Abstract
Objective:
Integrin α11β1 is a collagen receptor specific to fibroblasts that regulates myofibroblast differentiation. We sought to determine whether α11β1 is expressed in myometrium and fibroids and whether tissue expression varies.
Design:
Comparison of α11 in human myometrium and fibroids using Western blot and RNA in situ hybridization.
Materials and Methods:
Specimens were obtained from fibroid and myometrium. For Western blots, we used a polyclonal antibody to integrin α11. RNA in situ hybridization was performed using a custom RNA probe for α11 subunit.
Results:
Myometrium and fibroids express α11 integrin, with expression 2-fold greater in fibroids. The RNA probe offers a more precise method compared to Western blot using polyclonal human antibody.
Conclusions:
The difference in expression in myometrium and fibroids suggests that α11 is involved in the formation of myofibroblasts and fibroid development.
Keywords: integrin α11β1, collagen receptor, fibroids, RNA in situ hybridization
Background
Uterine fibroids are the most common tumors in reproductive-age women and arise from uterine smooth muscle. There is an abundance of collagen in these benign tumors1–3 and when compared to collagen fibrils observed in myometrium, adjacent fibroid tumors contain fibroid collagen with fibril structure orientation consistent with fibrosis.1 Several investigators have described fibroblasts in the extracellular matrix (ECM) microenvironment of the smooth muscle cells of fibroids.4 These fibroblasts demonstrated distinct protein expression as determined by immunostaining properties. Zaitseva et al4 reported that α-smooth muscle actin (αSMA)-negative fibroblast cells have a different morphology compared to similar cells in the myometrium. As demonstrated by coculture, fibroid-derived fibroblasts stimulate fibroid cell proliferation, collagen type I production, and activate both receptor tyrosine kinases and transforming growth factor β (TGFβ) receptor signaling5 and are thus profibrotic cells. Additionally, the cells in the Zaitseva study did not stain with desmin, suggesting that they were of nonmuscle origin. Together, these studies raise intriguing questions regarding the fibrosis observed in uterine fibroids.
Fibroid cells have striking characteristics that can be considered as myofibroblast-like cells.6 Myofibroblasts are responsible for the overabundance of collagen noted in fibrotic tissue. Fibroids appear to be resistant to apoptosis and contain a large amount of disoriented collagen fibrils.1,6 Interestingly, prior studies using electron microscopy imaging of fibroids <3 mm in size reported findings which suggested that 2 types of cells were present, including cells that “closely resembled immature smooth muscle cells or myofibroblasts.”7 Myofibroblasts were first observed in wound granulation tissue by electron microscopy almost 38 years ago8 and are involved in tissue regeneration, tissue fibrosis, and other processes such as development. Fibroids grow by a fibrotic process and contain a number of myofibroblast-like cells and respond in a unique manner to mechanical strain and compression and express active ρ.9,10
Integrins are cell adhesion molecules consisting of α- and β-chains that are noncovalently associated to form heterodimers. Of the 24 integrin αβ-heterodimers, 9 contain an interactive domain called the αI domain. There are 4 collagen-binding integrins (α1β1, α2β1, α10β1, and α11β1),11 that interact with the I domain of collagen at the collagen-associated glycine-phenylalanine-hydroxyproline-glycine-glutamate-arginine motif. As the β1 subunit is not involved in collagen binding, it is customary for experiments to be conducted using 1 of the 4 α subunits.
