice a day, or DMPA-SC 104 mg/0.65 mL (subcutaneous injection at weeks 1 and 12).
Patients
Eligible patients were women aged 18 to 49 years with a laparoscopically documented diagnosis of endometriosis within 7 years of screening and a total composite pelvic signs and symptoms score (CPSSS, based on a Biberoglu and Behrman scale16) ≥6 with a dysmenorrhea score and a nonmenstrual pelvic pain score of at least moderate (≥2) at screening and baseline and with at least 7 days of electronic diary (e-Diary) entries prior to randomization. The daily e-diary was used to record the visual analog scale (VAS) score for pelvic pain, bleeding diary, and the occurrence of hot flashes. Patients also had to agree to use 2 forms of nonhormonal contraception (eg, condom with spermicide) throughout the 48-week study. Patients were excluded if they had been administered a GnRH agonist or antagonist, danazol, or DMPA within 12 months of screening and had a history of unresponsiveness to GnRH agonist or antagonist therapy. In addition, patients having a BMD with either lumbar spine of femur T-scores below −1.5 at screening, as determined by dual-energy x-ray absorptiometry (DXA), were excluded. Patients were excluded if they had used oral contraceptives or other oral hormonal therapy within 1 month of screening.
Randomization and Blinding
Patients were randomized using an interactive voice response system. Treatment assignments were made according to a computer-generated randomization schedule with assignments occurring immediately before the first dose of study drug. Study drug was initiated within 2 to 7 days after the onset of menstrual bleeding. Patients, the investigator, and study personnel (with the exception of the unblinded person who administered DMPA-SC) were blinded to the patient’s treatment assignment throughout the study. Blinding was achieved by using a double-dummy design. All patients received elagolix and/or matching placebo as 2 identical tablets to be taken in the morning and evening. In addition, all patients received DMPA-SC or saline solution as a subcutaneous injection at the beginning of week 1 and at the end of week 12. Patients, the investigator, and all study personnel remained blinded to the patient’s treatment allocation during the posttreatment follow-up period. After all patients had completed the week 24 assessments, the study sponsor was unblinded to individual patient data.
Changes in BMD
The primary end point in this study was the percentage change from baseline in BMD for the spine and femur (total hip) at week 24. The BMD changes at other timepoints (weeks 12 and 48) and reversibility of changes in BMD following the discontinuation of treatment at weeks 48 and 72 were included as secondary end points. Bone mineral density of the spine and femur was measured by DXA in duplicate at screening and week 24 and single scans at weeks 12 and 48 (or early termination). A central laboratory (Bio-Imaging Technologies Inc, Newtown, Pennsylvania) was used to analyze all DXA scans.
Pelvic Pain and Quality-of-Life Assessments
Secondary objectives also included efficacy assessments demonstrating noninferiority of at least 1 dose regimen of elagolix to DMPA-SC in the reduction of the dysmenorrhea and nonmenstrual pelvic pain components of the CPSSS.
The CPSSS, based on the Biberoglu and Behrman scale, with 5 components addressing dysmenorrhea, dyspareunia, nonmenstrual pelvic pain, pelvic tenderness, and pelvic induration was assessed at screening, baseline, and at the end of weeks 4, 8, 12, 16, 20, and 24 and during follow-up weeks 28, 36, and 48 or early termination. Each component was scored on a scale of 0 to 3 (0 = absent, 1 = mild, 2 = moderate, and 3 = severe).
Additional secondary objectives included the changes from baseline in pelvic pain using VAS and the use of analgesics during the study. The VAS for pelvic pain, with a horizontal line on which the left extreme was labeled “no pain” and the right extreme was labeled “worst pain ever felt,” was used to monitor pain daily. The VAS was scored on a scale of 0 (no pain) to 100 (worst pain ever felt). Patients indicated the worst level of pain felt over a 24-hour period by “ticking” the horizontal line on their e-Diary at approximately the same time each day. Monthly mean VAS values were calculated from e-Diary data (a month was equal to the scheduled interval between visits, which was approximately 28 days). Analgesic use was collected as part of concomitant medications on a case report form that was administered at screening and at each scheduled visit.
Quality of life was assessed using the endometriosis health profile 5 (EHP-5)17 core questionnaire with 5 categories (pain, control and powerlessness, emotional well-being, social support, and self-image). Responses were scored on a 0 to 100 scale (0 = never, 25 = rarely, 50 = sometimes, 75 = often, and 100 = always).
Safety and Laboratory Parameters
Standard safety and tolerability assessments were evaluated as secondary end points. Blood samples for E2 measurements were collected at baseline and at the end of weeks 4, 8, 12, 16, 20, and 24 and during follow-up at weeks 28, 32, 36, 40, 44, and 48 (or early termination). Estradiol was measured by a highly sensitive and specific liquid chromatography with tandem mass spectrometry method (MDS Pharma Services, Lincoln, Nebraska). The lower limit of quantitation was 2.5 pg/mL, and the interday precision was ≤6.0% and the accuracy ranged from 5.6% to 0.3%. The description of the method used for N-telopeptide measurement is presented in the Supplemental Material (available at http://rs.sagepub.com/supplemental). Standard safety clinical laboratory assessments were performed by a central laboratory (ICON Laboratories, Farmingdale, New York).
Uterine Bleeding and Hot Flash Assessments
Patients recorded the presence and intensity of their menstrual bleeding (light, moderate, or heavy) in their daily e-Diary throughout the 48-week study. In addition, start dates for the first and second posttreatment menses were collected during posttreatment study visits. The number and intensity (mild, moderate, or severe) of hot flashes were also recorded using the daily e-Diary.
Statistical Methods
The planned study sample size of 240 patients (n = 80 per group) provided an 80% probability that the lower bound of the 2-sided 95% confidence interval (CI) around the treatment group mean percentage change from baseline in BMD was ≥−2.2% for BMD evaluations at week 24 (primary end point) and week 48. This calculation was based on an assumed common standard deviation of 2.5% and 3.2% for the spine and femur BMD percentage change from baseline, respectively, and an assumed mean BMD percentage change from baseline of −1.2% and −0.5% for the spine and femur, respectively, at weeks 24 and 48. The −2.2% mean percentage change from baseline in BMD was selected to reflect recommendations from the Food and Drug Administration (FDA; internal communication).
