Abstract
Introduction
Several cluster-randomized HIV prevention trials aim to demonstrate the population-level preventive impact of antiretroviral therapy (ART). 2013 World Health Organization guidelines raising the ART initiation threshold to CD4<500/µL could attenuate these trials’ effect size by increasing ART usage in control clusters.
Methods
We used a computational model to simulate strategies from a hypothetical cluster-randomized HIV prevention trial. The primary model outcome was the relative reduction in 24-month HIV incidence between control (ART offered with CD4 below threshold) and intervention (ART offered to all) strategies. We assessed this incidence reduction using the revised (CD4<500/µL) and prior (CD4<350/µL) control ART initiation thresholds. Additionally, we evaluated changes to trial characteristics that could bolster the incidence reduction.
Results
With a control ART initiation threshold of CD4<350/µL, 24-month HIV incidence under control and intervention strategies was 2.46/100PY and 1.96/100PY, a 21% reduction. Raising the threshold to CD4<500/µL decreased the incidence reduction by more than one-third, to 12%. Using this higher threshold, moving to a 36-month horizon (vs. 24-month), yearly control-strategy HIV screening (vs. biannual), and 2-monthly intervention-strategy screening (vs. biannual), resulted in a 31% incidence reduction, similar to effect size projections for ongoing trials. Alternate assumptions regarding cross-cluster contamination had the greatest influence on the incidence reduction.
Conclusions
Implementing the 2013 WHO HIV treatment threshold could substantially diminish the incidence reduction in HIV population prevention trials. Alternative HIV testing frequencies and trial horizons can bolster this incidence reduction, but could be logistically and ethically challenging. The feasibility of HIV population prevention trials should be reassessed as implementation of treatment guidelines evolves.
Keywords: HIV, highly-active antiretroviral therapy, prevention, randomized controlled trials as topic
INTRODUCTION
Over the past decade, antiretroviral therapy (ART) for HIV infection has greatly reduced HIV-related morbidity and mortality in resource-limited settings.1–5 In 2011, the HPTN 052 trial demonstrated that effective treatment could also dramatically reduce the risk of HIV transmission in stable serodiscordant sexual partnerships.6 But while the individual preventive impact of ART is clear, questions remain regarding the population-level effect of treatment-as-prevention in different settings. Ecological studies have shown conflicting results regarding the impact of ART scale-up on HIV incidence.7–10 The only non-ecological evidence of the population-level effect of increasing ART coverage comes from a prospective population cohort in KwaZulu-Natal, South Africa. Controlling for behavioral, demographic, economic, and environmental risk factors, individual HIV acquisition risk declined significantly with increasing ART coverage both in the surrounding community,11 and among household members of the opposite sex.12
To quantify the preventive benefits of expanded ART access more conclusively, and to assess the feasibility of intensive treatment expansion programs, several trials of treatment-as-prevention are ongoing13–15 or planned.16,17 In general, these trials aim to randomize geographic clusters of a population to initiate ART based on national guidelines (control, typically CD4<350/µL) vs. immediately upon diagnosis (intervention); most also incorporate frequent routine HIV screening into the intervention. These trials are designed to assess differences in HIV incidence between treatment strategies, and several are powered to evaluate incidence reductions of around 30%.13,14
In June 2013, the World Health Organization (WHO) released updated guidelines for the use of ART, raising the recommended threshold for ART initiation from CD4<350/µL to CD4<500/µL in asymptomatic patients.18 This recommendation may prompt a change in national guidelines and trial protocols, leading to greater usage of ART in control clusters, and potentially attenuating the difference in incidence between trial arms. Due to the longitudinal impact of policy changes, the incidence in the control arm, and the incidence reduction between arms, might be lower than planned, and could thus require some compensation in trial design characteristics. We aimed to: 1) quantify the impact that the revised ART initiation threshold could have on the outcomes of cluster-randomized treatment-as-prevention trials, and 2) assess how changes in trial characteristics could be used to augment the observed incidence reduction in the context of this policy change.
METHODS
Analytic overview
We used two integrated HIV models to evaluate the outcomes of cluster-randomized population prevention trials. We first used the Cost-Effectiveness of Preventing AIDS Complications International (CEPAC-I) model,5,19,20 a patient-level microsimulation, to project survival, CD4 count, and HIV RNA trajectories of infected individuals. These trajectories then served as input to the HIV Dynamic Epidemic Framework (H-DEF) transmission model,21 a dynamic, open-cohort epidemic simulation.
We loosely modeled the base case of our analysis after a simplified version of the Agence Nationale de Recherches sur le Sida (ANRS) 12249 trial, an ongoing cluster-randomized population prevention trial conducted in rural KwaZulu-Natal, South Africa; the trial is powered for an effect size of 34%, based on previous detailed modeling analyses.13* Per trial protocol, we simulated two treatment strategies: 1) delayed ART initiation (control, at CD4<350/µL) vs. 2) immediate ART initiation (intervention, at HIV diagnosis). Under both strategies, simulated individuals were offered home-based HIV screening at six-month intervals. We projected HIV transmissions over a 24-month horizon under the two strategies, and evaluated the relative reduction in cumulative HIV incidence between the two strategies (hereafter referred to as incidence reduction). Next, we assessed the impact that raising the control ART initiation threshold to CD4<500/µL would have on this incidence reduction. We then evaluated how changes in the HIV screening frequencies and the time horizon of the trial could alter the incidence reduction in the context of this higher ART initiation threshold.
Transmission model
To simulate trial outcomes, we developed a deterministic model of HIV transmission dynamics (H-DEF; see Supplementary Material for details).21 The model tracks a population of HIV-infected and susceptible individuals in monthly time-steps over the course of an evolving epidemic. The degree of infectivity of individuals with HIV is dependent on their HIV RNA level, with higher levels associated with increased infectivity,22 but is independent of age and sex; during an initial period of acute infection, infectivity is increased by a user-specified multiple of chronic infection infectivity. In each month, the model projects the number of new HIV infections based on: 1) collective HIV RNA levels (and thus infectivity) of the HIV-infected population, 2) the current prevalence of HIV, and 3) two calibration parameters controlling HIV-RNA specific transmission rates and sexual mixing patterns in the simulated population (Figure S1). Individual characteristics of the HIV-negative population (age, sex, etc.) are not modeled explicitly; instead, outside data23,24 are used to project population dynamics over time. Newly-infected individuals are then removed from this HIV-negative population, incorporated into the HIV-infected population, and are able to transmit HIV during subsequent monthly time-steps.
The H-DEF model itself does not simulate the disease progression of individuals with HIV. Instead, it relies on the CEPAC International microsimulation to provide trajectories of survival, HIV RNA, CD4 count, and ART status, all of which are used within the transmission model (Figure S1). As HIV testing and treatment policies are changed within the CEPAC International model, these outcome trajectories change, and the epidemic ramifications are projected by H-DEF.
A common concern in population prevention trial design is that of cross-cluster contamination – that is, the possibility that participants in a control cluster may sexually mix with those in an intervention cluster (and vice versa). To simulate this contamination, H-DEF permits a designated fraction of total infectivity from the control participants to be applied to the susceptible population in the intervention clusters, and vice versa. This feature captures the effects of participants having sexual partners outside of their own cluster type.