Recent studies indicate that integrin α11β1 is a collagen receptor specific to fibroblasts and regulates myofibroblast differentiation. During tooth eruption, α11β1 is needed on fibroblasts of the periodontal ligament and is also the major collagen receptor on cultured mouse embryonic fibroblasts.12 Furthermore, α11β1 has been shown to regulate the autocrine secretion of insulin-like growth factor II in fibroblasts of tumor stroma.13 Finally, α11β1 is regulated by mechanical strain and by members of the TGF-β superfamily involving activin A and Smad3, suggesting that α11β1 regulates profibrotic myofibroblast differentiation and mediates matrix metalloproteinase 13-dependent collagen lattice contraction by fibroblasts in mice.14–16
Our research goal is to determine whether integrin α11β1 is expressed in uterine fibroid tissue as well as in adjacent myometrium. We surmise that the expression of integrin α11 in fibroid and myometrial tissues differs and thus hypothesizes that α11β1 is involved in the development of the fibroid cell phenotype. Our ultimate goal is to provide evidence of the presence of the collagen-binding integrin α11 in myometrium and its differential expression in fibroids to stimulate further research of its role in the development of these common benign tumors that contribute to considerable morbidity and health care costs.17
Materials and Methods
Patients
The research protocol was approved by the institutional review board of Duke University Medical Center and patients were approached and enrolled prior to undergoing hysterectomy. A total of 9 premenopausal patients undergoing hysterectomy for symptomatic uterine fibroids were enrolled.
Tissue Isolation
Hysterectomy specimens were first evaluated by the Department of Pathology. Following this, fibroid tissue and myometrium were obtained by careful sharp dissection as described previously.1,18 Briefly, at the time of hysterectomy, tissue specimens were obtained at the edge of a single fibroid, taking care to avoid the capsule and adjacent myometrium. The adjacent myometrium was obtained 1 cm away from the fibroid capsule to ensure that there would be no compressive force from the fibroid on that tissue. We utilized tissue from 5 patients for the Western blots and tissue from 4 patients for the RNA in situ hybridization.
Western Blot
In order to initially determine whether uterine and fibroid tissue expressed α11 integrin, Western blot analysis was performed. The tissue specimens were pulverized, homogenized in radioimmunoprecipitation assay buffer (50 mmol/L Tris-HCl, 150 mmol/L NaCl, 1% Triton X-100, 0.5% sodium deoxycholate, and 0.1% sodium dodecyl sulfate), and then centrifuged at 12 000 rpm for 15 minutes at 4°C. Protein concentrations were estimated via Bradford protein assay. A total of 60 ug of protein from each sample was separated via a 4% to 15% Tris-HCl polyacrylamide gel (BioRad, Hercules, CA) and transferred to a polyvinylidene difluoride membrane. The blot was incubated with polyclonal antibodies to integrin α11 (1:500) and glyceraldehyde 3-phosphate dehydrogenase (GAPDH; 1:2500) at 4°C overnight, then goat antirabbit horseradish peroxidase (HRP) secondary antibody for 1 hour. The blot was incubated in SuperSignal West Pico Chemiluminescent Substrate (Thermo Fisher Scientific, Waltham, MA) and briefly exposed to film. Quantification of the signals was performed using ImageJ software.19 Integrin α11 band intensities were normalized to GAPDH band intensities.
RNA In Situ Hybridization
The tissues were immediately fixed for 24 hours in 10% neutral-buffered formalin at room temperature, embedded in paraffin, cut in 5 um sections and placed on SuperFrost plus slides. Tissue samples were fixed and slides were baked at 60° for 1 hour and then dried. The tissue sections were dewaxed with xylene and rehydrated through an ethanol series. RNA in situ hybridization for α11 integrin messenger RNA was performed manually using RNAscope 2.0 FFPE reagent kit (Advanced Cell Diagnostics, Inc, Hayward, California) and RNAscope Probe specific to the sequence region spanning 2515-3465 of the gene encoding ITGA11 according to the manufacturer’s instructions. Briefly, 5 µm formalin-fixed, paraffin-embedded tissue sections were pretreated with heat and protease prior to hybridization with the target oligonucleotide probes. Preamplifier, amplifier, and HRP-labeled oligonucleotides were then hybridized sequentially, followed by chromogenic precipitate development with 3,3′-diaminobenzidine. Each sample was quality controlled for RNA integrity with a RNAscope probe specific to PPIB RNA and for background with a probe specific to bacterial dapB RNA. Gallbladder tissue was also used as a control. Specific RNA staining signal was identified as brown, punctate dots.
