Abstract
Proteins are sensitive to oxidation, and oxidized proteins are excellent substrates for degradation by proteolytic enzymes such as the Proteasome and the mitochondrial Lon protease. Protein labeling is required for studies of protein turnover. Unfortunately, most labeling techniques involve 3H or 14C methylation which is expensive, exposes researchers to radioactivity, generates large amounts of radioactive waste, and allows only single-point assays because samples require acid-precipitation. Alternative labeling methods, have largely proven unsuitable, either because the probe itself is modified by the oxidant(s) being studied, or because the alternative labeling techniques are too complex or too costly for routine use. What is needed is a simple, quick, and cheap labeling technique that uses a non-radioactive marker, that binds strongly to proteins, is resistant to oxidative modification, and emits a strong signal. We have devised a new reductive method for labeling free carboxyl groups of proteins with the small fluorophore 7-amino-4-methycoumarin (AMC). When bound to target proteins, AMC fluoresces very weakly but when AMC is released by proteinases, proteases, or peptidases, it fluoresces strongly. Thus, without acid-precipitation, the proteolysis of any target protein can be studied continuously, in multiwell plates. In direct comparisons, 3H-labeled proteins and AMC-labeled proteins exhibited essentially identical degradation patterns during incubation with trypsin, cell extracts, and purified proteasome. AMC-labeled proteins are well-suited to study increased proteolytic susceptibility following protein modification, since the AMC-protein bond is resistant to oxidizing agents such as hydrogen peroxide and peroxynitrite, and is stable over time and to extremes of pH, temperature (even boiling), freeze-thawing, mercaptoethanol, and methanol.
Keywords: Protein Labeling, Fluorescent Label, Non-radioactive Label, Oxidative stress, Ubiquitin-Proteasome System, Protein Degradation, Protein Oxidation, Protein Modification, Protein Degradation, Proteolysis, Protein Turnover
INTRODUCTION
The free radical/oxidative stress field has a long history of papers devoted to lipid peroxidation and DNA oxidation, but the study of protein oxidation and, particularly, altered proteolytic susceptibility has not been studied by very many laboratories. Reasons for this apparent reluctance to measure protein degradation as a consequence of oxidative stress may well include the difficulty, expense, and (even) danger of the available methods. Basically, until now, if one wanted to study how oxidation may change the proteolytic susceptibility of any given purified protein (or mixture of protein substrates), one needed to be willing to use radioactive labels, or tracers. For many laboratories, the complicated protein labeling techniques, radioactive isotope training and licenses or permits, radioactive waste disposal problems, potential dangers to lab. workers, and the high costs of radioactive techniques have proven to be major barriers to the study of protein oxidation and proteolysis.
The use of 3H and 14C labeling of proteins by in vitro reductive methylation has become the major tool by which to measure the proteolytic degradation of a wide range of protein substrates by purified proteolytic enzymes, cell lysates, and cell extracts. Such 3H and 14C labeled protein substrates are also widely used to assess the effects of protein modifications, such as oxidation, denaturation, methylation, acetylation, etc., on proteolytic susceptibility and rates of turnover. In addition, the specificity of various proteolytic enzymes for putative substrates has frequently been tested using 3H and 14C labeled proteins[1–17]. The process of in vitro reductive methylation with 3H and 14C, however, has many drawbacks. The use of radioactive materials, with all the attendant exposure risks for experimenters and their colleagues, and the difficulties and ethical considerations of radioactive waste procedures rank high on the list of drawbacks. Additionally, the costs both of purchasing radionucleotides and disposing of them are extremely high. Proteolytic assays with 3H and 14C labeled protein substrates require a labor-intensive trichloloracetic acid (TCA) precipitation step, so that undegraded (TCA-insoluble) proteins can be separated from TCA-soluble degradation products; This further increases the volume of radioactive waste, limits the number of samples that may be analyzed, increases experimental error, and forces an absolute endpoint to the assay with the result that true time courses cannot be measured.
Fluorometric peptidase assays, in which a fluorophore covalently linked to a small peptide sequence is cleaved by a protease/protienase, provides a solution to all the above radiolabeling problems, and fluorogenic peptides are widely used to measure peptidase activities. Such fluorogenic peptidase measurements are based on the increase in fluorescence as the fluorophore is released from the peptide by proteolytic cleavage. TCA precipitation is not required, thus enabling continuous readings to be made, as well as permitting a greater number of assays to be performed. While this technology has been highly valuable in measuring the cleavage of short peptide sequences[6, 17, 18], it is only a primitive model with which to test the activities of complete proteinases which target whole proteins rather than short peptides. Additionally many proteinases are selective for various modified (e.g. oxidized) forms of their protein substrates, and such selectivity cannot be measured by peptide hydrolysis[19].
A solution would seem to be that of adapting the fluorescent labeling technique for peptides to work with intact proteins, but there has been limited success in modifying this technology to measure the degradation of whole proteins. Two techniques have been described for attaching fluorophores onto proteins FITC labeling has been used to label casein[20], hemoglobin[21] and BSA[22]. However, FITC-labeled proteins are highly unstable and so must be precipitated and stored in 50% ammonium sulfate then transferred out of solution, just prior to use. These steps are major drawbacks, and present considerable contamination risks as well as limiting the time over which assays can be performed[22]. The assay is further limited by a strong dependency on pH for the sensitivity of the fluorophore, making assays of strongly acidic proteases like pepsin, or strongly alkaline proteases like protienase K, impractical[23]. In addition, for measuring proteolysis, this technique is, like radio-labeling, limited by the requirement for TCA precipitation which makes it labor intensive, error prone and extremely limited to small-size experiments[20]. The second technique describes labeling of either casein or BSA with BODIPY[23]. This technique provides a number of advantages over both FITC labeling and radio-labeling, though it also has several drawbacks. For example, BODIPY has a very small separation between excitation and emission wavelengths (503/512) when compared to other fluorophores such as AMC (365/444) which makes it extremely difficult to detect the signal without highly specialized equipment, The label is relatively large and complex (389Da-634Da, depending on type of BODIPY label) compared to the small [3H]formaldehyde label (32Da) used in radio-labeling, this raises some concerns about modification of the protein during BODIPY labeling. BODIPY is also relatively expensive for very small quantities, when compared with other fluorophores. Finally, there are only a small number of assays for which BODIPY has been described. Thus, most studies of protein degradation continue to rely on in vitro radio-labeling (3H or 14C) of purified protein substrates, using the technique of reductive methylation developed by Jentoft and Dearborn[5].
While in vitro radio-labeling of protein substrates is something we would like to avoid, it occurred to us that reductive methylation remains an efficient and relatively mild procedure by which to attach a label to a protein. In addition, the careful experiments of Jentoft and Dearborn[5] demonstrated the high stability of such adducts, and thousands of studies over the past 30 years have verified the usefulness of reductively methylated protein substrates. We, therefore, set out to test whether we could take the fluorophore 7-amino-4-methylcoumarin (AMC), which is a small molecule (MW 175) that is commonly used in the substrates of peptidase activity assays (e.g. Suc-LLVY-AMC), and adduct it to protein substrates by an alternative reductive technique. We were also encouraged by preliminary experiments which indicated that AMC should be resistant to oxidation by agents such as hydrogen peroxide and peroxynitrite, that are widely used in free radical research. Thus, we attempted to generate stable AMC-labeled proteins by a simple and rapid method that could be used to measure protein degradation by proteolytic enzymes, in diverse studies of protein modification, including exposure to oxidative stress.
MATERIALS & METHODS
AMC Labeling of Protein Substrates
The protein substrates used for AMC labeling were as follows: Hemoglobin from Sigma-Aldrich (St Louis, MO,USA) catalogue #H-2500, Superoxide Dismutase from Calbiochem (San Diego, CA, USA) catalogue #574594, Catalase from Calbiochem (San Diego, CA, USA) catalogue #219001, and Bovine Serum Albinum from thermo-Fisher (Waltham, Massachusetts, USA) catalogue #BP1605-100. In all cases, 5mg of protein were dissolved in 1ml of 0.1M Hepes buffer to which was added 500μM of AMC (Calbiochem, San Diego, CA, USA, catalogue #164545), as well as 20mM sodium cyanoborohydride (final concentration) from Sigma-Aldrich (St Louis, MO, USA, catalogue #S8628-25G). Solutions were incubated at room temperature for 2 hours, then extensively dialyzed though a 10,000 M.W.C.O centrifugal filter (Millipore, Carrigtwohil, Ireland, catalogue #4321) and a buffer exchange was performed with proteolysis buffer (50mM Tris/HCl pH7.8, 20mM KCl, 5mM magnesium acetate, 0.5mM DTT). Protein content was then determined using the BCA assay kit (Thermo Scientific, Rockford, IL, USA, catalogue #PI-23225).