The safety analysis set included all patients who received at least 1 dose of study drug. Adverse events (AEs) and discontinuations due to AEs were summarized by the treatment group. The change from baseline in laboratory values, vital sign values, and electrocardiogram (ECG) was summarized by the treatment group.
The intent-to-treat (ITT) analysis set included all randomized patients who received at least 1 oral dose of study drug, an initial subcutaneous injection, and either reported at least 7 e-Diary VAS values or provided an assessment of either dysmenorrhea or nonmenstrual pelvic pain at week 4 or later during the treatment phase. Descriptive statistics were calculated for the change from baseline in the monthly mean VAS. Descriptive statistics were calculated for the EHP-5 category scores.
The percentage change from baseline in spine and femur BMD at week 24 (primary end point) was assessed using a 1-way analysis of variance model. The absence of significant bone loss was supported if the lower bounds of the CIs for the mean percentage change in BMD were ≥−2.2% for both the spine and femur at week 24, which was selected to reflect recommendations from the FDA (internal communication).
Changes from baseline in total CPSSS and individual components were summarized with descriptive statistics. The treatment period change from baseline data was analyzed using a mixed-effects repeated measures (MERMs) analysis of covariance model, which included fixed effects for treatment, time, treatment-by-time interaction, a random effect for patient, and terms for baseline value and the baseline-by-time interaction. Least square (LS) means were calculated from each MERM model.
A responder analysis using the dysmenorrhea and nonmenstrual pelvic pain components of the CPSSS as variables was performed. Patients were classified as responders if they reported a ≥1 point reduction from baseline. The results from the week 24 responder analysis were used to establish whether either or both elagolix dosing regimens were noninferior to DMPA-SC for efficacy. Statistical noninferiority was defined when the lower bound of the 95% 2-sided CI for the difference between an elagolix dose and DMPA-SC in the response rate was no less than −20% at week 24 for both dysmenorrhea and nonmenstrual pelvic pain.
Serum E2 concentrations were summarized at each scheduled visit by the treatment group using descriptive statistics. The number and percentage of patients using any analgesic were identified by a manual review of listings of concomitant medications and summarized by the treatment group. The statistical methods for the evaluation of changes in N-telopeptide concentrations, monthly mean VAS, and EHP-5 are included in the Supplemental Material.
Results
The flow of patients through the trial is shown in Supplemental Figure 1. A total of 252 patients were randomized; 84 patients each received elagolix 150 mg every day, elagolix 75 mg twice a day, or DMPA-SC 104 mg/0.65 mL every day 12 weeks. Overall, 56 patients in the elagolix 150 mg every day group, 62 patients in the elagolix 75 mg twice a day group, and 51 patients in the DMPA-SC group completed the treatment period. The entire study, including week 48 DXA scan, was completed by 32 patients in the elagolix 150 mg every day group, 54 patients in the elagolix 75 mg twice a day group, and 37 patients in the DMPA-SC group.
Demographics and baseline characteristics were similar in all 3 groups (Table 1). The mean age of the study population was 31.6 years, mean BMI was 26.0 kg/m2, and the average number of months since surgical diagnosis of endometriosis was 53.0.
Table 1.
Demographics and Baseline Characteristics.
| Variable | Elagolix 150 mg Every Day (N = 84) | Elagolix 75 mg Twice a Day (N = 84) | DMPA-SC |
|---|---|---|---|
| Age, mean (SEM), years | 32.4 (0.8) | 31.4 (0.7) | 31.6 (0.4) |
| Race, n (%) | |||
| Caucasian | 68 (81.0) | 70 (83.3) | 65 (77.4) |
| African American | 6 (7.1) | 10 (11.9) | 10 (11.9) |
| Hispanic | 8 (9.5) | 4 (4.8) | 6 (7.1) |
| Other | 2 (2.4) | 0 (0.0) | 3 (3.6) |
| Weight, mean (SEM), kg | 72.1 (1.7) | 68.9 (1.5) | 70.9 (1.6) |
| Body mass index, mean (SEM), kg/m2 | 26.5 (0.5) | 25.4 (0.5) | 26.2 (0.5) |
| Length of screening phase menstrual cycle,a mean (SEM), days | 29.4 (0.8) | 28.3 (0.4) | 29.4 (0.6) |
| Stage of endometriosis,b,c n (%) | |||
| I | 21 (25.0) | 15 (17.9) | 23 (27.4) |
| II | 18 (21.4) | 20 (23.8) | 26 (31.0) |
| III | 17 (20.2) | 33 (39.3) | 22 (26.2) |
| IV | 15 (17.9) | 5 (6.0) | 6 (7.1) |
| Unknown | 13 (15.5) | 11 (13.1) | 7 (8.3) |
| Months since diagnosis of endometriosis,d mean (SEM) | 53.4 (5.5) | 54.1 (6.1) | 51.6 (5.3) |
Abbreviations: DMPA-SC, subcutaneous depot medroxyprogesterone acetate; SEM, standard error of the mean.
aThe number of days from the start date of the menses before screening to the start date of the menses before first dose.
bStage of endometriosis: 1: minimal, few, or superficial implants are evident; II: mild, more implants, and deeper involvement; III: moderate, more implants, with ovaries affected, and the presence of adhesions; IV: severe, as stage III but with multiple and more dense adhesions (based on the Revised American Society of Reproductive Medicine [ASRM] Classification of Endometriosis, 1996).
cStage and depth of endometriosis are based on evaluation at the time of laparoscopy and not at baseline.
dNumber of months relative to the date of informed consent.