A total of 3 to 4 slides were processed for each tissue sample and 3 areas of each slide at a magnification of 10× were chosen at random for staining quantification. Furthermore, each chosen 10× area was subdivided into quadrants for accurate cell counting. Data collected included total number of cells per area, total number of stained cells, and staining score of each cell. Staining quantification was performed using standardized criteria of stained dots per cell correlating with a staining score. Cells with no staining received a staining score of 0; cells with 1 to 3 stained dots received a score of 1; 4 to 10 dots received a score of 2; and greater than 10 dots received a score of 3. All slides were scrutinized by all 3 authors separately, with cell counting and quantification performed by 1 author. Slides in which the staining process was unsuccessful (defined as less than 2% of all cells stained) were discarded from the analysis.
Statistical Analysis
Statistical analysis of the percentage of stained cells, intensity of stained cells, and overall intensity of the total cells counted was performed using a paired student t test (GraphPad, GraphPad software, San Diego, California). The averages, standard error of the mean, and P values were calculated.
Results
Western Blot
Our findings demonstrated that integrin α11 is expressed in both myometrium and fibroid tissue (Figure 1). In 2 paired fibroid/myometrium samples (patient 1 and patient 4), the expression of α11 was elevated or equivalent while in the remaining paired samples (patients 2, 3, and 5), the expression of α11 was decreased, which consistent with what is known about differential protein expression during the growth and development of uterine fibroids.20
Figure 1.
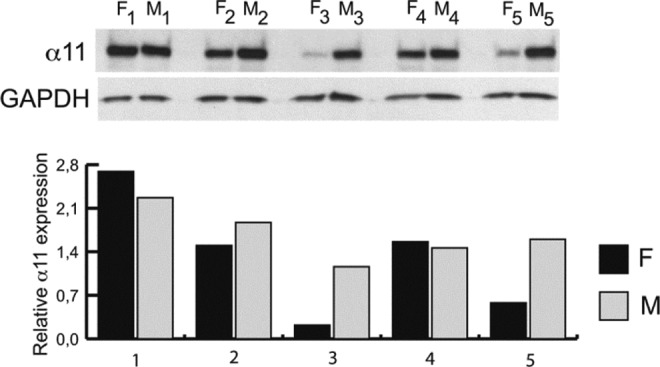
Western blot. Protein levels of α11 in 5 patient-paired samples of fibroid (F) and myometrium (M), with values normalized to GAPDH.
RNA In Situ Hybridization
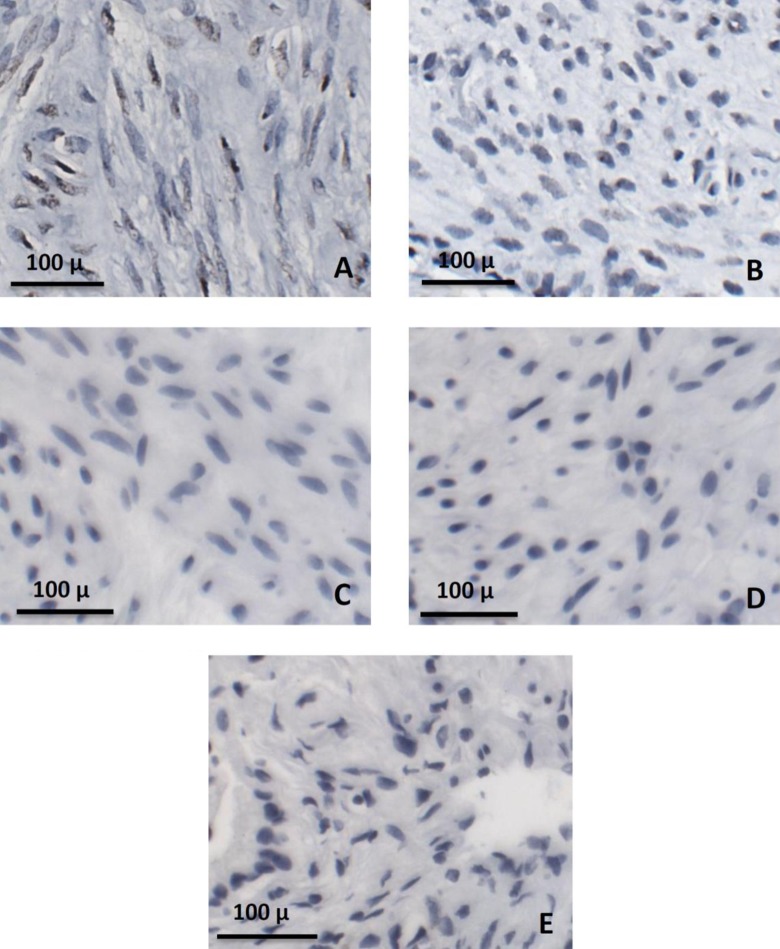