Disease model
CEPAC International is a stochastic microsimulation of HIV disease progression, testing, and treatment (see Supplementary Material for details).5,19,20 The model generates a cohort of HIV-infected individuals by drawing randomly from independent distributions of age, sex, HIV RNA, and CD4 count. In the absence of treatment, individuals experience a monthly decline in CD4 count, increasing the risk of opportunistic diseases (ODs) and HIV-related mortality.
Previously-unidentified individuals can be diagnosed with HIV in two ways: 1) presentation with an OD, or 2) periodic routine testing. In simulating population prevention trials, other mechanisms of HIV testing (antenatal care, workplace programs, etc.) are assumed to be captured under periodic routine testing. Participants diagnosed via presentation with an OD are assumed to accept testing and link to care. In the case of routine testing, however, individuals have a user-defined probability of being offered/accepting the HIV test, and a probability of linking to care following a positive test. Those who fail to link are eligible for subsequent testing.
Following diagnosis/linkage, simulated patients are monitored with CD4/HIV RNA tests at user-specified frequencies. ART is initiated when a patient’s observed CD4 count reaches a user-defined treatment threshold (specific to each trial strategy), or in the case of tuberculosis or WHO Stage 3–4 disease; pregnancy is not simulated. Once on ART, patients are stratified by adherence level: highly-adherent patients have a high probability of achieving virologic suppression, while poorly-adherent patients have a low probability.25 While suppressed, patients experience monthly gains in CD4 count; they are also subject to a monthly probability of regimen failure and viral rebound. Failure leads to CD4 decline; when failure is diagnosed based on observed immunologic and/or virologic criteria,18,26 patients are switched to 2nd-line ART or an adherence intervention is initiated. Any patient in care may be lost to follow-up; this risk declines with increasing adherence. Those who are lost to follow-up while on ART are assumed to discontinue therapy, but may resume treatment upon return to care. Lost patients continue to contribute to HIV transmission rates within the simulated population.
Input parameters
Transmission model inputs
HIV RNA-specific transmission rates (per 100PY with HIV) were derived from a meta-analysis of heterosexual serodiscordant couple studies (Table 1).22 We incorporated a 3-month acute HIV infection phase, during which infectivity is increased 26-fold relative to chronic infection.27 Per estimates in the ANRS trial protocol, we assumed 10% cross-cluster contamination13 (i.e. 10% of participant’s sexual partners will be outside of their own cluster type), and varied this widely in sensitivity analysis.
Table 1.
Model input parameters and sensitivity analysis ranges
| Variable | Base case value | Range | Reference |
|---|---|---|---|
| HIV transmission | |||
| Transmissions per 100 PY with HIV | |||
| < 500 copies/mL | 0.16 | 0 – 0.64 | 22 |
| 501–3,000 copies/mL | 2.06 | ||
| 3,001–10,000 copies/mL | 4.17 | ||
| 10,001–30,000 copies/mL | 8.12 | ||
| > 30,000 copies/mL | 9.03 | ||
| Calibrated transmission rate multipliera | 1.96 | 1.57 – 2.35 | |
| Acute infection | |||
| Duration (months) | 3 | 2 – 6 | 27,60 |
| Infectivity relative to unsuppressed | 26 | 18 – 38 | |
| Cross-cluster sexual contacts, % | 10 | 0 – 20 | |
| HIV test characteristics | |||
| Test interval, months | 6 | 13 | |
| Probability of test offer, % | 90 | 40 – 99b | Assumption based on ref. 32 |
| Probability of test consent, % | 80 | ||
| Probability of linkage to care (if positive), % | 70 | 40 – 99 | |
| Baseline ART adherence, % | |||
| Adherence <50% | 6 | 43c | |
| 50% ≤ Adherence < 95% | 57 | ||
| Adherence ≥ 95% | 37 | ||
| ART efficacy | |||
| HIV RNA suppressed at 6 months, overall, %d | 80 | 70 – 87e | 43,44c |
| Adherence <5% | 0 | ||
| Adherence = 50%f | 46 | ||
| Adherence >95% | 91 | 80 – 100 | |
| Virologic failure rate after 6 months, per 100 PY | |||
| Adherence <5% | 150 | 70 – 230g | 43,44c |
| Adherence = 50%f | 72 | ||
| Adherence >95% | 1.6 | 0.8 – 2.3g | |
| Loss to follow-up | |||
| Loss to follow-up rate, on ART, per 100 PY | |||
| Adherence <5% | 18 | 9 – 74g | 44,47c |
| Adherence = 50%f | 10 | ||
| Adherence >95% | 1.8 | 0.9 – 7.2g | |
| Loss to follow-up rate, pre-ART, per 100 PY | |||
| All adherence levels | 18 | 4 – 18 | 47,48c |
Note: ART: antiretroviral therapy; PY: person-years.
The transmission rate multiplier is used to increase transmission rates observed among heterosexual serodiscordant couples to rates reflective of the general population.
For sensitivity analysis, test offer and consent are combined into a single probability with base case value of 72%.
Model input value derived from primary data described in reference.
Overall suppression will be lower for second-line ART, as poorly-adherent patients are more likely to fail first-line and initiate second-line.
Only suppression at adherence >95% is varied in sensitivity analysis; overall suppression range indicates the range of overall suppression rates produced by variation in suppression at adherence >95%.
Values for suppression, virologic failure, and loss to follow-up are linearly interpolated for adherence values between 5% and 95%; the value for 50% is provided.
In sensitivity analysis, rates are varied in concert at high and low adherence levels.
Natural history
CD4-specific rates of morbidity and mortality were from the Cape Town AIDS Cohort.28 Patients in care received cotrimoxazole prophylaxis at CD4<200/µL,29 generally reducing OD risk.30,31
HIV testing
Per trial protocol, HIV tests were offered every six months in both control and intervention clusters.13 In each six-monthly round of screening, we assumed 90% of individuals would be offered an HIV test, 80% would consent to the test, and 70% of those testing positive would link to care, consistent with testing experience from preliminary trial data.32 We account for the “window period” of infection by assuming that HIV tests give negative results during the first month after infection (due to acute infection), and have 100% sensitivity thereafter.33,34
Population
At baseline, modeled adult (≥15 years) HIV prevalence was 23%, consistent with data from KwaZulu-Natal.35,36 Thirty-three percent of HIV-infected individuals were assumed to be undiagnosed (with 3% acutely infected),37 41% diagnosed but not on ART, 25% on 1st-line ART, and 1% on 2nd-line ART, based on data from the Africa Centre Demographic Information System and other South African sources (Figure S2).38–40 Among these baseline states, simulated mean CD4 varied from 380 (SD 190) cells/µL for those on ART to 560 (SD 230) cells/µL for those with acute infection (Table 2).37,41,42 We assumed that individuals who are eligible for but not on ART at baseline would initiate therapy with a monthly probability of 15%, reflecting enhanced efforts to provide access to ART in the context of a trial.13
Table 2.