Bone Mineral Density and N-Telopeptide
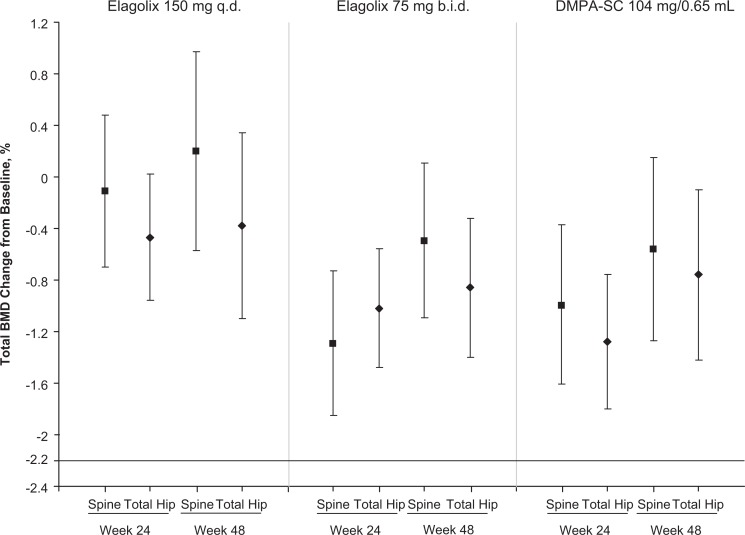
The mean percentage change from baseline and 95% CIs for the spine and total hip at weeks 24 and 48 are shown in Figure 1. For the primary end point, both doses of elagolix (150 mg every day and 75 mg twice a day) were comparable to DMPA-SC. In patients who received elagolix 150 mg every day, the mean percentage change from baseline (95% CI) in BMD was −0.11% (−0.70-0.48) for the spine and −0.47% (−0.96-0.02) for the femur. In patients who received elagolix 75 mg twice a day, the mean change from baseline in BMD was −1.29% (−1.85 to −0.74) for the spine and −1.02% (−1.48 to −0.56) for the femur. For the DMPA-SC group, the mean change from baseline at week 24 was −0.99% (−1.61 to −0.37) for the spine and −1.29% (−1.80 to −0.77) for the femur. The lower bounds of the 95% CIs were ≥−2.2% for the spine and femur for both elagolix treatment and DMPA groups at week 24 and week 48 (Figure 1). Minimal changes from baseline in blood concentrations of N-telopeptide were observed in all treatment groups (see Supplemental Material).
Figure 1.
Bone mineral density least square (LS) mean change from baseline ± 95% confidence interval at weeks 24 and 48.
Estradiol Concentrations
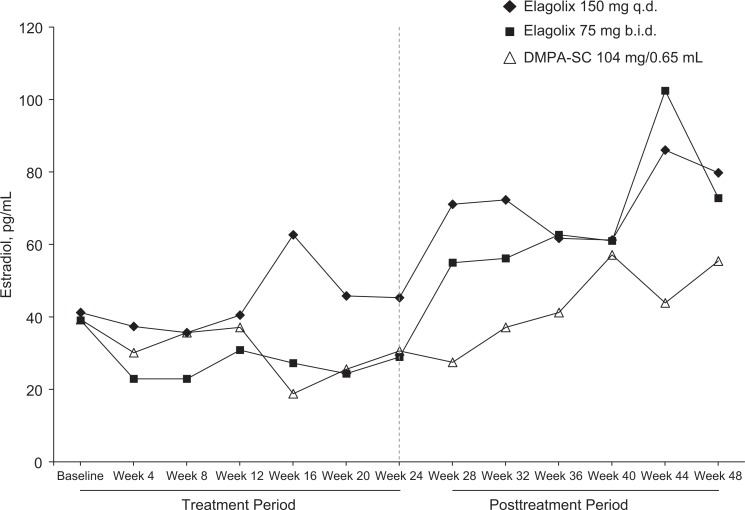
Median E2 levels throughout the study are plotted in Figure 2. Baseline estradiol, which was measured within 2 to 7 days after the onset of menses, was comparable in all 3 treatment groups (41.1, 39.1, and 39.3 pg/mL in the elagolix 150 mg every day, elagolix 75 mg twice a day, and DMPA-SC groups, respectively). During the treatment period, (from weeks 4 to 24), median E2 concentrations ranged from 36 to 63 pg/mL in the elagolix 150 mg every day group, from 23 to 31 pg/mL in the elagolix 75 mg twice a day group, and from 19 to 37 pg/mL in the DMPA-SC group. After withdrawal of study drug (weeks 28-48), median E2 concentrations increased starting at week 28 in the elagolix groups consistent with a return of normal menstrual cycles. In the DMPA-SC group, E2 levels increase more slowly starting at week 32 compared to the elagolix groups.
Figure 2.
Median estradiol concentrations through week 48.
Pain Assessments and Analgesic Use
Overall, the LS mean change (standard error of the mean) from baseline for the total CPSSS at week 24 was −5.5 ± 0.34, −5.2 ± 0.32, and −5.3 ± 0.36 for the elagolix 150 mg every day, elagolix 75 mg twice a day, and DMPA-SC groups, respectively. In all 3 treatment groups, clinically meaningful (defined per protocol as a reduction in mean value from baseline of at least 4 points) and statistically significant improvements over baseline were observed.