For RNA in situ hybridization, 35 slides from 3 different patients were analyzed, with a total of 10 195 myometrial cells counted and quantified. For the fibroid tissue, a total of 4686 fibroid cells from 20 slides were counted and underwent staining quantification. For our controls, 12 slides from 1 patient were used for both the control for myometrium and fibroid tissue, with a total of 1985 myometrial cells and 1941 fibroid cells counted. A total of 2192 cells of gallbladder from 12 slides were also analyzed. Representative sections of each tissue type are presented in Figure 2A-E. All sections of fibroid (Figure 2A) and myometrial tissue (Figure 2B) that were processed and analyzed stained positive for α11β1 as can be identified with brown staining within the cells. There was no evidence of staining for α11β1 in either the fibroid control (Figure 2C) or in the myometrium control (Figure 2D). Similarly, the gallbladder tissue had no evidence of α11β1 staining (Figure 2E).
Figure 2.
RNA in situ hybridization. Representative images of α11 staining in both fibroid (A) and myometrium (B) specimens, with brown coloring within cells representing positive staining for α11. Fibroid and myometrium controls (C and D, respectively), as well as gallbladder tissue (E) did not stain positive for α11.
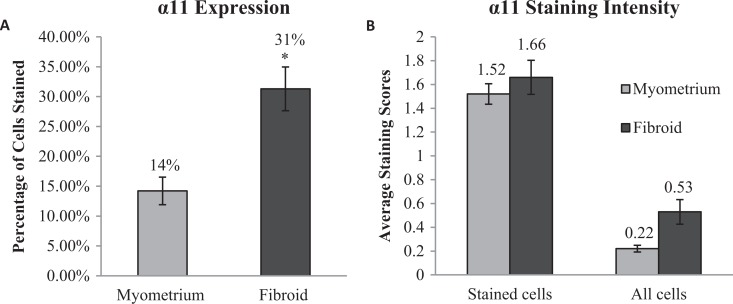
In the myometrium samples, 14.2% of total cells stained positive for α11β1 while more than twice the cells in the fibroid tissue (31.3%) were stained, with a significant P value of <.005 (Figure 3A). Of the cells that were stained in each tissue type, there was no statistically significant difference in the average intensity as samples in myometrium had an average intensity score of 1.52 compared to 1.66 in fibroid tissue. When the average intensity of all cells counted per tissue type was compared, there was a 2-fold difference between myometrium and fibroid (intensity scores of 0.22 and 0.53, respectively) although this was not found to be statistically significant (P value = .12; Figure 3B).
Figure 3.
Quantification of α11 staining in myometrium and fibroid samples. Of 10 195 myometrial cells, 1427 (14%) stained positive for α11, while 1453 of 4 686 fibroid cells (31%) stained positive (A); *p-value < 0.05. The intensity of the stained cells was similar in both myometrium and fibroid, and while the average intensity of all cells in each sample trended towards greater intensity in fibroid samples, this was not statistically significant (B).