Model input parameters and sensitivity analysis ranges, baseline population characteristics
| State | Proportion of HIV-infected population at baseline,a %37,38 |
Range | Mean (SD) baseline CD4, cells/µLb |
Range |
|---|---|---|---|---|
| Acute infection | 3 | 1.5 – 4.5 | 560 (230) | 450 – 750 |
| Undiagnosed, chronic | 30 | 15 – 45 | 430 (270) | 300 – 600 |
| Diagnosed, off ART | 41 | 20 – 60 | 390 (220) | 250 – 500 |
| On first-line ART | 25 | 12 – 37 | 380 (190) | 250 – 500 |
| On second-line ART | 1 | 0 – 5 | 380 (190) | 250 – 500 |
Note: ART: antiretroviral therapy.
ART efficacy
Six-month virologic suppression was assumed to be 91% for patients with adherence >95% (defined by medication possession ratio), and 0% with adherence <5%; linear interpolation was used for intermediate adherence levels.43 This leads to ∼80% overall suppression at six months, consistent with outcomes in KwaZulu-Natal.44 After six months, virologic failure rates ranged from 1.6 to 150/100PY, depending on adherence. Patients with virologic rebound on 1st-line ART received an adherence intervention, leading to resuppression for ∼50% of them (Supplementary Material).45 Those who did not resuppress were switched to a 2nd-line regimen, after which there were two additional opportunities to resuppress with adherence interventions of decreasing efficacy.46 Though previous failure to suppress on a regimen is not explicitly tracked in the model, failed patients have lower probabilities of regaining suppression after an increasing number of failure events. This feature is intended to capture some of the effects of resistance. The development of ART resistance is not explicitly modeled.
Loss to follow-up
We used adherence-specific relative rates of loss to follow-up,47 and calibrated the absolute rates to match the 4% loss at 12 months on ART observed in KwaZulu-Natal.44 As individuals at highest risk for loss to follow-up are removed from the in-care population over time, overall rates of loss to follow-up decline, resulting in 7% lost at 24 months. Patients ineligible for ART due to high CD4 count are subject to a higher loss-to-follow-up risk across all adherence levels, consistent with the 45% pre-ART retention observed in KwaZulu-Natal.48 Lost patients were assumed to have a 0.75% monthly probability of returning to care, and a 50% probability of return upon experiencing an acute OD.
Transmission model calibration
Before modeling the cluster-randomized trial, the transmission model was calibrated to historical HIV prevalence and incidence trends in KwaZulu-Natal35,36, 49 using two calibration parameters governing: 1) HIV RNA-specific transmission rates, and 2) sexual mixing patterns in the population. The first parameter (Table 1) is used to increase the transmission rates observed in serodiscordant couple studies to a level reflective of the overall population, while the second accounts for the effects of nonrandom sexual mixing (Table S1; see Supplementary Material for more details).
Alternative trial characteristics
We examined several potential changes to trial protocol that could bolster a trial’s incidence reduction in the context of an ART initiation threshold of CD4<500/µL for the control strategy. These included: alternative settings, characterized by differing frequencies of HIV screening in the control strategy (every 6, 12, 24, or 36 months); increasing the frequency of HIV screening in the intervention strategy (every 1, 2, 4, or 6 months); and extending the trial duration (24, 36, or 48 month horizons). For reporting purposes, we selected combinations of these changes to create three composite scenarios:
Initial: six-monthly screening in both strategies and a 24-month horizon, reflecting the initial design of the ANRS 12249 trial13
Intensified: two-monthly screening in the intervention strategy, yearly screening in the control strategy and a 36-month horizon
Maximal: monthly screening in the intervention strategy, three-yearly screening in the control strategy, and a 48-month horizon
While testing intervals as short as 1 month in the intervention strategy may not be feasible, these extreme frequencies serve to provide an upper limit on the trial incidence reduction.
Certain model parameters were derived from sub-Saharan African countries other than South Africa. To assess the impact of input parameter uncertainty and cross-country variability on our conclusions, we projected HIV incidence while varying model parameters in both trial strategies over the ranges shown in Tables 1, 2 and S1. Additionally, we selected several model parameters that could most plausibly be affected by the implementation of immediate ART (linkage to care, initial suppression on 1st-line ART, loss to follow-up) and varied these parameters by a factor of ±20% under the intervention strategy only.
RESULTS
Calibration
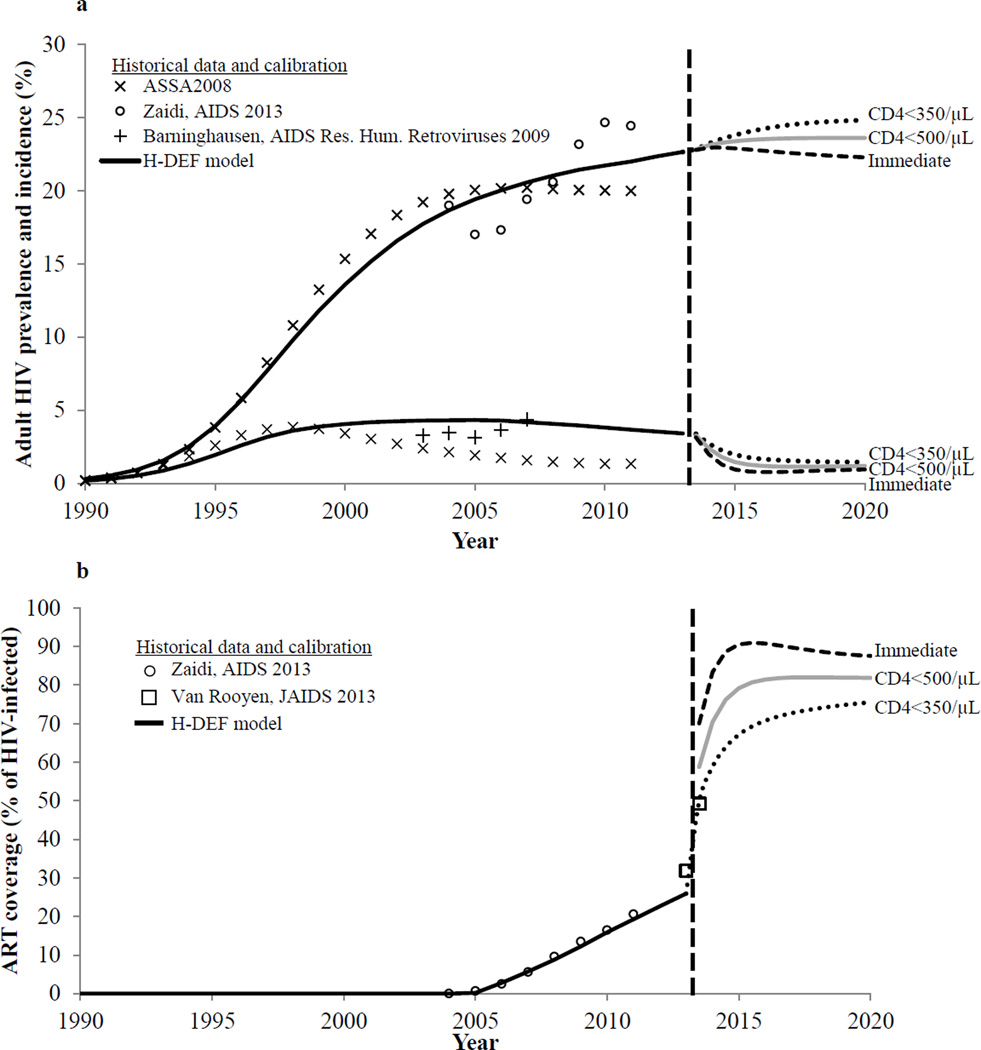
The model was able to produce a close fit to historical prevalence, incidence, and ART rollout data from 1990–2011 in KwaZulu-Natal (Figure 1). At the baseline of the analysis in 2013, projected HIV incidence was 3.41/100PY, consistent with published data estimates from KwaZulu-Natal.11,49,50 Six months after initiating the trial treatment strategies, ART coverage increased to 50% when using an initiation threshold of CD4<350/µL, consistent with levels achieved in an intensive home-based testing program in KwaZulu-Natal.51
Figure 1.