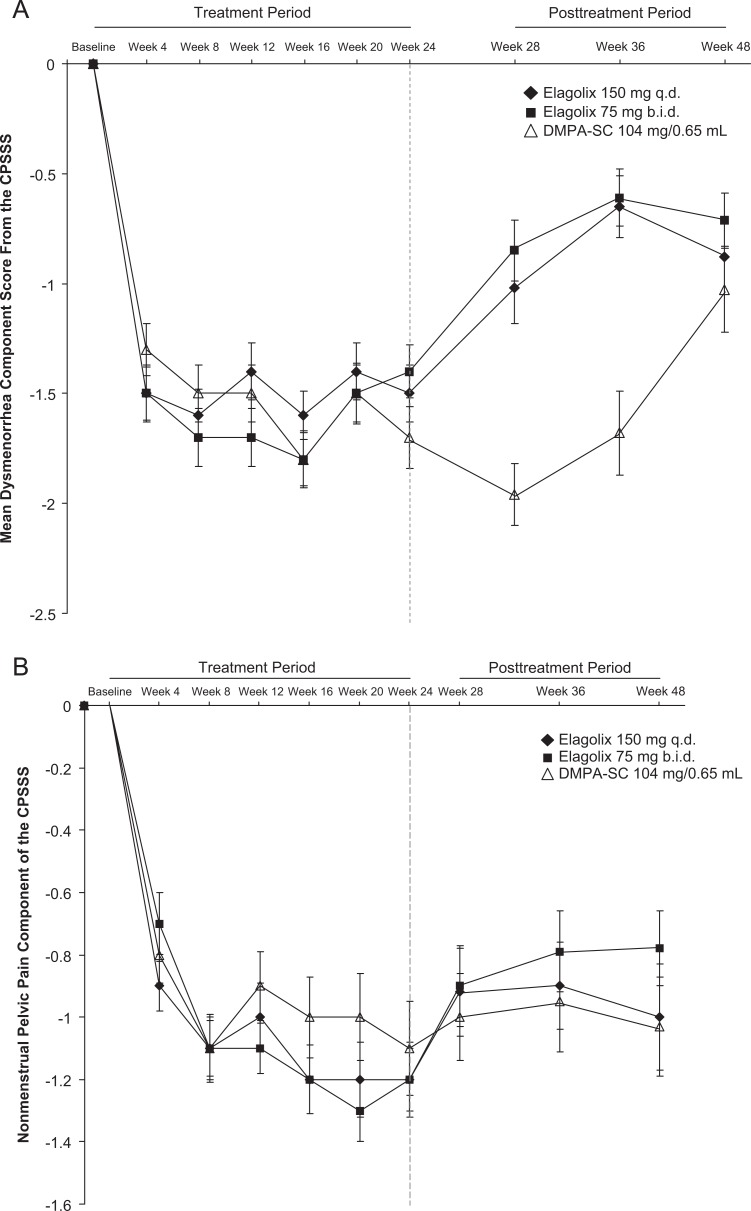
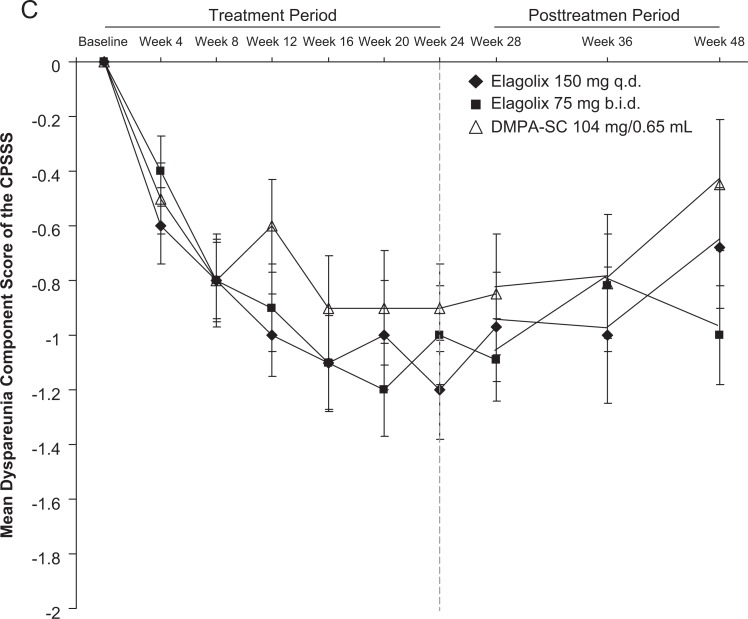
The mean changes from baseline for the dysmenorrhea, nonmenstrual pelvic pain, and dyspareunia components of the CPSSS are shown in Figure 3. For mean dysmenorrhea, onset of improvement (achieving a greater than 1-point improvement) was rapid, occurring by week 4, for all 3 groups. After withdrawal of study drug, dysmenorrhea rapidly returned toward baseline, but remained reduced compared with baseline at week 48. For mean nonmenstrual pelvic pain score, all groups reached onset of improvement by week 8. After withdrawal of study drug, mean improvements in all groups were maintained through week 48. For mean dyspareunia score, onset of improvement was slower, by week 12 for the 150 mg every day group and week 16 for the 75 mg twice a day group. Patients in the DMPA-SC group achieved ≤0.9 point mean reduction. After withdrawal of study drug, return toward baseline, mean improvements maintained through week 36 in all groups.
Figure 3.
Composite pelvic signs and symptoms score (CPSSS) mean ± standard error of the mean (SEM) component scores for (A) dysmenorrhea, (B) nonmenstrual pelvic pain, and (C) dyspareunia.
The results of the protocol prespecified efficacy analysis for responders, based on the ITT analysis set including patients with data at week 24, are presented in Table 2. For both the dysmenorrhea and nonmenstrual pelvic pain components of the CPSSS, the lower bound of 2-sided 95% confidence bound was no worse than −20% for the difference between the elagolix 150 mg every day treatment group and the DMPA-SC treatment group, indicating the protocol prespecified noninferiority criterion was met for the 150 mg every day group for both end points.
Table 2.
Composite Pelvic Signs and Symptoms Score Responder Analysis for Dysmenorrhea and Nonmenstrual Pelvic Pain at Week 24.
| Elagolix 150 mg Every Day (N = 84) | Elagolix 75 mg Twice a Day (N = 84) | DMPA-SC (N = 83) | |
|---|---|---|---|
| Dysmenorrhea | |||
| N | 57 | 65 | 51 |
| Responders,a N (%) | 49 (86.0) | 48 (73.8) | 44 (86.3) |
| Difference in response rates (elagolix dose—DMPA-SC) | −0.3 | −12.4 | NA |
| 95% CI for difference | (−13.4-12.7) | (−26.7-1.8) | NA |
| P valueb | 0.963 | 0.101 | NA |
| Nonmenstrual pelvic pain | |||
| N | 57 | 65 | 51 |
| Responders,a N (%) | 49 (86.0) | 50 (76.9) | 39 (76.5) |
| Difference in response rates (elagolix dose—DMPA-SC) | 9.5 | 0.5 | NA |
| 95% CI for difference | (−5.2-24.2) | (−15.1-16.0) | NA |
| P valueb | 0.205 | 0.954 | NA |
Abbreviations: CI, confidence interval; DMPA-SC, subcutaneous depot medroxyprogesterone acetate.
aA patient was considered a responder if a 1-point (or greater) decrease from baseline was reported at week 24.
bTwo-sided P values from Pearson chi-square statistic for the comparison of response rates between each elagolix dose regimen and DMPA-SC.
There were a number of patients, however, who did not complete 24 weeks of treatment with the highest discontinuation rate in the DMPA-SC treatment group. A secondary analysis using last observation carried forward (ie, imputing missing week 24 with week 12 data for patients who discontinued) was performed, and the protocol-prespecified noninferiority criteria were also met for the 150 mg every day group for both dysmenorrhea and nonmenstrual pelvic pain.