In some experiments samples were pre-treated with either N-(3-Dimethylaminopropyl)-N′-ethylcarbodiimide hydrochloride (Sigma-Aldrich, MO, USA, catalogue# E6383-1G) to block free protein carboxyl groups, with sulfo-N-hydroxysulfosuccinimide-acetate (Pierce, Rockford, IL, USA, catalogue #26777) to block free protein amino groups, or with tryptamine (Sigma-Aldrich, MO, USA, catalogue# 193747-10G) to disrupt potential non-covalent interactions in protein hydrophobic pockets.
[3H] Labeling of Protein Substrates
Tritium-labeled hemoglobin ([3H]Hb) and BSA ([3H]BSA) were generated in vitro as previously described [1–4, 6] using the [3H]formaldehyde and sodium cyanoborohydrate method of Jentoft and Deaborn [5]. Proteins were then extensively dialyzed.
Cell Culture – Murine Embryonic Fibroblasts
Murine embryonic fibroblasts (MEF) from ATCC (Manassas, VA, USA, catalogue #CRL-2214) were grown in Dulbecco’s Modified Eagle’s Medium (DMEM, Mediatech, Manassas, VA, catalogue #10-013-CV) supplemented with 10% Fetal Bovine Serum (Hyclone, Logan, UT, catalog #SH30070.03). Cells were incubated at 37°C under 5% CO2 and ambient oxygen. To generate cell lysates, MEF were grown to confluence then washed twice with PBS, cells were then scraped using a cell lifter, and centrifuged at 5,000g for 5 minutes. The cells were then re-suspended in proteolysis buffer and subjected to 3 freeze-thaw cycles at −20°C. The lysates were then centrifuged at 10,000g for 10 minutes, after which the supernatants were retained (the pellets discarded) and protein content was determined by BCA assay.
Proteolysis Assay – Common Procedures
Proteolysis was measured by incubation of 1μg of AMC-labeled protein substrate or [3H]-labeled protein substrate in 100ul of proteolysis buffer containing either dissolved Trypsin (VWR, West Chester, PA, USA, catalogue #100504-332), Chymotrypsin (Sigma-Aldrich, MO, USA, catalogue #C-7762), Pepsin (Thermo-Fisher, Waltham, Massachusetts, USA, catalogue #P53), Proteinase K (Oncor, Gaithersburg, MD, USA, catalogue #S4508), purified 20S proteasome (Biomol, Plymouth Meeting, PA, USA, catalogue #PW8720-0050), or lysate generated from MEF cells as above. In each experiment, pH was adjusted appropriately for the proteinase studied, and samples were incubated at 37°C for 4 hours.
Proteolysis of AMC-labeled Proteins by Fluorescence Assay
This procedure was used with AMC-labeled proteins. It should be noted that free AMC is soluble in water, and that it fluoresces strongly. AMC adducted to proteins, by reductive methylation, fluoresces only minimally (just enough to detect weakly in gel assays) but when liberated by proteolysis it again fluoresces strongly. During incubations described above under “Proteolysis Assay – Common Procedures,” fluorescence was measured every 10 minutes at an emission wavelength of 444nM, with excitation at 390nM, in a Fluoroskan Ascent Microplate Fluorometer (Thermo Fisher, Waltham, Massachusetts, USA, catalogue #5210480). Fluorescence emission was compared with a standard curve of the fluorescence of known concentrations of free AMC, between 5nM and 5mM, to quantify the moles of AMC released into solution.
Proteolysis of [3H]-labeled Proteins by Radioactive Liquid Scintillation Assay
Following incubations described above under “Proteolysis Assay – Common Procedures,” remaining intact protein was precipitated by addition of 20% trichloroacetic acid and 3% BSA (as carrier) as previously described [2, 17, 18, 24, 25]. Percent protein degraded was estimated by release of acid soluble counts into the TCA supernatants, measured by liquid scintilatation, in which Percent Protein Degraded = (acid-soluble counts – background counts) × 100.
SDS and Native Page Gels
For SDS Page gels, samples were mixed with 25% Nupage loading Dye (Invitrogen, Carlsbad, CA, USA, catalogue# NP0007) containing 5% 2-mercaptoethanol, Samples were boiled for 3 minutes then added to a 12% Tris-glycine SDS page gel. (VWR, West Chester, PA, USA, catalogue# 12001-042) and run at 80V for 2hr. In experiments where gel fluorescence was analyzed, gels were placed in a chamber and exposed to an excitation wavelength of 365nM. Silver staining was performed using silverSNAP stain kit II (Waltham, Massachusetts, USA, catalogue# 24612), as described in the product manual. For Commassie staining, gels were incubated in commassie stain (0.1% Coomassie blue R350, 10% methanol 10% acetic acid) for 30 minutes and then repeatedly washed in de-stain solution (10% Methanol, 10% acetic acid) until excess stain was removed. In the case of Native Page Gels, samples were mixed with a loading buffer of 25% glycerol/Brilliant Blue solution. Samples were then run on a 12% Native gels prepared exactly as described in the instructions for preparation of a 12% SDS-Page gels in (Biorad, Hercules, CA, USA, Catalogue# 161-0154) with the exception that 10% SDS was not added to the gel.
RESULTS
Reductively binding AMC to protein carboxyl groups
We hypothesized that sodium cyanoborohydride (NaCNBH3), which is commonly used to label proteins with either H3 or C14 linked formaldehyde, could be used to label proteins with AMC by promoting the formation of a carbon-nitrogen bond between the exposed amine group in the AMC molecule and free carboxyl groups of target proteins (Supplementary Figure. 1).
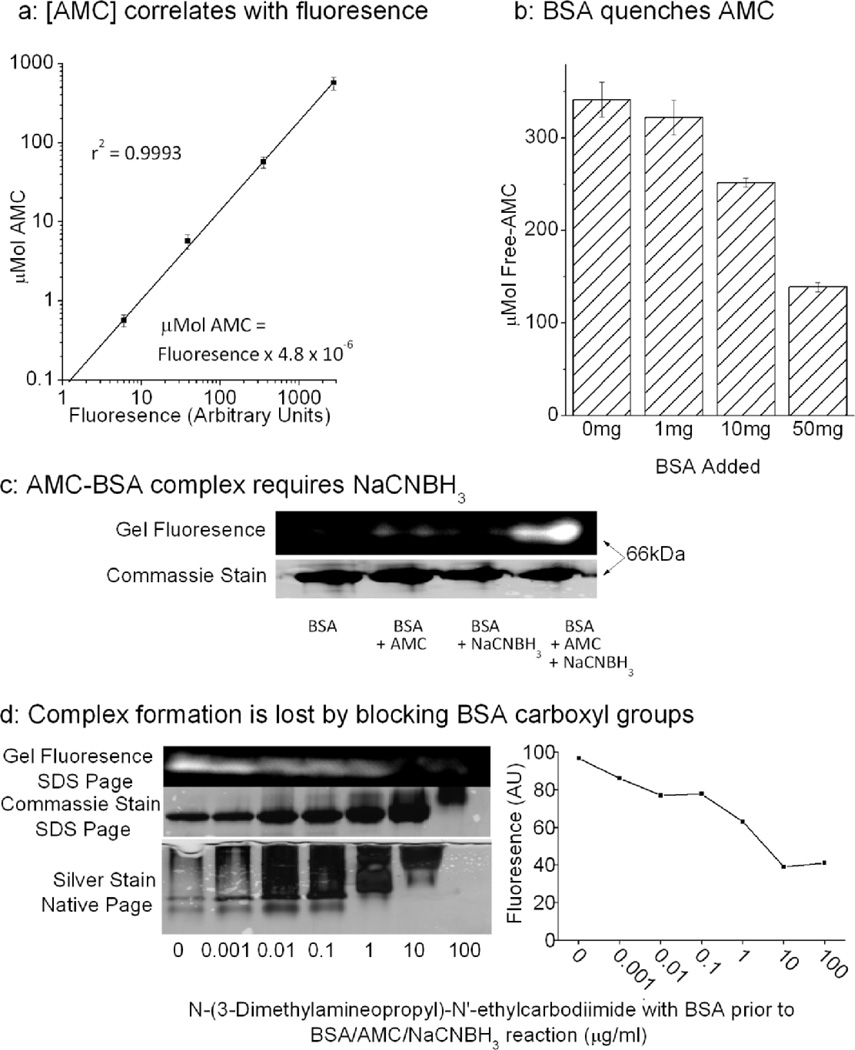
We observed a linear correlation between the concentration of free AMC in solution and it’s fluorescence (Figure. 1a), this enabled us to convert fluorescence readings directly to AMC concentrations. We predicted that incubation of AMC with the protein BSA and the reducing agent NaCNBH3 should result in a reductive labeling reaction, in which the AMC label becomes attached to carboxyl groups on the protein. Binding to proteins could be expected to quench AMC fluorescence. To test this we incubated AMC with increasing concentrations of BSA in the presence of NaCNBH3 (Figure. 1b) and saw a BSA concentration-dependent loss of fluorescence.