H-DEF model calibration and projections. Figure 1a depicts historical and projected HIV prevalence and incidence in KwaZulu-Natal from 1990 to 2020 in the H-DEF model, compared to three outside data sources: 1) the ASSA2008 model,36 2) adjusted HIV prevalence estimates from the Africa Center Demographic Information System (Zaidi),35 and 3) HIV incidence estimates from a large population-based longitudinal HIV surveillance study.49 Though the ASSA2008 model gives both lower prevalence and lower incidence estimates than the model calibration, ASSA2008 is reported to underestimate both parameters.61 Figure 1b depicts ART coverage in KwaZulu-Natal from 2004 to 2020 in the H-DEF model compared to 1) estimates from the Africa Center Demographic Information System (Zaidi),35 and 2) initial and six-month ART coverage estimates from a home-based testing program in KwaZulu-Natal (Van Rooyen),51 used as a rubric for how rapidly ART coverage could increase in the context of home-based testing within a trial. In both figures, the dashed vertical line indicates the start of the simulated trial. To the right of the vertical line are model projections of adult HIV prevalence and incidence and ART coverage with ART initiation criteria of CD4 < 350/µL (dotted line), CD4 < 500/µL (solid gray line), and immediate (dashed line).
Initial trial design
Over the 24-month horizon, we projected an HIV incidence of 2.46/100PY with the control strategy and 1.96/100PY with the intervention, for an incidence reduction of 21%. If the ART initiation threshold in control clusters were raised to CD4<500/µL, 24-month HIV incidence would fall for both strategies (due to cross-cluster contamination): 2.17/100PY with the control strategy and 1.90/100PY with the intervention, decreasing the incidence reduction between strategies by more than a third (incidence reduction = 12%, Table 3, top).
Table 3.
Base case results
| Control strategy ART threshold |
Control | Intervention | Relative reduction (%) |
|---|---|---|---|
|
Cumulative HIV incidence at 24 months (/100 PY) |
|||
| CD4 < 350/µL | 2.46 | 1.96 | 21b |
| CD4 < 500/µL | 2.17 | 1.90a | 12 |
|
HIV-infected individuals off ART at 12 months (%) |
|||
| CD4 < 350/µL | 41 | 17 | 59 |
| CD4 < 500/µL | 29 | 17 | 43 |
ART: antiretroviral therapy; PY: person-years
Due to cross-cluster contamination, incidence in the intervention strategy is affected by the ART initiation threshold in the control strategy.
The relative reduction in HIV incidence between intervention and control strategies is equivalent to trial effect size.
With a control ART threshold of CD4<350/µL, 41% of HIV-infected individuals in control clusters were not on ART after 12 months; in the intervention clusters, only 17% were not on ART, for a relative reduction in individuals off ART of 59%. Raising the ART threshold in the control strategy attenuated the differences in ART coverage between the strategies; with a threshold of CD4<500/µL, the relative reduction in individuals off ART fell to 43% (Table 3, bottom).
Sensitivity analysis
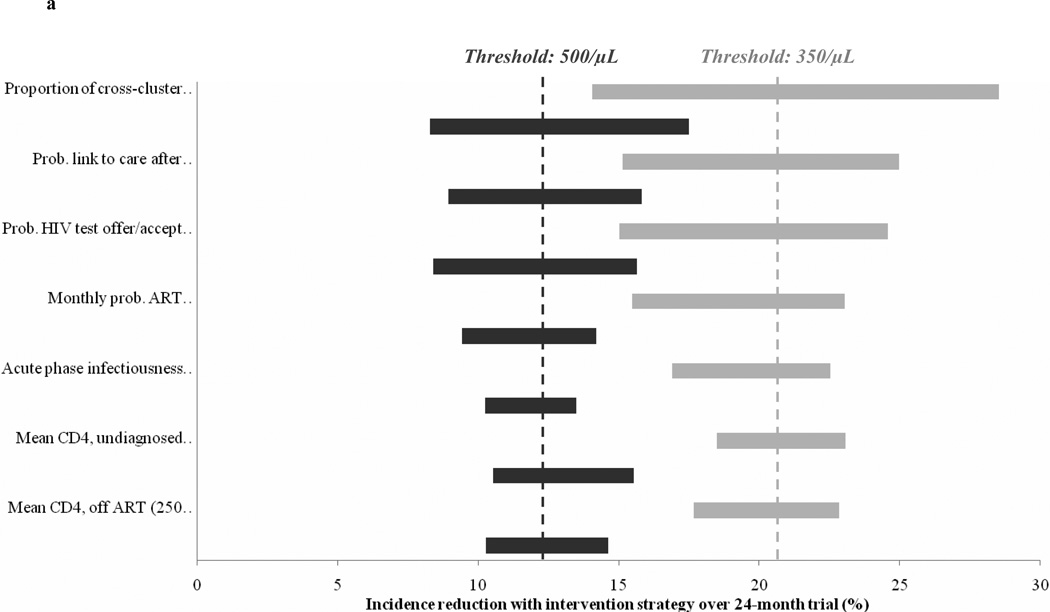
Raising the control ART threshold to CD4<500/µL consistently decreased the incidence reduction across wide variation in input parameters (Figure 2a). At all parameter values evaluated, the incidence reduction was lower with a threshold of CD4<500/µL than with a threshold of 350/µL; one-third to one-half of the total incidence reduction was lost when using the higher ART initiation threshold.
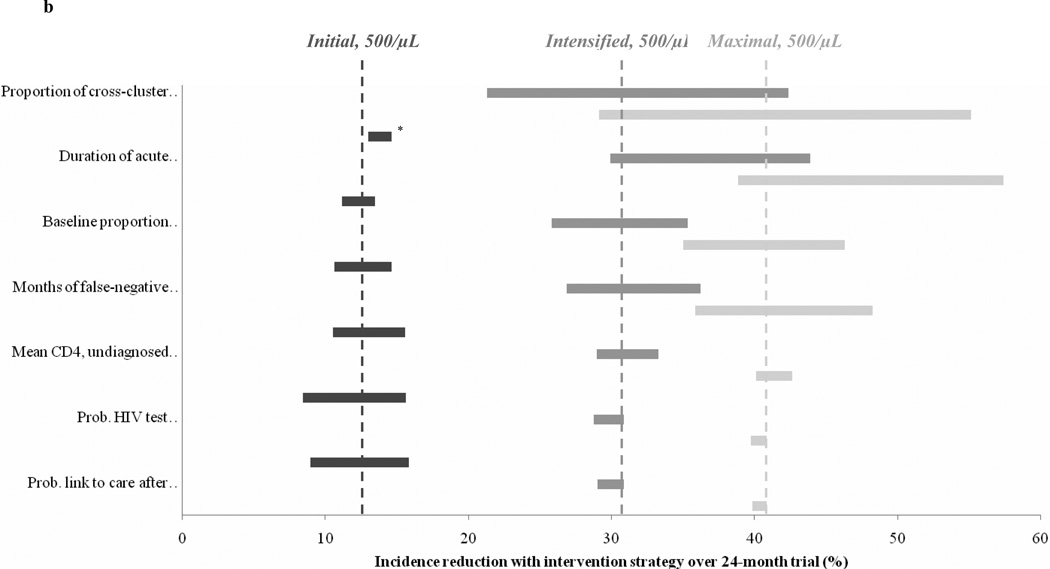
Figure 2.