All treatment groups demonstrated improvement from baseline in the monthly mean VAS for pelvic pain throughout the treatment period, with elagolix 75 mg twice a day having more pronounced effects compared to elagolix 150 every day and DPMPA-CS groups (Supplemental Figure 2). Overall, the 3 treatment groups had comparable improvement at the end of 24 weeks of treatment in all 5 core dimensions of EHP-5 (see Supplemental Table 1).
Analgesic use was similar in all 3 treatment groups before, during, and after treatment. A greater percentage of DMPA-SC patients used opioids prior to treatment (28.9%) and during treatment (33.7%) compared with patients in the elagolix 150 mg every day (21.4% and 23.8%) and elagolix 75 mg twice a day groups (19.0% and 25.0%).
Safety Assessments
The most common AEs in elagolix recipients were headache, nausea, and nasopharyngitis, while the most common AEs in DMPA patients were headache, nausea, upper respiratory tract, and mood swings.
Adverse events during the treatment period that occurred in >5% of patients or that led to discontinuation are shown in Table 3. Although the overall incidence of AEs was similar in the 3 treatment groups during the treatment period (weeks 1-24; 79.8%, 84.5%, and 79.8% of patients in the elagolix 150 mg every day, elagolix 75 mg twice a day, and the DMPA-SC groups, respectively), a higher percentage of patients in the DMPA-SC treatment group discontinued due to an AE (4.8%, 8.3%, and 16.7% in the elagolix 150 mg every day, elagolix 75 mg twice a day, and the DMPA-SC groups, respectively). There was no consistent single AE in the elagolix group that led to discontinuation. In the DMPA-SC group, the most common AE leading to discontinuation was menorrhagia at 4.8%.
Table 3.
Adverse Events in >5% of Patients and Discontinuations Due to Adverse Events.
| Elagolix 150 mg Every Day (N = 84) | Elagolix 75 mg Twice a Day (N = 84) | DMPA-SC (N = 83) | |
|---|---|---|---|
| Adverse event, N (%) | |||
| Overall | 72 (85.7) | 74 (88.1) | 75 (89.3) |
| Headache | 22 (26.2) | 23 (27.4) | 15 (17.9) |
| Nausea | 16 (19.0) | 13 (15.5) | 13 (15.5) |
| Nasopharyngitis | 9 (10.7) | 18 (21.4) | 9 (10.7) |
| Upper respiratory tract infection | 8 (9.5) | 10 (11.9) | 10 (11.9) |
| Sinusitis | 7 (8.3) | 7 (8.3) | 6 (7.1) |
| Pharyngolaryngeal pain | 7 (8.3) | 7 (8.3) | 3 (3.6) |
| Mood swinds | 7 (8.3) | 6 (7.1) | 10 (11.9) |
| Influenza | 7 (8.3) | 5 (6.0) | 2 (2.4) |
| Acne | 7 (8.3) | 2 (2.4) | 7 (8.3) |
| Back pain | 6 (7.1) | 10 (11.9) | 4 (4.8) |
| Anxiety | 6 (7.1) | 4 (4.8) | 4 (4.8) |
| Urinary tract infection | 5 (6.0) | 8 (9.5) | 5 (6.0) |
| Vaginal mycosis | 5 (6.0) | 4 (4.8) | 3 (3.6) |
| Fatigue | 5 (6.0) | 3 (3.6) | 6 (7.1) |
| Ovarian cyst | 5 (6.0) | 0 (0.0) | 1 (1.2) |
| Diarrhea | 4 (4.8) | 9 (10.7) | 8 (9.5) |
| Arthralgia | 4 (4.8) | 9 (10.7) | 2 (2.4) |
| Insomnia | 4 (4.8) | 7 (8.3) | 4 (4.8) |
| Nasal congestion | 4 (4.8) | 6 (7.1) | 3 (3.6) |
| Migraine | 4 (4.8) | 4 (4.8) | 5 (6.0) |
| Dizziness | 3 (3.6) | 6 (7.1) | 8 (9.5) |
| Depression | 3 (3.6) | 6 (7.1) | 4 (4.8) |
| Sinus congestion | 3 (3.6) | 5 (6.0) | 5 (6.0) |
| Cough | 2 (2.4) | 6 (7.1) | 3 (3.6) |
| Abdominal distension | 2 (2.4) | 0 (0.0) | 6 (7.1) |
| Dyspepsia | 1 (1.2) | 5 (6.0) | 3 (3.6) |
| Discontinuation due to adverse events during the treatment phase | |||
| Blood pressure increased | 1 (1.2) | 0 (0.0) | 0 (0.0) |
| Dehydration | 1 (1.2) | 0 (0.0) | 0 (0.0) |
| Endometriosis | 1 (1.2) | 0 (0.0) | 0 (0.0) |
| Headache | 1 (1.2) | 0 (0.0) | 1 (1.2) |
| Mood swings | 1 (1.2) | 0 (0.0) | 1 (1.2) |
| Ovarian cyst | 1 (1.2) | 0 (0.0) | 0 (0.0) |
| Pelvic pain | 1 (1.2) | 0 (0.0) | 1 (1.2) |
| Affect lability | 0 (0.0) | 2 (2.4) | 0 (0.0) |
| Dizziness | 0 (0.0) | 1 (1.2) | 1 (1.2) |
| Fall | 0 (0.0) | 1 (1.2) | 0 (0.0) |
| Hot flash | 0 (0.0) | 1 (1.2) | 0 (0.0) |
| Hypertension | 0 (0.0) | 1 (1.2) | 0 (0.0) |
| Migraine | 0 (0.0) | 1 (1.2) | 0 (0.0) |
| Weight decreased | 0 (0.0) | 1 (1.2) | 0 (0.0) |
| Menorrhagia | 0 (0.0) | 0 (0.0) | 4 (4.8) |
| Metorrhagia | 0 (0.0) | 0 (0.0) | 3 (3.6) |
| Abdominal distension | 0 (0.0) | 0 (0.0) | 1 (1.2) |
| Abdominal pain | 0 (0.0) | 0 (0.0) | 1 (1.2) |
| Adnexa uteri pain | 0 (0.0) | 0 (0.0) | 1 (1.2) |
| Genital pruritus | 0 (0.0) | 0 (0.0) | 1 (1.2) |
| Nausea | 0 (0.0) | 0 (0.0) | 1 (1.2) |
| Scotoma | 0 (0.0) | 0 (0.0) | 1 (1.2) |
| Suicidal ideation | 0 (0.0) | 0 (0.0) | 1 (1.2) |
| Discontinuation due to adverse events during the posttreatment phase | |||
| Ovarian dysgeminoma stage 1 | 1 (1.2) | 0 (0.0) | 0 (0.0) |
| Pelvic pain | 0 (0.0) | 0 (0.0) | 1 (1.2) |
Abbreviation: DMPA-SC, subcutaneous depot medroxyprogesterone acetate.