Figure 1. AMC can be conjugated to free Carboxyl groups on Proteins.
(a) Linear correlation between free AMC concentration, from 100nM to 1mM, and fluorescence. Here different concentrations of AMC, dissolved in proteolysis buffer, were incubated at 37°C on 96-well plates. Fluorescence was analyzed at an emission wavelength of 444nM, with excitation wavelength of 390nM. Values are means ± SE, n = 3. (b) Addition of increasing amounts of BSA to AMC in the presence of NaCNBH3 progressively quenches the fluorescence of AMC. Here 0–50mg of BSA was added to 100μM AMC and 20mM NaCNBH3 and incubated for 1hr at 37°C. Free AMC content was determined with reference to a standard curve of known AMC concentrations. Values are means ± SE, n = 3. (c) Here 50mg/ml of BSA was incubated with 1mM AMC in the presence or absence of 20mM NaCNBH3, and then run on a 12% SDS Page gel. A fluorescent BSA-AMC complex was readily observed at ≈66kDa (the approximate size of BSA), using an excitation wavelength of 365nM and an emission wavelength of 444 nm, when all three reagents were present, but could only be faintly discerned in the absence of NaCNBH3. A silver stain was later performed. (d) N-(3-Dimethylamineopropyl)-N'-ethylcarbodiimide (1ng/ml to 100μg/ml) which blocks free carboxyl groups[26], was incubated with 50mg of BSA for 1hr. BSA was extensively dialyzed then then prepared as in panel (c). Increasing concentrations of N-(3-Dimethylamineopropyl)-N'-ethylcarbodiimide, caused a progressive decrease in BSA’s electrophoretic mobility, and loss of fluorescence at 66kDa; a representative gel is shown to the left of the panel, and fluorescence is quantified in the graph to the right.
To determine whether binding was actually occurring, we next ran SDS PAGE of BSA treated with AMC ± NaCNBH3 (Figure. 1c). A very weakly fluorescent band was observed at the molecular size of BSA (≈66kDa) when AMC was incubated with BSA, but a much stronger 660Kda fluorescent band was seen when the protein was reacted with both AMC and NaCNBH3 together. This implies that the binding of fluorophore to protein requires a reductive step. It is also clear that although protein-bound AMC can be detected by fluorescence, the fluorescence yield (brightness) of protein-bound AMC is only a fraction of that seen with free AMC. To test if AMC actually binds to free-carboxyl groups, as hypothesized, we incubated 50mg of BSA with 1ng-100μg of N-(3-Dimethylamineopropyl)-N'-ethylcarbodiimide, which effectively blocks exposed carboxyl groups[26]. After one hour of incubation we extensively dialyzed samples to remove any free N-(3-Dimethylamineopropyl)-N'-ethylcarbodiimide, then attempted to react the BSA with AMC and NaCNBH3. Both SDS PAGE and native gels of BSA showed clear proof of dose-dependent protein carboxyl group blocking by N-(3-Dimethylamineopropyl)-N'-ethylcarbodiimide, as evidenced by decreased electrophoretic mobility, as the protein became progressively more electropositive with treatment. The same carboxyl blocking conditions prevented the formation of BSA-AMC adducts, as shown by gradual loss of the fluorescent band at 66kDa (Figure. 1d and quantified in Figure. 1e).
To test whether exposed amine groups on the protein might react with the carboxyl group on the fluorophore, we used 0.5–50mM of Sulfo-NHS-Acetate to block exposed amine groups on BSA. Despite blocking the majority (80%) of free amine groups we saw no significant change in the fluorescence of the BSA/AMC complex (Supplementary Figure. 2a). This implies that the complex formed between AMC and BSA is independent of exposed protein amine groups.
Another possibility was that AMC might be sequestered in protein hydrophobic pockets by non-covalent interactions. To test this we performed a competition experiment with tryptamine to compete with AMC for non-covalent binding sites on the protein, and measured the effect of tryptamine on quenching of AMC by BSA (Supplementary Figure. 3a). We also tested the ability of the BSA/AMC complex to function as a substrate for proteolysis (Supplementary Figure. 3b). Despite using a 100 fold excess of tryptamine (at which concentration, protein structure was probably disrupted) we were only able to block 30% of the association between AMC and BSA, and tryptamine had minimal effects on the effectiveness of BSA as a proteolytic substrate. These results imply that non-covalent interactions do not play a significant role in AMC binding to proteins.
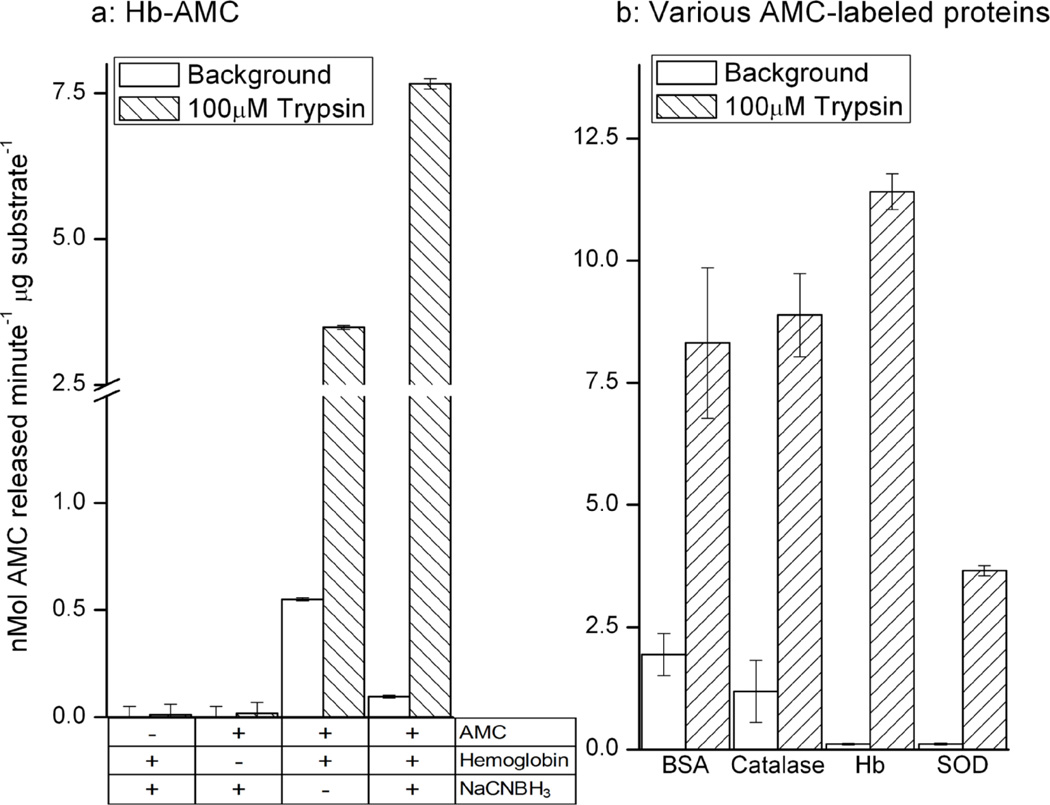
Next, we incubated Hb with NaCNBH3 alone, AMC alone, or AMC and NaCNBH3 then extensively dialyzed the samples to remove any Free AMC or NaCNBH3. As with BSA-AMC (above) we found that Hb formed a stable adduct with AMC (Figure. 2a). To further test the versatility of the labeling process, we repeated the above experiments using hemoglobin (Hb), catalase, and superoxide dismutase (SOD) as substrates and obtained essentially the same results, generating stable AMC-protein adducts (Figure. 2b).
Figure 2. Proteolysis of AMC-labeled Proteins by Trypsin.