Sensitivity analysis on model input parameters. Observed incidence reduction is shown for varying values of select model input parameters; the seven parameters with the greatest effect on projected results are shown in the figure. The width of each horizontal bar indicates the variation in incidence reduction when varying a particular model parameter over the range (corresponding left to right) denoted in the label in parentheses on the vertical axis; wider bars indicate that the incidence reduction is more sensitive to that particular parameter. Dashed vertical lines indicate the incidence reduction observed with base case inputs. Figure 2a shows the incidence reduction with the Initial scenario at control ART initiation thresholds of CD4 < 350/µL (light gray) and CD4 < 500/µL (dark gray). Figure 2b shows the incidence reduction with the Initial (dark gray), Intensified (medium gray), and Maximal (light gray) scenarios at a control ART initiation threshold of CD4 < 500/µL.
*For the Initial scenario, the incidence is greater with acute infection duration of 2 months or 6 months, compared to the base case value of 3 months.
Alternative trial characteristics
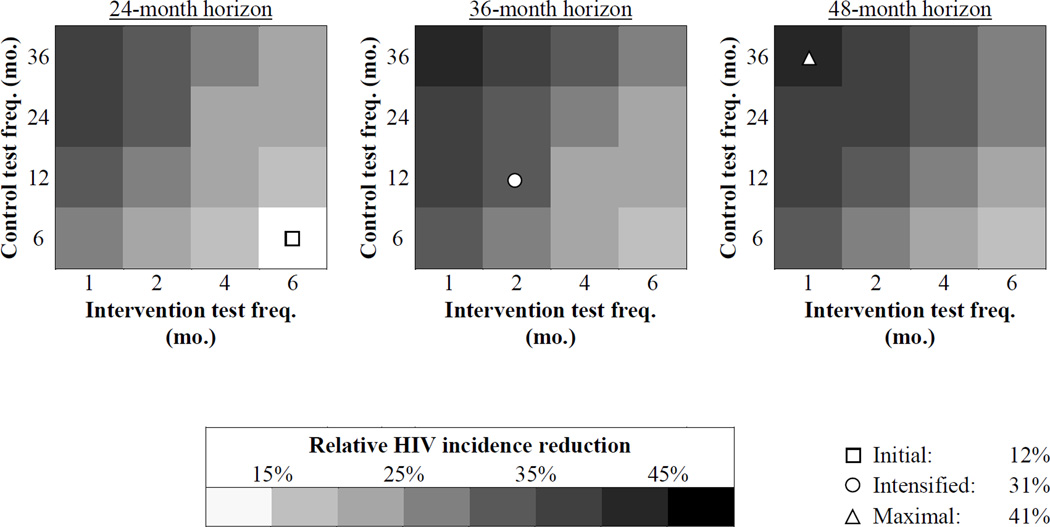
In general, greater incidence reductions were associated with settings with less frequent HIV screening in the control strategy, more frequent screening in the intervention strategy, and longer trial time horizon (Figure 3). The Intensified scenario (Figure 3, middle panel, circle) produced an incidence reduction of 31%; the Maximal scenario (Figure 3, right panel, triangle) produced an incidence reduction of 41%. Similar trends with respect to the trial design parameters were observed when using an ART initiation threshold of CD4<350/µL (S5). Notably, the three scenarios responded differently to the change from an ART initiation threshold of CD4<350/µL to 500/µL: the incidence reduction in the Initial scenario decreased by a relative 40% (21% to 12%), that of the Intensified scenario decreased by 17% (37% to 31%), and that of the Maximal scenario decreased by only 9% (45% to 41%).
Figure 3.
Incidence reduction with varying trial characteristics. Projected trial incidence reduction with an ART initiation threshold of CD4 < 500/µL and varying combinations of control strategy HIV test frequency (6 – 36 months), intervention strategy HIV test frequency (1 – 6 months), and trial horizon (24 – 48 months). The color at each point in the figure denotes the projected incidence reduction with that combination of trial design characteristics. Three scenarios are highlighted for further analysis (see Methods for descriptions of these scenarios): 1) Initial, square; 2) Intensified, circle; 3) Maximal, triangle. The predicted incidence reduction for each of these scenarios is shown in the legend.
Sensitivity analysis
For all three of the trial design scenarios we selected, the parameter with the greatest impact on the incidence reduction was related to cross-cluster contamination (Figure 2b). When contamination was varied between 0–20%, the incidence reduction in the Initial scenario (at a threshold of CD4<500/µL) ranged from 17% to 8%; the incidence reduction in the Intensified scenario ranged from 42% to 21%; and the incidence reduction in the Maximal scenario ranged from 55% to 29%. The impact of other parameters differed by trial scenario. For example, variation in the likelihood of test offer/acceptance (40–99%) or in linkage to care after a positive test (40–99%) both altered the incidence reduction in the Initial scenario over a range of 7%. The Intensified and Maximal scenarios were comparatively insensitive to these parameters, with variation in the incidence reduction of 2% or less. Conversely, variation in the proportion of the population with undiagnosed HIV at baseline altered the incidence reduction in the Intensified and Maximal scenarios over ranges of 10% and 11%, while that of the Initial scenario varied by less than 3%.
When varied in the intervention strategy alone, we found that 20% increases or decreases in the rate of loss to follow-up did not alter the incidence reduction in any of the scenarios by more than 1% compared to the base case. However, variation in linkage-to-care or 1st-line ART suppression in the intervention strategy alone did markedly affect the incidence reduction. At an ART threshold of CD4<500/µL, reductions in these parameters led to 2–7% absolute decreases in the incidence reduction; increases in these parameters increased the incidence reduction by 1–4% compared to the base case (Table S2).
DISCUSSION
On occasion, policy changes are based on best possible evidence that falls short of the “gold standard” randomized clinical trial. When this occurs, clinical trials designed to provide that evidence can become that much more challenging to conduct, as evidenced by this study.