Discussion
This study is novel and the first to report that α11β1, a collagen-binding integrin, is expressed in both human myometrial and fibroid tissue. Although we were able to demonstrate the presence of α11β1 in both myometrium and fibroids via Western blot, utilizing RNA probe in situ technology allowed us to obtain a more precise method of analysis with less nonspecific binding of the probe. The probe sets are hybridized to the oligonucleotide probe in pairs, with each pair providing a binding site for the preamplifier provided by the manufacturer. The temperature used in our methods allowed hybridization for the target probe pairs but not for individual target probes. This procedure is critical since it prevents a signal from the unpaired target probes that might be hybridized to a nonspecific RNA. We found that this technique provides a highly sensitive and specific assay while preserving the tissue and cellular architecture of the myometrium and fibroids. With the specific RNA in situ probe, we were able to demonstrate a 2-fold increase in α11β1 expression in fibroid tissue relative to myometrial tissue in 3 patients. It is likely, however, that differential protein expression patterns within samples exist due to the dynamic growth and degeneration patterns observed in fibroids, and unfortunately, fibroid size and growth pattern data were not available due to the nature of the tissue collection protocol. As this is the first report of α11β1 expression in fibroid tissue, continued studies investigating the characterization of α11β1 protein expression patterns during fibroid growth and degeneration would be more informative. Additionally, given the low number of patients from which samples were obtained for this initial investigation, increasing both sample size and patient number with future studies is merited now that expression of this integrin in both myometrial and fibroid tissues has been established.
In all tissue types studied previously, the collagen-binding integrin α11β1 is expressed in fibroblasts and myofibroblasts and is considered to be a marker for fibrosis.12–15 Thus, we conclude the cells expressing α11β1 integrin in this study are fibroblast-derived cells. We did not stain for αSMA as it was not within our scope of this current study to determine the expression patterns of α11β1 in a specific cell lineage or cell type. However, future studies with colocalization of αSMA and α11β1 staining would further determine expression of α11β1 in both uterine fibroblasts and myofibroblasts. A previous study of uterine cervical tissue has shown expression of α11β1 integrin in the uterine cervical tissue of nonpregnant and timed pregnant Sprague-Dawley rats. Importantly, in that study, α11β1 expression increased progressively through gestation until day 18 and then decreasing through day 22, seemly parallel to the increase in progesterone levels during rat gestation.21 The cervix during gestation and parturition undergoes dynamic remodeling and presumably α11β1 plays a role in the maintenance of cervical resistance throughout pregnancy.21 Progesterone appears to be the dominant sex steroid affecting the growth of fibroids, a fact supported by increased mitotic rates in fibroids during the secretory phase of the menstrual cycle as well as the clinical response to progesterone antagonist, mifepristone, of inhibiting fibroid growth.6 It could be speculated that progesterone modulation of α11β1in uterine tissue might contribute to fibroid growth through a mechanism or mechanisms that could cause the apoptotic resistance observed in these tumors.
Studies have demonstrated that α1, α2, α3, α4, α5, and α6 integrins were expressed in myometrial and fibroid cell lines.22 The α6 integrin, a laminin-binding integrin, was increased 1.91 ± 11 in fibroid cells. The investigators did not examine whether or not α10 or α11 was expressed in these cell lines. They also report that the β1 integrin, the integrin subunit which forms a heterodimer with the collagen-binding α subunits, was increased 2.25 ± 0.32-fold in fibroid cells compared to myometrium. It is interesting to speculate that the increase in β1 subunit may be due to an increase in α11.22
Our findings are significant in that this is the first evidence that α11 integrin is present in human uterine tissue in vivo, adding to the growing body of literature demonstrating that uterine myometrial smooth muscle cells are a unique phenotype compared to cells from other tissues. The distinct difference in expression of α11β1 integrin in myometrial cells versus myofibroblasts-like cells suggests that this integrin is involved in differentiation or apoptosis pathways leading to formation of myofibroblasts.