(a) Incubation of 1mg/ml of hemoglobin with 100μM AMC and 20mM NaCNBH4 followed by extensive dialysis produced a stable and sensitive substrate for measuring protease activity, in which 10μg/ml of Hb-AMC was combined with 10μM trypsin. Free AMC content was determined with reference to a standard curve of known AMC concentrations. Values are means ± SE’s, n = 3. (B) AMC labeling of BSA, catalase, Hb, or superoxide dismustase (SOD) generates valid substrates substrates for trypsin digestion, as measured by liberation of fluorescent AMC. All assay conditions (including trypsin concentration) were identical to those in panel (a), and each substrate protein was used at a final concentration of 10μg/ml. Free AMC content was determined with reference to a standard curve of known AMC concentrations. Values are means ± SE’s, n = 3.
Utility of AMC-labeled proteins as proteolytic substrates
We next incubated the Hb-AMC substrate with the protease trypsin to determine its usefulness as a proteolytic substrate (Figure. 2A). Trypsin released an extremely large amount of AMC fluorophore from Hb, removing any remaining doubt that the fluorophore had actually been successfully adducted to the protein. Reaction of Hb with AMC alone produced a Hb-AMC proteolytic substrate with high background release of AMC, and about a six-fold increase in AMC liberation following incubation with trypsin. In contrast, use of the full labeling procedure, with NaCNBH3 to increase the strength of the adduct, produced a more stable Hb-AMC proteolytic substrate with only one-sixth the background AMC release, but with an 80-fold increase in AMC liberation after trypsin digestion (Figure. 2a). To test the broad applicability of the AMC labeling technique to measure degradation of proteins in general, we bound the AMC fluorophore to BSA, catalase, hemoglobin, and superoxide dismutase, and observed that all of the AMC-labeled proteins were effective and sensitive substrates for proteolysis by trypsin, as measured by release of fluorescent AMC (Figure. 2B).
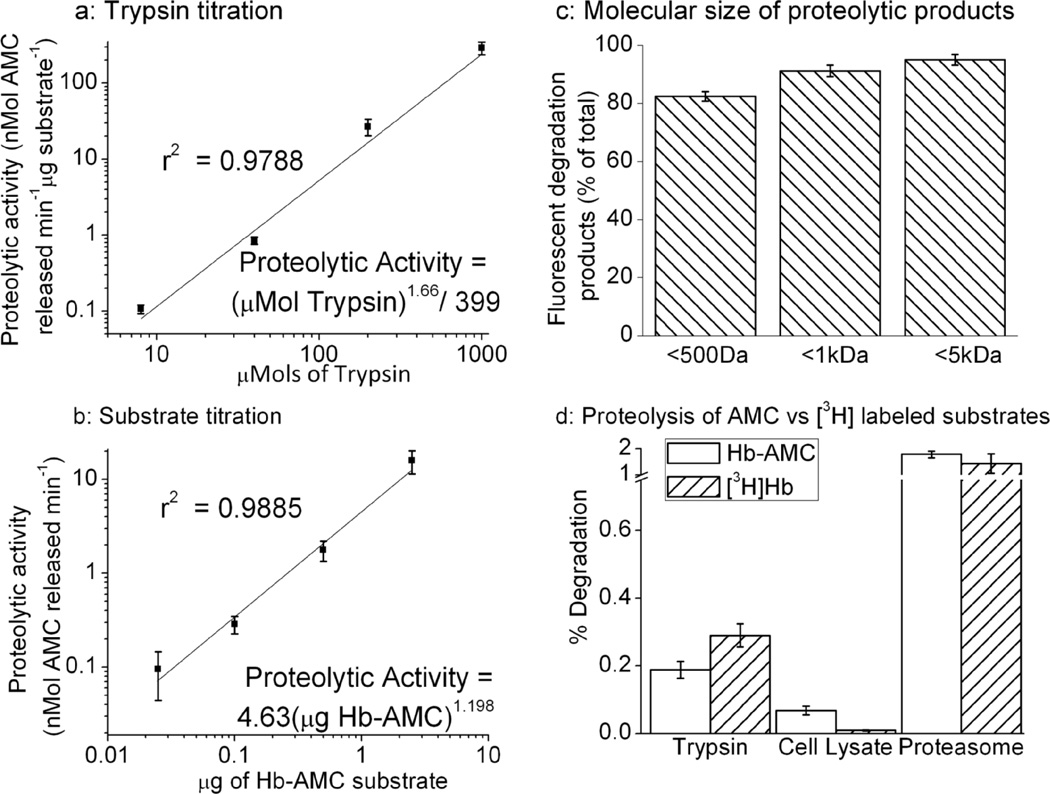
Effective and reliable proteolytic substrates exhibit linear increases in degradation when exposed to linear increases in protease concentration (at least over a fairly wide and useful range), and when substrate concentration is increased in the presence of non-limiting protease activity. To determine the usefulness and reliability of AMC-labeled protein substrates, we assayed AMC release over a wide range of trypsin concentrations and a wide range of substrate concentrations, using Hb-AMC as a model substrate. We observed a linear relationship between proteolytic activity (AMC liberation) and trypsin concentration between 320nM – 1mM trypsin concentrations (Figure. 3A), and 25ng – 2.5μg of Hb-AMC substrate (Figure. 3B), when plotted using log-log scales. With these results we were able to plot linear regression curves with correlation coefficients close to unity: indicating excellent statistical reliability.
Figure 3. Protease and Substrate Titration, and Particle Size of Proteolytic Degradation Products.
(a) A linear relationship between the concentration of protease and AMC release is seen at trypsin concentrations between 320nM-1mM, using an Hb-AMC protein concentration of 10μg/ml. (b) A linear relationship between the concentration of Hb-AMC substrate and proteolytic activity (AMC release) is seen between 25ng – 2.5μg of Hb-AMC All other conditions in both Panels A and B were as described in the legend to Fig. 3 and, in both panels, values are means ± SE’s, n = 3. (c) Dialysis of partially digested Hb-AMC substrate shows that the majority of liberated fluorescent AMC-products consist of particles smaller than 500Da. For this experiment, Hb-AMC (10μg/ml) was incubated with 10μM trypsin at 4°C for 24hr in dialysis tubing, to generate sufficient fluorescent products to measure, but also to preclude complete digestion of the substrate. Values are means ± SE’s, n = 4, for which the fluorescence of controls was subtracted. (d) Hb was labeled with AMC, or with tritium, by reductive labeling in both cases, as described in Materials & Methods. Protein degradation was measured in panel (a) by AMC fluorescence, and in panel (b) by release of acid-soluble [3H] counts by liquid scintillation, as described in Materials & Methods. Background fluorescence or radioactivity were measured in the absence of protease (proteolysis buffer alone), and proteolysis was measured by increased fluorescence or acid-soluble radioactivity after incubation with either 10μM Trypsin, 1μg/ml purified 20S proteosome, or 150μg/ml MEF cell lysate. Percent degradation of Hb-AMC is reported as the percentage of total fluorescence that could be released from Hb-AMC after exhaustive proteolytic digestion (not shown), whereas percent degradation of [3H]Hb is reported as the percent of total (initial) radioactive counts released into TCA-soluble form by proteolysis. All values are means ± SE’s, n = 3.
At this point it seemed clear that free AMC is strongly fluorescent whereas the fluorescence of protein-bound AMC is mostly (but not completely) quenched, and that trypsin-mediated AMC release from AMC-labeled proteins reflects protein degradation. We next wanted to determine the size(s) of protein-AMC degradation products that actually produce fluorescent signals. To study this we partially digested a sample of Hb-AMC. We then dialyzed the sample through <5kDa, <1kDa and <500Da size exclusion membranes into a 500X volume of proteolysis buffer. Dialysis through a 500Da filter caused an ≈80% reduction in signal, compared to a ≈90% reduction with a 1kDa filter and a ≈95% reduction with a 5kDa filter (Figure. 3C). From this we concluded that the majority (80%) of fluorescent products are smaller than 500Da, while another 15% are particles between 500Da and 5kDa, and only some 5% of the signal comes from peptides larger than 5kDa. These results seem quite consistent with proteolysis assays using radio-labeled protein substrates, in which a TCA precipitation step is routinely used to precipitate remaining intact protein, and peptides larger than about 5kDa, so that soluble radioactivity reflects free amino acids and only very small peptides[25]
We also considered it important to directly compare the sensitivity of proteolytic measurements using the AMC-labeled substrates we generated with that of traditional radio-labeled substrates[5]. Thus, we assessed the degradation of Hb-AMC versus [3H]Hb following incubation with various, widely studied proteolytic systems. Our results reveal broadly comparable sensitivity for both substrates, with trypsin, MEF cell lysates, and purified 20S proteasome (Figure. 3d).