Using a dynamic mathematical modeling approach, we projected that implementing the ART initiation threshold of CD4<500/µL suggested by the new WHO guidelines18 reduces the incidence reduction in HIV population prevention trials by one-third to one-half. Altered characteristics – especially more frequent HIV screening in the intervention strategy compared to the control strategy – produced greater incidence reductions. Notably, we projected that a trial scenario with 2-monthly intervention-strategy screening and yearly control-strategy screening would produce a 31% incidence reduction at 36 months, consistent with the effect size that several population prevention trials are currently powered to detect.13,14
While we found that differential HIV screening frequencies could efficiently amplify the observed incidence reduction, we recognize that implementing this will not be straightforward: increasing testing frequency in intervention arms will be logistically challenging (and could lead to lower test acceptance), and maintaining reduced testing frequencies in control arms may pose ethical concerns.52 Because HIV screening is also the method of ascertaining trial outcomes, differential screening frequencies will also need to be accounted for when analyzing results. Moreover, the bolstered incidence reduction may come at the cost of interpretability of results. A trial whose strategies differ only in their choice of ART initiation threshold is definitively a trial of treatment-as-prevention, and differences between the two strategies can be attributed to the ART initiation threshold alone. With differential screening rates incorporated as well, some of the incidence reduction would clearly be due to HIV testing; this would now be a trial of test-and-treat. Further, while we did not evaluate additional preventive interventions (male circumcision, condom provision, prevention of mother-to-child transmission, etc.), some planned cluster-randomized trials will incorporate these into combined interventions, with some varying interventions – including testing frequency – across more than two arms.14,15 Extrapolating from our results, we can expect that the effect of these combination interventions may prove relatively robust to changes in ART initiation threshold. However, it will be essential to weigh the benefits of this bolstered incidence reduction against the potential to further complicate the interpretation of trial results.
In sensitivity analysis, we found that implementing the new ART initiation threshold produced consistent decreases in the incidence reduction across a wide range of parameter values; likewise, differences between the alternative trial scenarios we simulated were robust to parameter variation. However, absolute incidence reductions were particularly sensitive to variation in certain parameters. Reductions in the rate of HIV test acceptance, the likelihood of linkage-to-care after a positive test, and the baseline proportion of the population with undiagnosed HIV attenuated the differences in ART coverage between the two trial strategies, and thus decreased the incidence reduction. This result is consistent with findings from prior trials of HIV prevention strategies such as microbicides, in which “overlap” of an intervention to the control arm has been a common barrier to achieving a significant incidence reduction.53 In this case, overlap is manifested in increasing control arm ART coverage; and in our analysis, a greater overlap (i.e. a smaller difference in ART coverage between strategies) was associated with a diminished incidence reduction. These findings imply that ongoing monitoring of ART coverage will prove valuable in interpreting the results of population prevention trials.
Cross-cluster contamination also proved to be highly influential on trial outcomes. This finding highlights the importance of selecting trial settings in which communities are relatively sexually isolated (such as rural KwaZulu-Natal),13 and it suggests that a priori measurement of the likelihood of contamination (via survey or genetic linkage testing) will be critical to assessments of the feasibility of a given trial. Settings in which a high degree of contamination is likely may be suitable for studying interventions targeted to negative individuals,54 but will likely be inappropriate for interventions targeted to persons with HIV.
This analysis has several limitations. First, we note that the clinical trial strategies simulated here are only loosely based on the ANRS 12249 trial; our results should not be interpreted as projections of the potential outcome of that trial specifically. Due to the complexity of our combined disease-progression and transmission models, we have not performed direct trial simulations and did not make effect size/sample size calculations. Next, our model of HIV transmission is neither age- nor sex-stratified and does not explicitly account for behavioral factors such as concurrent sexual partnerships or sexual networks. Likewise, sex differences in HIV testing frequency and ART uptake are not explicitly modeled. Thus, if trial interventions are rolled-out unevenly to individuals who differ in these predictors of transmission risk, our model may fail to capture the implications for HIV incidence.55 For example, current ART coverage is comparatively low among more sexually-active younger individuals;35 increasing coverage in this group could lead to a disproportionately large reduction in incidence. Additionally, we did not model the potential for expanded HIV treatment and/or poor ART adherence to produce an increase in transmitted drug resistance; while transmitted resistance could affect long-term outcomes of expanded ART policies,56 this omission is unlikely to bias our short-term incidence projections. Finally, we note that the value of expanded HIV treatment and prevention programs will depend on their long-term population-level costs and clinical impact, not just their short-term impact on HIV transmission.57–59 Thus, the results of this analysis, and of the cluster-randomized trials we have modeled, will be an imperfect indicator of the full, long-term benefits of treatment-as-prevention.
Our modeling analysis suggests that increasing the control ART initiation threshold from CD4<350/µL to CD4<500/µL could substantially reduce the incidence reduction observed in HIV population prevention trials. While it may take some time for this change to be implemented in national treatment guidelines and trial protocols, it is critical to begin preparing for it now. The feasibility of ongoing trials may need to be reevaluated, and potential changes to trial protocol and sample size to preserve effect size should be considered.
Supplementary Material
Acknowledgments
The authors are grateful to Michael Girouard for his technical assistance.
Funding:
Agence Nationale de Recherches sur le SIDA et les hépatites virales (ANRS 12136, ANRS 12212), National Institute of Allergy and Infectious Diseases (NIAID, R01 AI058736, R01 AI093269, R01 HD058482, and UM1 AI068636). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Footnotes
The ANRS 12249 trial incorporates a phased approach to enrollment and follow-up in 22 clusters. For simplicity, we do not simulate this phased approach, and instead assume concurrent enrollment and follow-up.12
Disclosures:
Dr. Pei reports personal fees from United BioSource Corporation, outside the submitted work.
Dr. Weinstein reports personal fees from OptumInsight, outside the submitted work.
REFERENCES
- 1.Bor J, Herbst AJ, Newell M-L, Bärnighausen T. Increases in adult life expectancy in rural South Africa: valuing the scale-up of HIV treatment. Science. 2013;339:961–965. doi: 10.1126/science.1230413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Fairall L, Bachmann M, Louwagie G, et al. Effectiveness of antiretroviral treatment in a South Africa program: a cohort study. Arch Intern Med. 2008;168:86–93. doi: 10.1001/archinternmed.2007.10. [DOI] [PubMed] [Google Scholar]
- 3.Gargano JW, Laserson K, Muttai H, et al. The adult population impact of HIV care and antiretroviral therapy in a resource poor setting, 2003–2008. AIDS. 2012;26:1545–1554. doi: 10.1097/QAD.0b013e328353b7b9. [DOI] [PubMed] [Google Scholar]
- 4.Floyd S, Marston M, Baisley K, et al. The effect of antiretroviral therapy provision on all-cause, AIDS and non-AIDS mortality at the population level - a comparative analysis of data from four settings in Southern and East Africa. Tropical Medicine & International Health. 2012;17:e84–e93. doi: 10.1111/j.1365-3156.2012.03032.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.April MD, Wood R, Berkowitz BK, et al. The survival benefits of antiretroviral therapy in South Africa. J Infect Dis. 2014;209:491–499. doi: 10.1093/infdis/jit584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cohen M, Chen Y, McCauley M, et al. Prevention of HIV-1 infection with early antiretroviral therapy. N Engl J Med. 2011;365:493–505. doi: 10.1056/NEJMoa1105243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Birrell PJ, Gill ON, Delpech VC, et al. HIV incidence in men who have sex with men in England and Wales 2001-10: a nationwide population study. Lancet Infectious Diseases. 2013;13:313–318. doi: 10.1016/S1473-3099(12)70341-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Smith MK, Powers KA, Muessig KE, Miller WC, Cohen MS. HIV treatment as prevention: the utility and limitations of ecological observation. Plos Medicine. 2012;9:e1001260. doi: 10.1371/journal.pmed.1001260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wilson DP. HIV treatment as prevention: natural experiments highlight limits of antiretroviral treatment as HIV prevention. Plos Medicine. 2012;9:e1001231. doi: 10.1371/journal.pmed.1001231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Montaner JSG, Lima VD, Barrios R, et al. Association of highly active antiretroviral therapy coverage, population viral load, and yearly new HIV diagnoses in British Columbia, Canada: a population-based study. Lancet. 2010;376:532–539. doi: 10.1016/S0140-6736(10)60936-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tanser F, Bärnighausen T, Grapsa E, Zaidi J, Newell M-L. High coverage of ART associated with decline in risk of HIV acquisition in rural KwaZulu-Natal, South Africa. Science. 2013;339:966–971. doi: 10.1126/science.1228160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Vandormael A, Newell ML, Bärnighausen T, Tanser F. Use of antiretroviral therapy in households and risk of HIV acquisition in rural KwaZulu-Natal, South Africa, 2004-12: a prospective cohort study. Lancet Global Health. 2014;2:e209–e215. doi: 10.1016/S2214-109X(14)70018-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Iwuji CC, Orne-Gliemann J, Tanser F, et al. Evaluation of the impact of immediate versus WHO recommendations-guided antiretroviral therapy initiation on HIV incidence: the ANRS 12249 TasP (Treatment as Prevention) trial in Hlabisa sub-district, KwaZulu-Natal, South Africa: study protocol for a cluster randomised controlled trial. Trials. 2013;14:230. doi: 10.1186/1745-6215-14-230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hayes R, Ayles H, Beyers N, et al. HPTN 071 (PopART): rationale and design of a cluster-randomised trial of the population impact of an HIV combination prevention intervention including universal testing and treatment - a study protocol for a cluster randomised trial. Trials. 2014;15:57. doi: 10.1186/1745-6215-15-57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Botswana combination prevention project (BCPP) [Accessed 28 Mar 2014]; at http://clinicaltrials.gov/ct2/show/study/NCT01965470.