These findings further support that integrin α11β1 is involved in the regulation of myofibroblast differentiation. Fibroids are composed of an abundance of altered poorly organized collagen-rich matrix consistent with fibrosis formed by profibrotic myofibroblasts.15 A hallmark of fibroids is their increased tissue stiffness as well as the attenuated response of fibroid cells to mechanical clues.10 In mice, inhibition of α11β1-collagen interaction results in cell death suggesting that α11 may be a pivotal molecule in promoting the apoptotic resistance noted in growing fibroids.23 As the α11 integrin subtype acts as a mechanosensor to promote the myofibroblast differentiation,14 this suggests that inhibitors of α11β1 integrin could be considered potential drug targets for the treatment of uterine fibroids. Since the α subunit determines in general the amount of the receptor that will bind to the cell surface,24 our findings complete the fibroid integrin story initiated by others.22
Further work is indicated to determine the nature of these cells in the myometrium and the mechanisms that cause their increase in uterine fibroids. As we learn more about the mechanical forces at play between fibroid cells and their surrounding ECM, it is of interest to consider the phenomenon of integrin switching, a process that is well known in wound healing, development, and morphogenesis. In integrin switching, cells change the integrins expressed as they differentiate, migrate, change polarity, and other properties.24,25 This study strengthens the findings that fibroid growth occurs primarily from apoptotic resistance.26 Changes in expressed integrins could explain why some fibroids grow, others are stagnant, and others regress all within the same individual uterus. Although limited by a small sample size, our compelling results suggest that α11β1 integrin is involved in myofibroblast differentiation in the myometrium and plays a substantial role in the fibroid cell phenotype.
Acknowledgments
We thank Donald Gullberg, PhD, Friederike L. Jayes, DVM, PhD, Ning Lu, PhD, and Cedric Zeltz, PhD, for their generous assistance in the preparation of the manuscript.
Footnotes
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship and/or publication of this article: Charles Hammond Research Fund.
References
- 1. Leppert PC, Baginski T, Prupas C, Catherino WH, Pletcher S, Segars JH. Comparative ultrastructure of collagen fibrils in uterine leiomyomas and normal myometrium. Fertil Steril. 2004;82 suppl 3:1182–1187 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Ferenczy A, Richart RM, Okagaki T. A comparative ultrastuctural study of leiomyosarcoma, cellular leiomyoma, and leiomyoma of the uterus. Cancer. 1971;28(4):1004–1018 [DOI] [PubMed] [Google Scholar]
- 3. Stewart EA, Friedman AJ, Peck K, Nowak RA. Relative overexpression of collagen type I and collagen type III messenger ribonucleic acids by uterine leiomyomas during the proliferative phase of the menstrual cycle. J Clin Endocrinol Metabol. 1994;79(3):900–906 [DOI] [PubMed] [Google Scholar]
- 4. Zaitseva M, Vollenhoven BJ, Rogers PA. Retinoic acid pathway genes show significantly altered expression in uterine fibroids when compared with normal myometrium. Mol Hum Reprod. 2007;13(8):577–585 [DOI] [PubMed] [Google Scholar]
- 5. Moore AB, Yu L, Swartz CD, et al. Human uterine leiomyoma-derived fibroblasts stimulate uterine leiomyoma cell proliferation and collagen type I production, and activate RTKs and TGF beta receptor signaling in coculture. Cell Commun Signal. 2010;8:10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Leppert PC, Catherino WH, Segars JH. A new hypothesis about the origin of uterine fibroids based on gene expression profiling with microarrays. Am J Obstet Gynecol. 2006;195(2):415–420 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Konishi I, Fujii S, Ban C, Okuda Y, Okamura H, Tojo S. Ultrastructural study of minute uterine leiomyomas. Int J Gynecol Pathol. 1983;2(2):113–120 [DOI] [PubMed] [Google Scholar]