Stability of AMC-labeled proteins and resistance to denaturing agents
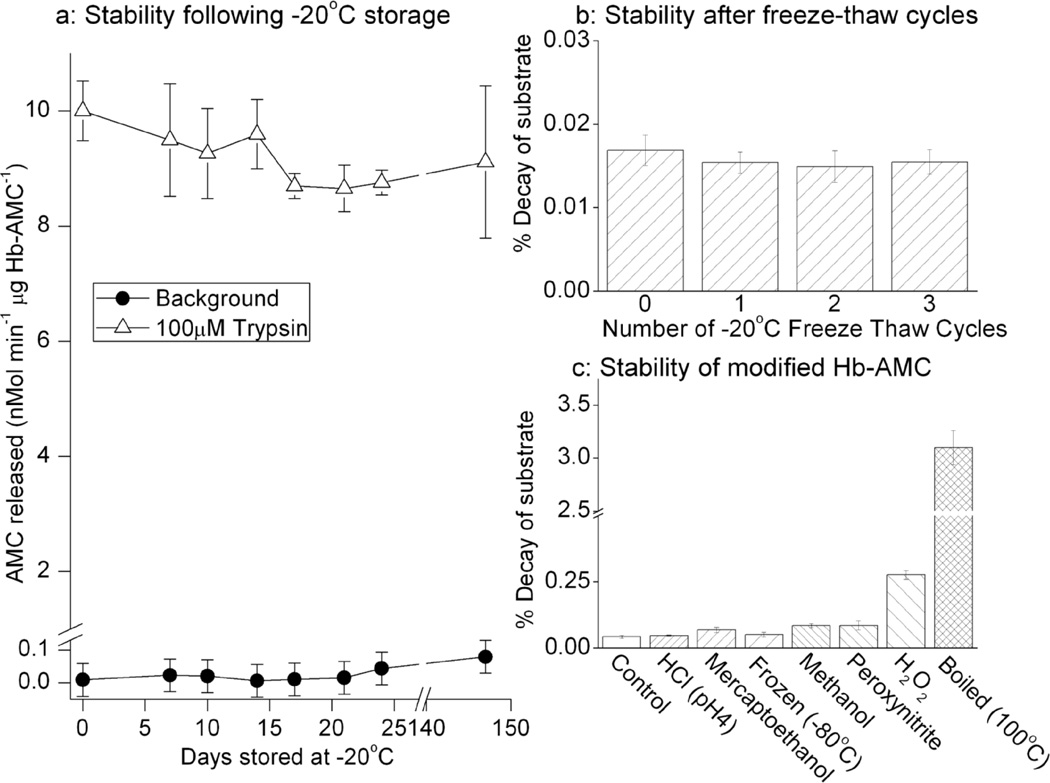
The stability of AMC-labeled substrates, the resistance of the AMC-protein linkage to various treatments, and the reproducibility of proteolytic assays after prolonged storage are important concerns in weighing the usefulness of our technique. To begin to test these matters, we stored Hb-AMC at −20°C and then periodically thawed samples and analyzed both their background release of free AMC (representing undesirable breakdown of the complex) and their proteolytic susceptibility during incubation with trypsin. In repeated trials over 150 days, both the background AMC release, and the trypsin-induced release of AMC varied by less than 15%, indicating that the substrate was quite stable and that samples can be stored for long period of time without significant changes in proteolytic susceptibility (Figure. 4a). As a harsher test of substrate stability we subjected Hb-AMC to repeated freeze thaw cycles and then measured background release of free AMC (Figure. 4b). This did not significantly affect the stability of the Hb-AMC complex.
Figure 4. Stability of AMC-labeled Hemoglobin After Frozen Storage or Denaturation.
(a) Hb-AMC was stored at −20°C for up to 21 weeks. At various time points, samples were thawed, and measurements of both background fluorescence (release of free AMC from the Hb-AMC complex) and liberation of fluorescent AMC by proteolytic digestion with trypsin were measured, as described in Fig. 3a. (b) The stability of Hb-AMC was tested with repeated −20°C freeze-thaw cycles, by measuring release of free AMC from the Hb-AMC complex (background fluorescence). c: Hb-AMC was incubated for 60minutes in dilute HCl at pH 4, 10% 2-mercaptoethanol, 70% methanol, 1mM peroxynitrite,or 1mM H2O2, or was boiled at 100°C for 60minutes or subjected to free-thawing at −80°C. Release of free AMC from the Hb-AMC complex (background fluorescence) was then measured in comparison with control (untreated Hb-AMC). In all three panels, values are means ± SE’s, n = 3.
We started this project because we wanted to find a new way to label proteins for studies of oxidation-induced changes in proteolytic susceptibility. In addition to oxidants, proteolytic substrates are often subjected to various other modifying or denaturing conditions, to test for effects on proteolytic susceptibility, so we considered it important to test the stability of AMC-labeled substrates over a range of harsh conditions. Hb-AMC was almost completely stable to incubation in 1mM H2O2, 1mM peroxynitrite, dilute HCl at pH 4, 10% 2-mercaptoethanol, freeze-thawing at −80°C, or exposure to 50% methanol. Even boiling (100°C) for 60 minutes only caused a 3.1% breakdown of the Hb-AMC complex (Figure. 4c).
Use of AMC-labeled protein substrates with acidic, neutral, and alkaline proteases
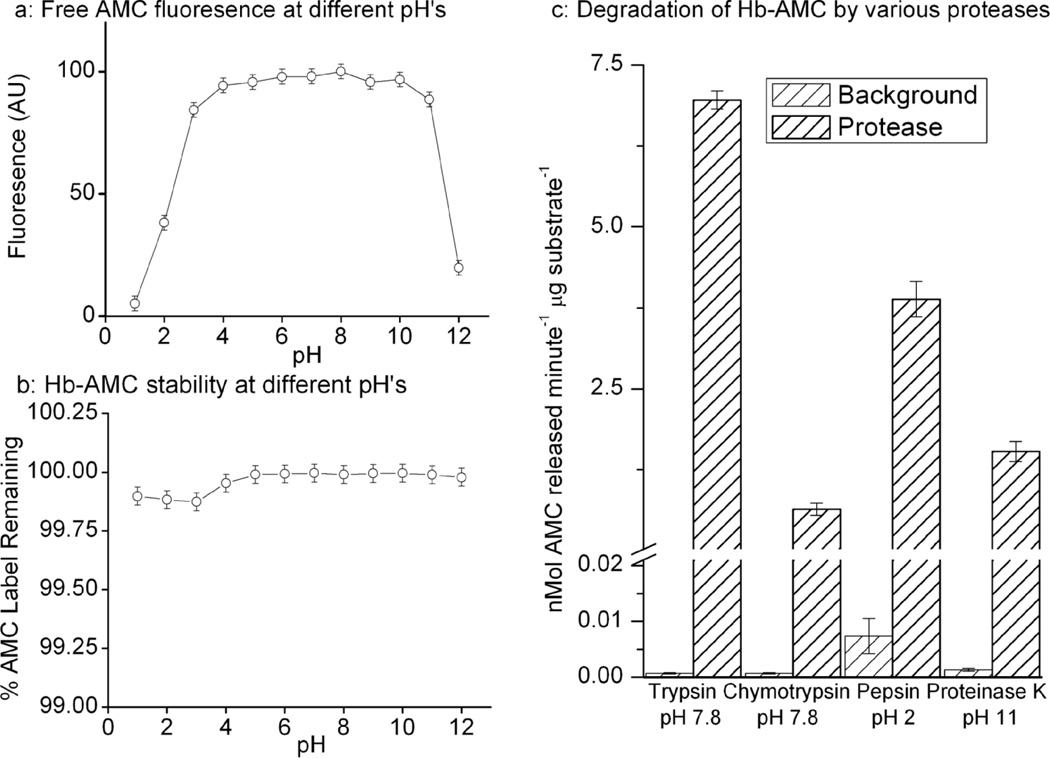
While many proteolytic enzymes have pH optima in the neutral to slightly alkaline range, others are „designed’ to function under strongly acidic or alkaline conditions. We, therefore, needed to test both the fluorescent properties of free AMC over a wide pH range, as well as the stability of protein-AMC complexes. The fluorescence of free AMC was unaffected by mildly acidic or alkaline conditions in a broad range from pH 3–11; highly acidic (below pH 2) or alkaline (above pH 11) conditions, however, significantly decreased AMC fluorescence (Figure. 5a). It should be noted that the fluorescence quenching effects of strong acid or base were completely reversed, with AMC fluorescence returning to normal levels, when pH was neutralized (not shown, but evident in the experiments of Figure. 5b below).