- 16.Kennedy CE, Kerrigan DD, Brahmbhatt H, et al. Setting the stage for combination HIV prevention: developing a strategic assessment to inform a cluster-randomized trial in Iringa, Tanzania; Washington, DC. XIX International AIDS Conference.2012. Jul 22–27, [Google Scholar]
- 17.HPTN studies in development. [Accessed 28 Mar 2014];HIV Prevention Trials Network. at http://www.hptn.org/research_studies/Developing.asp.
- 18.Consolidated Guidelines on the Use of Antiretroviral Durgs for Treating and Preventing HIV Infection. [Accessed 28 Mar 2014];World Health Organization. 2013 at http://apps.who.int/iris/bitstream/10665/85321/1/9789241505727_eng.pdf)
- 19.Goldie SJ, Yazdanpanah Y, Losina E, et al. Cost-effectiveness of HIV treatment in resource-poor settings - The case of Côte d'Ivoire. New England Journal of Medicine. 2006;355:1141–1153. doi: 10.1056/NEJMsa060247. [DOI] [PubMed] [Google Scholar]
- 20.Walensky RP, Wood R, Weinstein MC, et al. Scaling up antiretroviral therapy in South Africa: The impact of speed on survival. J Infect Dis. 2008;197:1324–U23. doi: 10.1086/587184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ross EL, Pei PP, Walensky RP, Losina E, Weinstein MC, Freedberg KA. Modeling HIV transmission dynamics in conjunction with a microsimulation of disease progression. Baltimore, MD: Society for Medical Decision Making; 2013. Oct 19–23, [Google Scholar]
- 22.Attia S, Egger M, Muller M, Zwahlen M, Low N. Sexual transmission of HIV according to viral load and antiretroviral therapy: systematic review and meta-analysis. AIDS. 2009;23:1397–1404. doi: 10.1097/QAD.0b013e32832b7dca. [DOI] [PubMed] [Google Scholar]
- 23.World dataBank: World Development Indicators (WDI), Health Nutrition and Population Statistics. [Accessed 28 Mar, 2014];World Bank. 2012 at http://databank.worldbank.org/ddp/home.do)
- 24.United Nations Department of Economic and Social Affairs. World population prospects: the 2010 revision. New York: United Nations; 2011. [Google Scholar]
- 25.Ross EL, Weinstein MC, Schackman BR, et al. The clinical role and cost-effectiveness of long-acting antiretroviral formulations. San Francisco: IDWeek; 2013. Oct 2–5, [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.The South African Antiretroviral Treatment Guidelines. Republic of South Africa Department of Health. [Accessed 28 Mar 2014]; at http://www.sahivsoc.org/upload/documents/2013%20ART%20Guidelines-Short%20Combined%20FINAL%20draft%20guidelines%2014%20March%202013.pdf.
- 27.Hollingsworth TD, Anderson RM, Fraser C. HIV-1 transmission, by stage of infection. J Infect Dis. 2008;198:687–693. doi: 10.1086/590501. [DOI] [PubMed] [Google Scholar]
- 28.Holmes CB, Wood R, Badri M, et al. CD4 decline and incidence of opportunistic infections in Cape Town, South Africa: implications for prophylaxis and treatment. J Acquir Immune Defic Syndr. 2006;42:464–469. doi: 10.1097/01.qai.0000225729.79610.b7. [DOI] [PubMed] [Google Scholar]
- 29.Clinical guidelines for the management of HIV & AIDS in adults and adolescents. Pretoria: National Department of Health; [Google Scholar]
- 30.Anglaret X, Chene G, Attia A, et al. Early chemoprophylaxis with trimethoprim-sulphamethoxazole for HIV-1-infected adults in Abidjan, Côte d'Ivoire: a randomised trial. Lancet. 1999;353:1463–1468. doi: 10.1016/s0140-6736(98)07399-1. [DOI] [PubMed] [Google Scholar]
- 31.Yazdanpanah Y, Losina E, Anglaret X, et al. Clinical impact and cost-effectiveness of co-trimoxazole prophylaxis in patients with HIV/AIDS in Côte d'Ivoire: a trial based analysis. AIDS. 2005;19:1299–1308. doi: 10.1097/01.aids.0000180101.80888.c6. [DOI] [PubMed] [Google Scholar]
- 32.Iwuji CC, Orne-Gliemann J, Newell ML, Dabis F. A cluster-randomised community-based trial of treatment as prevention in rural KwaZulu-Natal, South Africa: early findings; Vancouver, BC. 3rd International HIV Treatment as Prevention Workshop.Apr 22–25, 2013. [Google Scholar]
- 33.Cohen MS, Shaw GM, McMichael AJ, Haynes BF. Acute HIV-1 Infection. New England Journal of Medicine. 2011;364:1943–1954. doi: 10.1056/NEJMra1011874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Faraoni S, Rocchetti A, Gotta F, et al. Evaluation of a rapid antigen and antibody combination test in acute HIV infection. Journal of Clinical Virology. 2013;57:84–87. doi: 10.1016/j.jcv.2013.01.007. [DOI] [PubMed] [Google Scholar]
- 35.Zaidi J, Grapsa E, Tanser F, Newell ML, Bärnighausen T. Dramatic increases in HIV prevalence after scale-up of antiretroviral treatment: a longitudinal population-based HIV surveillance study in rural KwaZulu-Natal. AIDS. 2013;27:2301–2305. doi: 10.1097/QAD.0b013e328362e832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Summary Outputs, ASSA 2008 model. [Accessed 28 Mar 2014];Actuarial Society of South Africa. 2011 at http://aids.actuarialsociety.org.za/ASSA2008-Model-3480.htm)
- 37.Kranzer K, van Schaik N, Karmue U, et al. High prevalence of self-reported undiagnosed HIV despite high coverage of HIV testing: a cross-sectional population based sero-survey in South Africa. Plos One. 2011;6:e25244. doi: 10.1371/journal.pone.0025244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Long L, Fox M, Sanne I, Rosen S. The high cost of second-line antiretroviral therapy for HIV/AIDS in South Africa. AIDS. 2010;24:915–919. doi: 10.1097/QAD.0b013e3283360976. [DOI] [PubMed] [Google Scholar]
- 39.Tanser F, Hosegood V, Bärnighausen T, et al. Cohort profile: Africa Centre Demographic Information System (ACDIS) and population-based HIV survey. International Journal of Epidemiology. 2008;37:956–962. doi: 10.1093/ije/dym211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.UNAIDS. Global HIV/AIDS Response: Epidemic update and health sector progress towards Universal Access. Geneva: World Health Organization; 2011. [Google Scholar]