- 8. Schurch W, Seemayer TA, Lagace R, Gabbiani G. The intermediate filament cytoskeleton of myofibroblasts: an immunofluorescence and ultrastructural study. Virchows Arch A Pathol Anat Histopathol. 1984;403(4):323–336 [DOI] [PubMed] [Google Scholar]
- 9. Rogers R, Norian J, Malik M, et al. Mechanical homeostasis is altered in uterine leiomyoma. Am J Obstet Gynecol. 2008;198(4):474.e471–e411 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Norian JM, Owen CM, Taboas J, et al. Characterization of tissue biomechanics and mechanical signaling in uterine leiomyoma. Matrix Biol. 2012;31(1):57–65 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Popova SN, Lundgren-Akerlund E, Wiig H, Gullberg D. Physiology and pathology of collagen receptors. Acta Physiologica. 2007;190(3):179–187 [DOI] [PubMed] [Google Scholar]
- 12. Popova SN, Barczyk M, Tiger CF, et al. Alpha11 beta1 integrin-dependent regulation of periodontal ligament function in the erupting mouse incisor. Mol Cell Biol. 2007;27(12):4306–4316 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Zhu CQ, Popova SN, Brown ER, et al. Integrin alpha 11 regulates IGF2 expression in fibroblasts to enhance tumorigenicity of human non-small-cell lung cancer cells. Proc Natl Acad Sci U S A. 2007;104(28):11754–11759 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Carracedo S, Lu N, Popova SN, Jonsson R, Eckes B, Gullberg D. The fibroblast integrin alpha11beta1 is induced in a mechanosensitive manner involving activin A and regulates myofibroblast differentiation. J Biol Chem. 2010;285(14):10434–10445 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Talior-Volodarsky I, Connelly KA, Arora PD, Gullberg D, McCulloch CA. alpha11 integrin stimulates myofibroblast differentiation in diabetic cardiomyopathy. Cardiovasc Res. 2012;96(2):265–275 [DOI] [PubMed] [Google Scholar]
- 16. Barczyk MM, Lu N, Popova SN, Bolstad AI, Gullberg D. alpha11beta1 integrin-mediated MMP-13-dependent collagen lattice contraction by fibroblasts: evidence for integrin-coordinated collagen proteolysis. J Cell Physiol. 2013;228(5):1108–1119 [DOI] [PubMed] [Google Scholar]
- 17. Cardozo ER, Clark AD, Banks NK, Henne MB, Stegmann BJ, Segars JH. The estimated annual cost of uterine leiomyomata in the United States. Am J Obstet Gynecol. 2012;206(3):211.e211–219 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Catherino WH, Leppert PC, Stenmark MH, et al. Reduced dermatopontin expression is a molecular link between uterine leiomyomas and keloids. Genes Chromosomes Cancer. 2004;40(3):204–217 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Rasband WS, ImageJ U.S. National Institutes of Health, Bethesda Maryland, USA. http://imagej.nih.gov/ij/, 1997–2012 [Google Scholar]
- 20. Peddada SD, Laughlin SK, Miner K, et al. Growth of uterine leiomyomata among premenopausal black and white women. Proc Natl Acad Sci U S A. 2008;105(50):19887–19892 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Ji H, Long V, Briody V, Chien EK. Progesterone modulates integrin {alpha}2 (ITGA2) and {alpha}11 (ITGA11) in the pregnant cervix. Reprod Sci. 2011;18(2):156–163 [DOI] [PubMed] [Google Scholar]
- 22. Malik M, Segars J, Catherino WH. Integrin beta1 regulates leiomyoma cytoskeletal integrity and growth. Matrix Biol. 2012;31(7-8):389–397 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Popov C, Radic T, Haasters F, et al. Integrins alpha2beta1 and alpha11beta1 regulate the survival of mesenchymal stem cells on collagen I. Cell Death Dis. 2011;2:e186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Barczyk M, Carracedo S, Gullberg D. Integrins. Cell Tissue Res. 2010;339(1):269–280 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Larjava H, Salo T, Haapasalmi K, Kramer RH, Heino J. Expression of integrins and basement membrane components by wound keratinocytes. J Clin Invest. 1993;92(3):1425–1435 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Davis BJ, Risinger JI, Chandramouli GV, Bushel PR, Baird DD, Peddada SD. Gene expression in uterine leiomyoma from tumors likely to be growing (from black women over 35) and tumors likely to be non-growing (from white women over 35). PloS One. 2013;8(6):e63909. [DOI] [PMC free article] [PubMed] [Google Scholar]