There were no deaths during the study. Serious AEs were experienced by 3 patients in the elagolix 150 mg every day group (1 during the treatment period and 2 during the posttreatment period), 3 patients in the elagolix 75 mg twice a day group (2 during the treatment period and 1 during the posttreatment period), and 6 patients in the DMPA-SC group (3 during the treatment period and 3 during the posttreatment period; Supplemental Table 2). One patient in the elagolix 75 mg twice a day group experienced a serious AE of suicidal ideation, which occurred approximately 2.5 months after the initiation of study drug. The patient was admitted to the hospital, and study drug was interrupted for 3 days. The patient had a history of psychiatric hospitalizations and multiple suicide attempts. The event was judged by the investigator to be moderate and not related to the study drug, and the patient completed the study. A separate AE of suicidal ideation occurred in the DMPA-SC group, leading to study discontinuation. There were no clinically meaningful changes in laboratory safety parameters, vital sign measurements, and ECG readings throughout the study.
There were 9 pregnancies during the study, 3 that occurred during the treatment period (“on-treatment”) and 6 during the posttreatment period. Among the 3 on-treatment pregnancies, 1 patient was lost to follow-up (75 mg twice a day), 1 patient (150 mg every day) delivered an infant with a congenital anomaly of cleft palate, and 1 patient (150 mg every day) delivered an infant with aspiration pneumonia. The remaining 6 pregnancies (2 elagolix 150 mg, 3 elagolix 75 mg, and 1 DMPA-CS) occurred during the posttreatment period ranging approximately 2 to 12 months after the last dose of study drug. In all, 1 pregnancy was lost to follow-up, and the remaining 5 delivered healthy infants.
Hot Flash
During the 1-month screening period (prior to treatment), 1 or more episodes of hot flash was experienced by 47.6%, 39.3%, and 46.4% of patients randomized to receive elagolix 150 mg every day, elagolix 75 mg twice a day, and DMPA-SC groups, respectively. During the 6-month treatment period, the percentage of patients experiencing 1 or more episodes of hot flash rose in all groups to 71.1%, 82.1%, and 75.9% in the elagolix 150 mg every day, elagolix 75 mg twice a day, and DMPA-SC groups, respectively. On average, the number of hot flashes per day was low (0.1, 0.2, and 0.1 in the elagolix 150 mg every day, elagolix 75 mg twice a day, and DMPA-SC groups, respectively); 1 patient (75 mg twice a day dose) reported hot flashes as part of AEs leading to study discontinuation. The majority of hot flashes experienced in all groups were mild to moderate in intensity.
Uterine Bleeding
At baseline, the mean percentage of days with uterine bleeding was similar among the 3 treatment groups (28.1 ± 1.7%, 26.0 ± 1.5%, and 26.7 ± 1.9% for elagolix 150 mg every day, elagolix 75 mg twice a day, and DMPA-SC, respectively). During the treatment period, patients in the elagolix treatment groups had a lower percentage of days with any uterine bleeding compared with patients who received DMPA-SC. The mean percentages of days with any uterine bleeding were 15.5% ± 1.0%, 13.9% ± 1.3%, and 30.4% ± 2.4% for the elagolix 150 mg every day, elagolix 75 mg twice a day, and DMPA-SC groups, respectively. After treatment discontinuation, the mean percentage of days with uterine bleeding was similar among the treatment groups (22.0% ± 0.9%, 21.4% ± 1.3%, and 18.7% ± 2.8% for the elagolix 150 mg every day, elagolix 75 mg twice a day, and DMPA-SC groups, respectively). The onset of the first posttreatment menses was delayed in the DMPA-SC group compared with the elagolix groups. The mean number of days to the first posttreatment menses was 24.2 ± 1.5, 22.4 ± 1.2, and 72.1 ± 7.6 in the elagolix 150 mg every day, elagolix 75 mg twice a day, and DMPA-SC groups, respectively.
Discussion
The primary objective of this study was to evaluate the impact of elagolix on BMD. The mean percentage change from baseline in BMD was −0.11%, −1.29%, and −0.99% at the spine and −0.47%, −1.02%, and −1.29% at the femur (total hip) in patients who received elagolix 150 mg, elagolix 75 mg, and DMPA-SQ, respectively. In addition, the lower bound of the 95% CI for BMD mean percentage change from baseline was above the prespecified threshold of −2.2% for the spine and total hip in both elagolix treatment groups and the DMPA group at week 24, indicating that all 3 treatments had minimal impact on BMD. The elagolix 75 mg twice a day dose had greater impact on BMD, compared with 150 mg every day, which is consistent with a more profound suppression of E2 concentrations at this dose regimen. These data suggest that the elagolix 150 mg dose may have the potential for longer term use for the management of endometriosis-associated pain, without the need for add-back therapy.
The efficacy of elagolix on endometriosis-associated pain was demonstrated by improvements in both the CPSSS and VAS assessments. Elagolix 150 mg every day dose was statistically noninferior to DMPA, for reducing the dysmenorrhea and nonmenstrual pelvic pain components of the CPSSS at week 24. Similar effects were observed with regard to daily VAS assessments. Improvements in multiple measures of efficacy demonstrate the consistency of the effect of elagolix for improving endometriosis-associated pain.
There were also improvements in quality of life as measured by the EHP-5. The 3 treatment groups had comparable improvement at the end of 24 weeks of treatment in all 5 dimensions of EHP-5.