Figure 5. pH Profile of Fluorescence, Stability, and Proteolytic Susceptibility of Free AMC and Hb-AMC.
(a) The fluorescence of free AMC was measured in proteolysis buffer over a wide range of pH conditions. (b) Samples of Hb-AMC were incubated over a range of pH conditions for 4hr. The pH of each sample was then adjusted to pH 7.8 and AMC fluorescence was measured. Results are expressed as a percent of total AMC label originally incorporated into the Hb-AMC complex which was (separately) assessed by exhaustive proteolytic digestion of Hb-AMC, by incubation with 500μM trypsin for 4 hours. (c) Hb-AMC was incubated with 100μM trypsin, 10μM chymotrypsin, 100μM pepsin, or 100μM proteinase K (at the pH shown for each protease) for 4 hr at 37°C and proteolysis was measured by AMC release, as described in the legend to Fig. 3a. Values in all panels are means ± SE’s, n = 3.
We next wanted to determine the stability of protein-AMC adducts over the same broad range of pH. For these experiments, Hb-AMC was incubated for 4 hr, using the same pH conditions as in Figure. 5a, after which the pH of each sample was readjusted to pH 7.8 to assess the stability of the Hb-AMC complex, independent of any possible quenching effects of pH on the fluorophore. We found that the Hb-AMC complex was highly stable over the entire range from pH 1 – 12, with less than a 0.2% decrease in stability observed under any condition (Figure. 5b). We next wished to test the viability of protein-AMC complexes as substrates for proteases with widely different pH optima. As shown in Figure. 5c, Hb-AMC proved to be an excellent substrate for proteolysis with enzymes as diverse as pepsin at pH 2, proteinase K at pH 11, and trypsin or chymotrypsin at pH 7.8.
Use of AMC-labeling to detect the preferential degradation of modified proteins
While digestive enzymes such as trypsin, chymotrypsin, and elastase are very efficient at degrading both normal and modified proteins, major intracellular proteolytic enzymes, such as the Proteasome[1, 17] and the mitochondrial Lon protease[27] exhibit little activity against normal proteins while avidly degrading their modified or damaged forms. The landmark paper of Jentoft and Dearborn[5] demonstrated that reductive methylation is a relatively mild treatment and their work, backed-up by thousands of studies by other researchers in the past 30 years have verified that radiolabeling proteins (by reductive methylation) generates protein substrates that are not extensively modified or denatured. Despite the small size of the AMC fluorophore, we had to be concerned that AMC labeling of proteins might causes a degree of denaturation that would increase the proteolytic susceptibility of normal proteins, making it harder to determine if intentional (experimental) modifications to proteins, such as oxidation, affect their degradation. For a labeling technique to be useful in this regard, one would hope to see only minor degradation of the „normal’ labeled protein but significantly increased degradation of a suitably modified or denatured form by intracellular proteases.
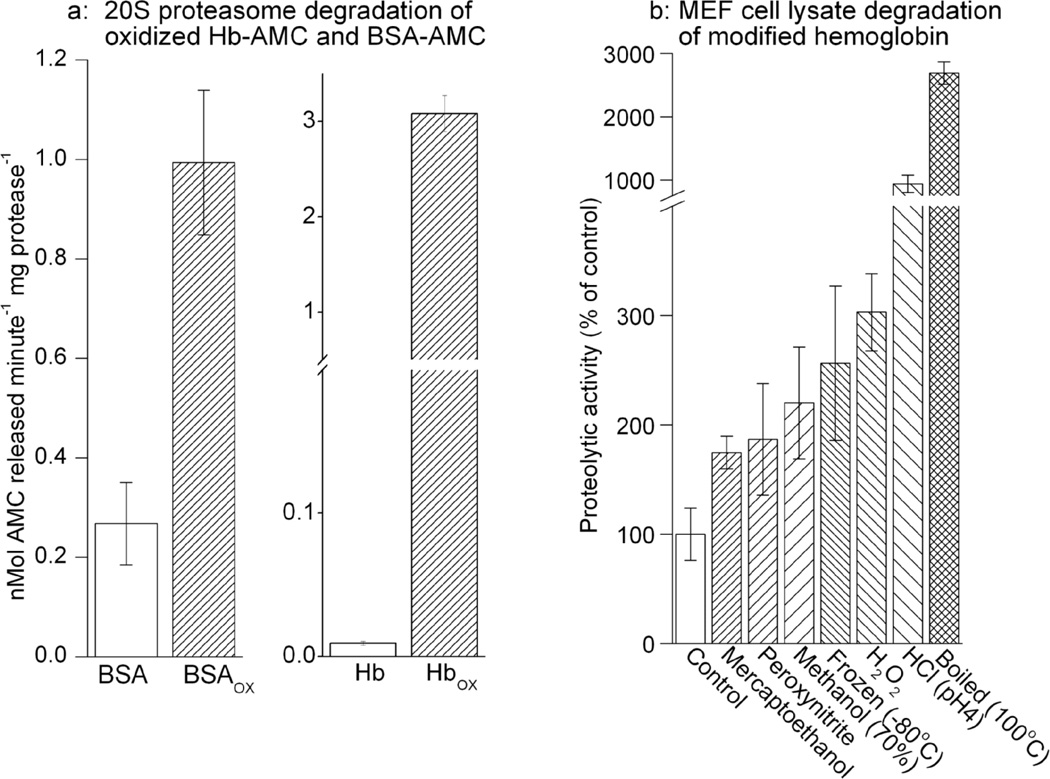
To test this we incubated both control and oxidized forms of Hb-AMC and BSA-AMC with purified 20S proteasome which selectively degrades oxidized proteins[1, 2, 19, 25]. Our results show that the unoxidized forms of BSA-AMC and Hb-AMC were rather poor substrates for the purified proteasome, but BSA-AMC’s susceptibility to proteasomal degradation increased some four-fold following mild oxidation with H2O2, whereas that of Hb-AMC increased by more than 300-fold (Figure. 6A). We additionally tested oxidation of Hb-AMC by peroxynitrite, and a number of other protein denaturing treatments including, boiling, freezing, low pH, methanol, and 2-mercaptoethanol. Both untreated (control) Hb-AMC and the variously treated Hb-AMC samples were then incubated with lysates of MEF cells for measurements of proteolysis. Cell lysates and extracts (which contain proteasome and many other intracellular proteolytic enzymes) are widely employed in many studies of intracellular proteolytic susceptibility[4, 17, 19, 28]. Oxidative modification of Hb-AMC, by H2O2 or peroxynitrite, significantly increased its degradation during (subsequent) incubation with MEF cell extracts, in comparison with unmodified (control) Hb-AMC; similar results were also obtained with other methods of Hb-AMC modification, including boiling, freeze-thawing; or exposure to HCl, methanol, or mercaptoethanol (Figure. 6b).
Figure 6. Proteolytic Susceptibility of Modified AMC-labeled Proteins.
(a) The capacity of 20S proteasome to degrade both the native and oxidized forms of Hb-AMC and BSA-AMC was measured. For both assays, 1μg/ml of purified 20S proteasome was combined with 10μg/ml of Hb-AMC, Hbox-AMC, BSA-AMC, or BSAox-AMC and incubated for 4hr at 37 oC. Protein degradation was then measured as per Fig. 3. Hbox-AMC, and BSAox were prepared by treating Hb-AMC and BSA-AMC with 1.0mM H2O2 followed by extensive dialysis. (b) The Capacity of MEF cell lysates to degrade various modified forms of Hb-AMC was measured. Hb-AMC was modified by incubation with dilute HCl at pH 4, 10% 2-mercaptoethanol, 70% methanol, 1mM peroxynitrite, or 1mM H2O2, or was boiled at 100°C for 60 minutes, or subjected to freeze-thawing at −80°C. The substrates were then extensively dialyzed and incubated with 150μg/ml of MEF cell lysates for 4hr. In both panels, values are means ± SE’s, n = 3.
DISCUSSION
Our studies describe a novel technique for in vitro protein labeling that is free of radio-isotopes. Although our technique contains a reductive step, it is quite distinct from the radio-labeling procedure originally described by Means and Feeney[29], and then subsequently adapted by Rice and Means[30] and Jentoft and Dearborn[5], in which either [14C] or [3H] formaldehyde forms a covalent linkage with free amino groups on target proteins, using the reducing agent NaBH4 or its milder variant NaCNBH3. In our method, the fluorophore AMC is reductively (NaCNBH3) conjugated with free protein carboxyl groups, and no methylation step is involved.