- 41.Lodi S, Phillips A, Touloumi G, et al. Time from human immunodeficiency virus seroconversion to reaching CD4+ cell count thresholds < 200, < 350, and < 500 cells/mm(3): assessment of need following changes in treatment guidelines. Clin Infect Dis. 2011;53:817–825. doi: 10.1093/cid/cir494. [DOI] [PubMed] [Google Scholar]
- 42.Malaza A, Mossong J, Bärnighausen T, Viljoen J, Newell M-L. Population-based CD4 counts in a rural area in South Africa with high HIV prevalence and high antiretroviral treatment coverage. PloS one. 2013;8:e70126. doi: 10.1371/journal.pone.0070126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Messou E, Chaix M-L, Gabillard D, et al. Association between medication possession ratio, virologic failure and drug resistance in HIV-1-infected adults on antiretroviral therapy in Côte d'Ivoire. J Acquir Immune Defic Syndr. 2011;56:356–364. doi: 10.1097/QAI.0b013e3182084b5a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Mutevedzi PC, Lessells RJ, Heller T, Bärnighausen T, Cooke GS, Newell M-L. Scale-up of a decentralized HIV treatment programme in rural KwaZulu-Natal, South Africa: does rapid expansion affect patient outcomes? Bulletin of the World Health Organization. 2010;88:593–600. doi: 10.2471/BLT.09.069419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Orrell C, Harling G, Lawn SD, et al. Conservation of first-line antiretroviral treatment regimen where therapeutic options are limited. Antiviral Therapy. 2007;12:83–88. [PubMed] [Google Scholar]
- 46.Rawizza H, Chaplin B, Meloni S, et al. High rates of re-suppression among patients with viral load failure on second-line ART in Nigeria; Atlanta, Georgia. 20th Conference on Retroviruses and Opportunistic Infections.2013. Mar 3–6, [Google Scholar]
- 47.Messou E, Kouakou M, Gabillard D, et al. Medication possession ratio: predicting and decreasing loss to follow-up in antiretroviral treatment programs in Côte d'Ivoire. J Acquir Immune Defic Syndr. 2011;57:S34–S39. doi: 10.1097/QAI.0b013e3182208003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Lessells RJ, Mutevedzi PC, Cooke GS, Newell M-L. Retention in HIV care for individuals not yet eligible for antiretroviral therapy: rural KwaZulu-Natal, South Africa. J Acquir Immune Defic Syndr. 2011;56:E79–E86. doi: 10.1097/QAI.0b013e3182075ae2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Bärnighausen T, Tanser F, Newell M-L. Lack of a Decline in HIV Incidence in a Rural Community with High HIV Prevalence in South Africa, 2003–2007. Aids Research and Human Retroviruses. 2009;25:405–409. doi: 10.1089/aid.2008.0211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Bärnighausen T, Wallrauch C, Welte A, et al. HIV incidence in rural South Africa: comparison of estimates from longitudinal surveillance and cross-sectional cBED assay testing. Plos One. 2008;3:e3640. doi: 10.1371/journal.pone.0003640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.van Rooyen H, Barnabas RV, Baeten JM, et al. High HIV Testing Uptake and Linkage to Care in a Novel Program of Home-Based HIV Counseling and Testing With Facilitated Referral in KwaZulu-Natal, South Africa. J Acquir Immune Defic Syndr. 2013;64:e1–e18. doi: 10.1097/QAI.0b013e31829b567d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Sugarman J, Grace WC. Ethics and the standards of prevention in HIV prevention trials. AIDS. 2010;24:2298–2299. doi: 10.1097/QAD.0b013e32833d4364. [DOI] [PubMed] [Google Scholar]
- 53.Padian NS, McCoy SI, Balkus JE, Wasserheit JN. Weighing the gold in the gold standard: challenges in HIV prevention research. AIDS. 2010;24:621–635. doi: 10.1097/QAD.0b013e328337798a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Boily M-C, Masse B, Alsallaq R, et al. HIV treatment as prevention: considerations in the design, conduct, and analysis of cluster randomized controlled trials of combination HIV prevention. Plos Medicine. 2012;9:e1001250. doi: 10.1371/journal.pmed.1001250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Boily MC, Lowndes CM, Alary M. Complementary hypothesis concerning the community sexually transmitted disease mass treatment puzzle in Rakai, Uganda. AIDS. 2000;14:2583–2592. doi: 10.1097/00002030-200011100-00022. [DOI] [PubMed] [Google Scholar]
- 56.Baggaley RF, Powers KA, Boily M-C. What do mathematical models tell us about the emergence and spread of drug-resistant HIV? Current Opinion in Hiv and Aids. 2011;6:131–140. doi: 10.1097/COH.0b013e328343ad03. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Granich R, Kahn J, Bennett R, et al. Expanding ART for treatment and prevention of HIV in South Africa: estimated cost and cost-effectiveness 2011–2050. PLoS One. 2012;7:e30216. doi: 10.1371/journal.pone.0030216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Bärnighausen T, Salomon JA, Sangrujee N. HIV treatment as prevention: issues in economic evaluation. Plos Medicine. 2012;9:e1001263. doi: 10.1371/journal.pmed.1001263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Walensky RP, Ross EL, Kumarasamy N, et al. Cost-effectiveness of HIV treatment as prevention in serodiscordant couples. N Engl J Med. 2013;369:1715–1725. doi: 10.1056/NEJMsa1214720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Powers K, Ghani A, Miller W, et al. The role of acute and early HIV infection in the spread of HIV and implications for transmission prevention strategies in Lilongwe, Malawi: a modelling study. Lancet. 2011;378:256–268. doi: 10.1016/S0140-6736(11)60842-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Johnson L. THEMBISA version 1.0: A model for evaluating the impact of HIV/AIDS in South Africa (working paper) [Accessed 17 July, 2014];Centre for Infectious Disease Epidemiology and Research, University of Cape Town. 2014 at http://webdav.uct.ac.za/depts/epi/publications/documents/THEMBISA%20version%201.0.pdf.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.