Although elagolix and DMPA-SC demonstrated minimal impact on BMD and similar efficacy on endometriosis-associated pain, there were some tolerability differences between the 2 treatments. Patients who received elagolix experienced a reduction in uterine bleeding during the treatment period, as evidenced by a lower number of days with bleeding, compared to the DMPA-SC group. The increase in uterine bleeding (mostly breakthrough bleeding and spotting) seen inpatients in this study who received DMPA-SC is a well-characterized side effect of treatment with progestins,2–4 which may be bothersome to some patients. Furthermore, a greater number of patients in the DMPA-SC group discontinued from the study as a result of AEs and 7 of the discontinuations were related to uterine bleeding (4 for menorrhagia [heavy menstrual bleeding] and 3 for metrorrhagia [irregular and acyclic bleeding]) compared with 0 in the elagolix treatment groups. After discontinuation of treatment, the return to menses was rapid in elagolix-treated patients (mean of 24.2 and 22.4 days in elagolix 150 and 75 mg groups, respectively) but was delayed in patients in the DMPA-SC group (mean of 72.1 days). This delay in the return to menses is another well-known effect of DMPA, and any delay in the return to a regular menstrual cyclicity may also be undesirable to women for any number of reasons (eg, culturally, psychologically, reproductive desires, etc).
Overall, there was a high rate of hot flashes, recorded as mild or moderate in all groups during the study, including a high rate during the screening period. The incidence of hot flashes did show a similar, modest increase in all 3 study groups during the treatment period. However, the incidence of hot flash was prompted for and recorded daily in the patient’s e-diary, which may have resulted in overreporting. Hot flashes did not result in a significant number of premature discontinuations. There was a low number of serious AEs, with 3 occurring in elagolix patients during the treatment period, and 3 in the DMPA group. All of these events, including the event of suicidal ideation, were judged by the investigator as unlikely or not related to treatment.
This study did not include a placebo control arm. However, this study was part of a series of phase 2 studies that evaluated elagolix for the treatment of endometriosis-associated pain and 2 other phase 2 studies included placebo-control arms.18,19 In addition, this study included an active comparator, DMPA-SC, with established efficacy for the management of endometriosis-associated pain.
Elagolix is an oral GnRH antagonist, which has the ability to rapidly suppress the pituitary-ovarian hormones in a dose-dependent manner, that is, from partial suppression at lower doses to full suppression at higher doses.8,9 However, elagolix is not a contraceptive, and ovulations may still occur during treatment with elagolix, particularly at doses which partially suppress the ovarian estrogen production. A review of all the data from the early clinical development program of elagolix to date estimates an annualized pregnancy rate of ∼5% for the 150 mg every day dose (AbbVie Inc data on file). Despite the protocol requirement for use of dual nonhormonal contraception, there were 3 pregnancies that occurred during the treatment period of this study, all in the elagolix-treatment arms.
Preclinical studies with elagolix have revealed no teratogenic effects at all doses studied (30-98× the clinically relevant dose; AbbVie Inc data on file). One of the pregnancy outcomes in this study was a cleft palate. An extensive review (eg, timing of elagolix exposure relative to organogenesis, absence of teratogenic findings in animal toxicological studies, background incidence of this abnormality, etc) of this congenital abnormality suggests that it is unlikely to be related to elagolix; however, the true relationship remains unknown.
The discontinuation rate in the elagolix treatment arms of this study (32.9%) is consistent with previous studies of endometriosis-associated symptoms, where discontinuation rates average ∼30% to 40%, but have ranged from 15% to 60%.3,20–25 During the treatment period, the discontinuation rates for all 3 treatment groups (elagolix and DMPA-SC) were similar.
In conclusion, data from this study demonstrate that all 3 treatment regimens had minimal impact on BMD for up to 24 weeks of treatment and showed comparable efficacy on endometriosis-related pain symptoms. Although further studies are warranted, data from this study suggest that elagolix has the potential to become a new treatment option for the management of premenopausal women with endometriosis-associated pain.
Acknowledgments
The authors would like to thank the following investigators: Bruce Akright, MD, Brian Allen, DO, Richard Beyerlein, MD, Arthur J. Donovan, MD, Micah S. Harris, MD, William Koltun, MD, Robin Kroll, MD, Brock McConnehey, DO, Frank Morgan, MD, Robert Lamar Parker, MD, David Portman, MD, Prescott Prillaman, MD, Ishrat Rafi, MD, Larry Seidman, DO, Robert Smith, MD, Barbara Soltes, MD, Michael Swor, MD, Daniel A. Tomlinson, MD, Michael Twede, MD, Arthur S. Waldbaum, MD, Debra Walland, MD, and Edward A. Zbella, MD.
Footnotes
Authors’ Note: Elagolix is being developed by AbbVie and Neurocrine Biosciences. Neurocrine Biosciences, AbbVie, and all authors participated in data analysis and interpretation. AbbVie provided funding for writing support. All authors contributed to the development of the content. The authors and AbbVie reviewed and approved the article; the authors maintained control over the final content. Medical writing support was provided by Amanda J. Fein, PhD, of AbbVie and Elaine Hanna, MA, contracted by AbbVie.
Declaration of Conflicting Interests: The author(s) declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: BC has been a consultant for AbbVie Inc. WPD has been a consultant for Neurocrine Biosciences, Inc and AbbVie Inc. JB, RJ, and CO are Neurocrine Biosciences, Inc employees and own Neurocrine Biosciences, Inc stock. KC and PJ are AbbVie Inc employees and own AbbVie Inc stock; EG is a former AbbVie Inc employee.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This study was designed and funded by Neurocrine Biosciences . BC has received grant support from Neurocrine Biosciences, Inc, also received research grants from Syneract and Evofem.
Supplemental Material: The online supplemental material is available at http://rs.sagepub.com/supplemental.