We have described a novel technique by which an inexpensive and stable AMC fluorophore-protein complex can be formed both quickly and simply by reductively adducting AMC to free carboxyl groups. We go on to demonstrate that this technique is applicable to a wide range of protein substrates, and that it can be used to measure proteolytic susceptibility with high sensitivity, comparable to that achieved with radio-labeled proteins. Finally, we show that AMC-protein adducts are stable to oxidation and various other denaturing conditions, and can be used to measure the increased proteolytic susceptibility of oxidatively modified proteins, as well as proteins modified by other denaturing treatments. In addition to their utility as proteolytic substrates, AMC-labeled proteins could also be used for any other project requiring sensitive detection of stably labeled proteins.
AMC labeling appears to generate substrates which are comparable to 3H or 14C labeled proteins in terms of versatility, stability and reproducibility, and which have several advantages over radiolabeling in terms of safety, labor and cost. Radio-isotopes can be hazardous to use, costly to store or discard, and require complicated and time-consuming training and use permits. Proteolysis assays with radio-labeled substrates require an acid precipitation and centrifugation step (to precipitate undegraded proteins) before sample supernatants are transferred to scintillation vials to quantify 3H or 14C release. These steps are highly work-intensive and error-prone, are a limit to sample numbers, and preclude continuous monitoring of individual samples over time. In comparison, fluorescence assays with AMC-labeled proteins can be easily performed on 96-well plates, with no TCA prcecipitation or centrifugation, and with continuous monitoring of proteolytic activity over (real) time.
AMC is relatively cheap, compared with radio-labeled formaldehyde. This makes the labeling process approximately 40 times cheaper than 3H or 14C labeling (based on label usage in Figure. 3c). The labeling procedure is also fast and easy, and requires no specialized equipment or training. These factors will now make it feasible for researchers to generate, store, and study whole libraries of labeled protein substrates. Finally, AMC’s fluorescent properties, and the AMC-protein bond are stable to oxidation, boiling, freezing, and other modifying or denaturing conditions, while the protein itself can still be modified. Thus AMC-labeled proteins can be used to measure changes in proteolytic susceptibility following oxidation, or any number of other protein modifying treatments.
Supplementary Material
(a) To test if reductive labeling might cause binding of AMC to lysine (epsilon) amino groups, and/or the N-terminus of proteins, we performed AMC labeling experiments with both native protein, and with protein in which free amino groups were blocked with 0.5–50mM sulfo-N-hydroxysulfosuccinimide-acetate (sulfo-NHS-acetate from Pierce, Rockford, IL, USA, catalogue #26777). BSA was suspended in 0.1M sodium carbonate buffer pH 8.5. 1.6μM BSA (representing 1mM of amino acids, based on a 607 amino acid sequence) was incubated with 0–50mM of sulfo-nhs-acetate (i.e., up to a 50 fold molar excess) for 1 hour at room temperature. Following preparation, samples were extensively dialyzed and a buffer exchange was performed with HEPES in preparation for AMC labeling. Free lysine content was determined through colorimetric analysis (absorbance at 250nm) following incubation of 1μl samples with 100μl of 2,4,6 trinitrobenzene sulfonic acid (Thermo-fisher, Waltham, Massachusetts, USA, catalogue #TS-28997) for 30 minutes. Values are means ± SE, n = 12. (b) treatment with 0.5–50mM sulfo-nhs-acetate appears to cause little significant change in gel fluorescence. Samples prepared as in panel (a) were incubated with AMC in the presence of NaCNBH3 and run on SDS Page gels as in Figure 2c–e. The BSA-AMC fluorescent band was unaffected by 0.5mM sulfo-NHS-acetate, which caused a 50% loss of free amino groups in panel (a). Even pretreatment with 5.0mM sulfo-NHS-acetate caused less than 10% loss of the BSA-AMC fluorescent band, despite causing more than 75% loss of free amino groups in panel (a). Significant loss of the BSA-AMC fluorescent band was only observed after pretreatment with 50mM sulfo-NHS-acetate, which caused extensive modification of the protein, as evidenced by altered mobility and blurred appearance in the silver stained samples.
(a) We incubated 1mM AMC and 0 – 1000μg of BSA with 20mM NaCNBH3, with and without 100mM tryptamine, for 2 hours at 37°C. Where tryptamine was used, it was first incubated with BSA for 30 minutes at 37°C prior to the addition of AMC and NaCNBH3. Fluorescence was measured as in Figure 1b. Values were plotted as a percent of fluorescence in the absence of BSA, and are means ± SE, n = 4. (b) We added 0–100mM Tryptamine to 1.6μM BSA (1mM of amino acids based on a 607 amino acid sequence) for 30 minutes. Next 1mM AMC and 20mM NaCNBH3 (final concentrations) were added. Samples were incubated for 2 hours, then dialyzed extensively with buffer exchanged for proteolysis buffer. Samples were then incubated with or without 100μM trypsin to measure proteolytic capacity as in Figure 3, values are means ± SE, n = 3. It is important to note that, in both panels, tryptamine was present when AMC + NaCNBH3 so that it could (potentially) compete with AMC labeling.
Acknowledgments
FUNDING
This research was supported by grant #RO1-ES003598, and by ARRA Supplement 3RO1-ES 003598-22S2, both from the NIH/NIEHS to KJAD.
ABBREVIATIONS USED
The abbreviations used are:
- AMC
the fluorophore 7-amino-4-methycoumarin
- Hb-AMC
AMC-labeled hemoglobin
- BSA-AMC
AMC-labeled bovine serum albumin
- SOD-AMC
AMC-labeled superoxide dismutase
- H2O2
hydrogen peroxide
- MEF
murine embryonic fibroblasts
- [3H]Hb
tritium-labeled hemoglobin
- Hbox
oxidized hemoglobin
- BSAox
oxidized bovine serum albinum
- TCA
trichloroacetic acid
- sulfo-NHS-acetate
sulfo-N-hydroxysulfosuccinimide-acetate
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
STATEMENT OF FINANCIAL INTEREST
The University of Southern California has filed a Preliminary Patent Application citing the technique of protein labeling, and the measurements of proteolysis, described in this paper. The authors share a partial financial interest in this patent application.
REFERENCES
- 1.Davies KJA. Degradation of oxidized proteins by the 20S proteasome. Biochimie. 2001;83:301–310. doi: 10.1016/s0300-9084(01)01250-0. [DOI] [PubMed] [Google Scholar]
- 2.Shringarpure R, Grune T, Mehlhase J, Davies KJA. Ubiquitin conjugation is not required for the degradation of oxidized proteins by proteasome. J Biol Chem. 2003;278:311–318. doi: 10.1074/jbc.M206279200. [DOI] [PubMed] [Google Scholar]
- 3.Grune T, Reinheckel T, Joshi M, Davies KJA. Proteolysis in cultured liver epithelial cells during oxidative stress. Role of the multicatalytic proteinase complex, proteasome. J Biol Chem. 1995;270:2344–2351. doi: 10.1074/jbc.270.5.2344. [DOI] [PubMed] [Google Scholar]
- 4.Grune T, Reinheckel T, Davies KJA. Degradation of oxidized proteins in K562 human hematopoietic cells by proteasome. J Biol Chem. 1996;271:15504–15509. doi: 10.1074/jbc.271.26.15504. [DOI] [PubMed] [Google Scholar]
- 5.Jentoft N, Dearborn DG. Labeling of proteins by reductive methylation using sodium cyanoborohydride. J Biol Chem. 1979;254:4359–4365. [PubMed] [Google Scholar]
- 6.Ullrich O, Reinheckel T, Sitte N, Hass R, Grune T, Davies KJA. Poly-ADP ribose polymerase activates nuclear proteasome to degrade oxidatively damaged histones. Proc Natl Acad Sci U S A. 1999;96:6223–6228. doi: 10.1073/pnas.96.11.6223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tanaka K, Waxman L, Goldberg AL. ATP serves two distinct roles in protein degradation in reticulocytes, one requiring and one independent of ubiquitin. J Cell Biol. 1983;96:1580–1585. doi: 10.1083/jcb.96.6.1580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kirschner RJ, Goldberg AL. A high molecular weight metalloendoprotease from the cytosol of mammalian cells. J Biol Chem. 1983;258:967–976. [PubMed] [Google Scholar]
- 9.Hershko A, Heller H, Eytan E, Kaklij G, Rose IA. Role of the alpha-amino group of protein in ubiquitin-mediated protein breakdown. Proc Natl Acad Sci U S A. 1984;81:7021–7025. doi: 10.1073/pnas.81.22.7021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cuervo AM, Dice JF. Age-related decline in chaperone-mediated autophagy. J Biol Chem. 2000;275:31505–31513. doi: 10.1074/jbc.M002102200. [DOI] [PubMed] [Google Scholar]
- 11.Netland PA, Dice JF. Red blood cell-mediated microinjection: methodological considerations. Anal Biochem. 1985;150:214–220. doi: 10.1016/0003-2697(85)90461-0. [DOI] [PubMed] [Google Scholar]
- 12.Farber JM, Levine RL. Sequence of a peptide susceptible to mixed-function oxidation. Probable cation binding site in glutamine synthetase. J Biol Chem. 1986;261:4574–4578. [PubMed] [Google Scholar]
- 13.Friguet B, Stadtman ER, Szweda LI. Modification of glucose-6-phosphate dehydrogenase by 4-hydroxy-2-nonenal. Formation of cross-linked protein that inhibits the multicatalytic protease. J Biol Chem. 1994;269:21639–21643. [PubMed] [Google Scholar]
- 14.Chondrogianni N, Tzavelas C, Pemberton AJ, Nezis IP, Rivett AJ, Gonos ES. Overexpression of proteasome beta5 assembled subunit increases the amount of proteasome and confers ameliorated response to oxidative stress and higher survival rates. J Biol Chem. 2005;280:11840–11850. doi: 10.1074/jbc.M413007200. [DOI] [PubMed] [Google Scholar]
- 15.Ferber S, Ciechanover A. Transfer RNA is required for conjugation of ubiquitin to selective substrates of the ubiquitin- and ATP-dependent proteolytic system. J Biol Chem. 1986;261:3128–3134. [PubMed] [Google Scholar]
- 16.Hershko A, Heller H, Elias S, Ciechanover A. Components of ubiquitin-protein ligase system. Resolution, affinity purification, and role in protein breakdown. J Biol Chem. 1983;258:8206–8214. [PubMed] [Google Scholar]
- 17.Pickering AM, Koop AL, Teoh CY, Ermak G, Grune T, Davies KJA. The immunoproteasome, the 20S proteasome, and the PA28αβ proteasome regulator are oxidative stressadaptive proteolytic complexes. Biochem J. 2010;432:585–594. doi: 10.1042/BJ20100878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Reinheckel T, Grune T, Davies KJA. The measurement of protein degradation in response to oxidative stress. Methods Mol Biol. 2000;99:49–60. doi: 10.1385/1-59259-054-3:49. [DOI] [PubMed] [Google Scholar]
- 19.Davies KJA. Protein damage and degradation by oxygen radicals. I. general aspects. J Biol Chem. 1987;262:9895–9901. [PubMed] [Google Scholar]
- 20.Twining SS. Fluorescein isothiocyanate-labeled casein assay for proteolytic enzymes. Anal Biochem. 1984;143:30–34. doi: 10.1016/0003-2697(84)90553-0. [DOI] [PubMed] [Google Scholar]
- 21.De Lumen BO, Tappel AL. Fluorescein-hemoglobin as a substrate for cathepsin D and other proteases. Anal Biochem. 1970;36:22–29. doi: 10.1016/0003-2697(70)90328-3. [DOI] [PubMed] [Google Scholar]
- 22.Voss EW, Jr., Workman CJ, Mummert ME. Detection of protease activity using a fluorescence-enhancement globular substrate. Biotechniques. 1996;20:286–291. doi: 10.2144/96202rr06. [DOI] [PubMed] [Google Scholar]
- 23.Jones LJ, Upson RH, Haugland RP, Panchuk-Voloshina N, Zhou M. Quenched BODIPY dye-labeled casein substrates for the assay of protease activity by direct fluorescence measurement. Anal Biochem. 1997;251:144–152. doi: 10.1006/abio.1997.2259. [DOI] [PubMed] [Google Scholar]
- 24.Grune T, Reinheckel T, North JA, Li R, Bescos PB, Shringarpure R, Davies KJA. Ezrin turnover and cell shape changes catalyzed by proteasome in oxidatively stressed cells. Faseb J. 2002;16:1602–1610. doi: 10.1096/fj.02-0015com. [DOI] [PubMed] [Google Scholar]
- 25.Pacifici RE, Davies KJA. Protein degradation as an index of oxidative stress. Methods Enzymol. 1990;186:485–502. doi: 10.1016/0076-6879(90)86143-j. [DOI] [PubMed] [Google Scholar]
- 26.Hoare DG, Koshland DE., Jr. A method for the quantitative modification and estimation of carboxylic acid groups in proteins. J Biol Chem. 1967;242:2447–2453. [PubMed] [Google Scholar]
- 27.Bota DA, Davies KJA. Lon protease preferentially degrades oxidized mitochondrial aconitase by an ATP-stimulated mechanism. Nat Cell Biol. 2002;4:674–680. doi: 10.1038/ncb836. [DOI] [PubMed] [Google Scholar]
- 28.Davies KJA, Goldberg AL. Oxygen radicals stimulate intracellular proteolysis and lipid peroxidation by independent mechanisms in erythrocytes. J Biol Chem. 1987;262:8220–8226. [PubMed] [Google Scholar]
- 29.Means GE, Feeney RE. Reductive alkylation of amino groups in proteins. Biochemistry. 1968;7:2192–2201. doi: 10.1021/bi00846a023. [DOI] [PubMed] [Google Scholar]
- 30.Rice RH, Means GE. Radioactive labeling of proteins in vitro. J Biol Chem. 1971;246:831–832. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(a) To test if reductive labeling might cause binding of AMC to lysine (epsilon) amino groups, and/or the N-terminus of proteins, we performed AMC labeling experiments with both native protein, and with protein in which free amino groups were blocked with 0.5–50mM sulfo-N-hydroxysulfosuccinimide-acetate (sulfo-NHS-acetate from Pierce, Rockford, IL, USA, catalogue #26777). BSA was suspended in 0.1M sodium carbonate buffer pH 8.5. 1.6μM BSA (representing 1mM of amino acids, based on a 607 amino acid sequence) was incubated with 0–50mM of sulfo-nhs-acetate (i.e., up to a 50 fold molar excess) for 1 hour at room temperature. Following preparation, samples were extensively dialyzed and a buffer exchange was performed with HEPES in preparation for AMC labeling. Free lysine content was determined through colorimetric analysis (absorbance at 250nm) following incubation of 1μl samples with 100μl of 2,4,6 trinitrobenzene sulfonic acid (Thermo-fisher, Waltham, Massachusetts, USA, catalogue #TS-28997) for 30 minutes. Values are means ± SE, n = 12. (b) treatment with 0.5–50mM sulfo-nhs-acetate appears to cause little significant change in gel fluorescence. Samples prepared as in panel (a) were incubated with AMC in the presence of NaCNBH3 and run on SDS Page gels as in Figure 2c–e. The BSA-AMC fluorescent band was unaffected by 0.5mM sulfo-NHS-acetate, which caused a 50% loss of free amino groups in panel (a). Even pretreatment with 5.0mM sulfo-NHS-acetate caused less than 10% loss of the BSA-AMC fluorescent band, despite causing more than 75% loss of free amino groups in panel (a). Significant loss of the BSA-AMC fluorescent band was only observed after pretreatment with 50mM sulfo-NHS-acetate, which caused extensive modification of the protein, as evidenced by altered mobility and blurred appearance in the silver stained samples.
(a) We incubated 1mM AMC and 0 – 1000μg of BSA with 20mM NaCNBH3, with and without 100mM tryptamine, for 2 hours at 37°C. Where tryptamine was used, it was first incubated with BSA for 30 minutes at 37°C prior to the addition of AMC and NaCNBH3. Fluorescence was measured as in Figure 1b. Values were plotted as a percent of fluorescence in the absence of BSA, and are means ± SE, n = 4. (b) We added 0–100mM Tryptamine to 1.6μM BSA (1mM of amino acids based on a 607 amino acid sequence) for 30 minutes. Next 1mM AMC and 20mM NaCNBH3 (final concentrations) were added. Samples were incubated for 2 hours, then dialyzed extensively with buffer exchanged for proteolysis buffer. Samples were then incubated with or without 100μM trypsin to measure proteolytic capacity as in Figure 3, values are means ± SE, n = 3. It is important to note that, in both panels, tryptamine was present when AMC + NaCNBH3 so that it could (potentially) compete with AMC labeling.