References
- 1. Carr BR. Endometriosis. In: Schorge JO, Schaffer JI, Halvorson LM, Hoffman B, Bradshaw KD, Cunningham FG, eds. Williams Gynecology. New York: McGraw-Hill; 2008:chapter 10 [Google Scholar]
- 2. Crosignani PG, Luciano A, Ray A, Bergqvist A. Subcutaneous depot medroxyprogesterone acetate versus leuprolide acetate in the treatment of endometriosis-associated pain. Hum Reprod (Oxford, England). 2006;21 (1):248–256 [DOI] [PubMed] [Google Scholar]
- 3. Schlaff WD, Carson SA, Luciano A, Ross D, Bergqvist A. Subcutaneous injection of depot medroxyprogesterone acetate compared with leuprolide acetate in the treatment of endometriosis-associated pain. Fertil Steril. 2006;85 (2):314–325 [DOI] [PubMed] [Google Scholar]
- 4. Vercellini P, Fedele L, Pietropaolo G, Frontino G, Somigliana E, Crosignani PG. Progestogens for endometriosis: forward to the past. Hum Reprod Update. 2003;9 (4):387–396 [DOI] [PubMed] [Google Scholar]
- 5. Batzer FR. GnRH analogs: options for endometriosis-associated pain treatment. J Minim Invasive Gynecol. 2006;13 (6):539–545 [DOI] [PubMed] [Google Scholar]
- 6. Olive DL. Gonadotropin-releasing hormone agonists for endometriosis. N Engl J Med. 2008;359 (11):1136–1142 [DOI] [PubMed] [Google Scholar]
- 7. Surrey ES. Gonadotropin-releasing hormone agonist and add-back therapy: what do the data show? Curr Opin Obstet Gynecol. 2010;22 (4):283–288 [DOI] [PubMed] [Google Scholar]
- 8. Struthers RS, Xie Q, Sullivan SK, et al. Pharmacological characterization of a novel nonpeptide antagonist of the human gonadotropin-releasing hormone receptor, NBI-42902. Endocrinology. 2007;148 (2):857–867 [DOI] [PubMed] [Google Scholar]
- 9. Struthers RS, Nicholls AJ, Grundy J, et al. Suppression of gonadotropins and estradiol in premenopausal women by oral administration of the nonpeptide gonadotropin-releasing hormone antagonist elagolix. J Clin Endocrinol Metab. 2009;94 (2):545–551 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Kaunitz AM, Darney PD, Ross D, Wolter KD, Speroff L. Subcutaneous DMPA vs. intramuscular DMPA: a 2-year randomized study of contraceptive efficacy and bone mineral density. Contraception. 2009;80 (1):7–17 [DOI] [PubMed] [Google Scholar]
- 11. Berenson AB, Rahman M, Breitkopf CR, Bi LX. Effects of depot medroxyprogesterone acetate and 20-microgram oral contraceptives on bone mineral density. Obstet Gynecol. 2008;112 (4):788–799 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Clark MK, Sowers M, Levy B, Nichols S. Bone mineral density loss and recovery during 48 months in first-time users of depot medroxyprogesterone acetate. Fertil Steril. 2006;86 (5):1466–1474 [DOI] [PubMed] [Google Scholar]
- 13. Shaarawy M, El-Mallah SY, Seoudi S, Hassan M, Mohsen IA. Effects of the long-term use of depot medroxyprogesterone acetate as hormonal contraceptive on bone mineral density and biochemical markers of bone remodeling. Contraception. 2006;74 (4):297–302 [DOI] [PubMed] [Google Scholar]
- 14. Kaunitz AM, Miller PD, Rice VM, Ross D, McClung MR. Bone mineral density in women aged 25-35 years receiving depot medroxyprogesterone acetate: recovery following discontinuation. Contraception. 2006;74 (2):90–99 [DOI] [PubMed] [Google Scholar]
- 15. Isley MM, Kaunitz AM. Update on hormonal contraception and bone density. Rev Endocr Metab Disord. 2011;12 (2):93–106 [DOI] [PubMed] [Google Scholar]
- 16. Biberoglu KO, Behrman SJ. Dosage aspects of danazol therapy in endometriosis: short-term and long-term effectiveness. Am J Obstet Gynecol. 1981;139 (6):645–654 [DOI] [PubMed] [Google Scholar]
- 17. Jones G, Jenkinson C, Kennedy S. Development of the short form endometriosis health profile questionnaire: the EHP-5. Qual Life Res. 2004;13 (3):695–704 [DOI] [PubMed] [Google Scholar]
- 18. Carr B, Chwalisz K, Jimenez R, Burke J, Jiang P, O'Brien C. A novel oral GnRH antagonist, elagolix, is effective for reducing endometriosis-associated pelvic pain: results of a 24-week randomized study. Fertil Steril. 2011;96 (3):S45 [Google Scholar]
- 19. Imani R T-CD, Jimenez R, Burke J, Kroll R, O'Brien C. Petal study: safety, tolerability and effectiveness of elagolix, an oral GnRH antagonist for endometriosis. Fertil Steril. 2009;92 (3):S111–S112 [Google Scholar]
- 20. Cosson M, Querleu D, Donnez J, et al. Dienogest is as effective as triptorelin in the treatment of endometriosis after laparoscopic surgery: results of a prospective, multicenter, randomized study. Fertil Steril. 2002;77 (4):684–692 [DOI] [PubMed] [Google Scholar]
- 21. Vercellini P, Cortesi I, Crosignani PG. Progestins for symptomatic endometriosis: a critical analysis of the evidence. Fertil Steril. 1997;68 (3):393–401 [DOI] [PubMed] [Google Scholar]
- 22. Overton CE, Lindsay PC, Johal B, et al. A randomized, double-blind, placebo-controlled study of luteal phase dydrogesterone (Duphaston) in women with minimal to mild endometriosis. Fertil Steril. 1994;62 (4):701–707 [DOI] [PubMed] [Google Scholar]
- 23. Lockhat FB, Emembolu JO, Konje JC. The evaluation of the effectiveness of an intrauterine-administered progestogen (levonorgestrel) in the symptomatic treatment of endometriosis and in the staging of the disease. Hum Reprod. 2004;19 (1):179–184 [DOI] [PubMed] [Google Scholar]
- 24. Hornstein MD, Surrey ES, Weisberg GW, Casino LA. Leuprolide acetate depot and hormonal add-back in endometriosis: a 12-month study. Lupron Add-Back Study Group. Obstet Gynecol. 1998;91 (1):16–24 [DOI] [PubMed] [Google Scholar]
- 25. Friedman AJ, Hornstein MD. Gonadotropin-releasing hormone agonist plus estrogen-progestin “add-back” therapy for endometriosis-related pelvic pain. Fertil Steril. 1993;60 (2):236–241 [DOI] [PubMed] [Google Scholar]