Abstract
We examined the antitumor and therapeutic potentials of paricalcitol, an analog of 1,25-dihydroxyvitamin D3 with lower calcemic activity, against uterine fibroids using in vitro and in vivo evaluations in appropriate uterine fibroid cells and animal models. We found that paricalcitol has potential to reduce the proliferation of the immortalized human uterine fibroid cells. For the in vivo study, we generated subcutaneous tumors by injecting the Eker rat-derived uterine leiomyoma cell line (ELT-3) rat uterine fibroid-derived cell line in athymic nude mice supplemented with estrogen pellets. These mice were administered with vehicle versus paricalcitol (300 ng/kg/d) or 1,25-dihydroxyvitamin D3 (500 ng/kg/d) for 4 consecutive weeks, and the data were analyzed. We found that while both paricalcitol and 1,25-dihydroxyvitamin D3 significantly reduced fibroid tumor size, the shrinkage was slightly higher in the paricalcitol-treated group. Together, our results suggest that paricalcitol may be a potential candidate for effective, safe, and noninvasive medical treatment option for uterine fibroids.
Keywords: paricalcitol; Zemplar; 1,25-dihydroxyvitamin D3; vitamin D3; uterine fibroids; leiomyoma
Introduction
Uterine fibroids (leiomyoma) are benign tumors of the myometrium and are the most common pelvic tumor in reproductive-age women. Human uterine fibroids that arise from the smooth muscle cells of the uterus are frequent benign clonal tumors among women. These tumors usually cause specific symptoms such as anemia due to the release of heavy menstrual bleeding, pelvic pressure, and bladder discomfort.1–3 Fibroids can also cause infertility, abortions, and other obstetric complications.4–7 Fibroids are the most commonly cited indication for hysterectomy in the United States.8 Fibroids appear only after menarche and proliferate, and they grow during the reproductive years and regress after menopause. Fibroids can also regrow even after hormone replacement therapy.9 Uterine fibroids are 3 to 4 times more prevalent in African American women than in caucasian women.10 There is currently no effective medicinal treatment for uterine fibroids, and thus, the cost for the treatment is estimated to be US$34.4 billion per year11 for the United States health care system.
When we take into account the social costs and associated long-term health problems, it is clear that better prevention and treatment options for women with uterine fibroids are urgently needed. Presently, the removal of whole uterus by surgery (hysterectomy), removal of uterine fibroid by surgery (myomectomy), and image-guided focused ultrasound thermal therapy are the primary treatment options for uterine fibroids.12–14 Recently, we and others have developed some novel noninvasive treatment options for uterine fibroids such as localized gene therapy, oral green tea extracts, and selective progesterone receptor modulators.13,15–18 However, it is indispensable to develop more effective and safe treatment options for uterine fibroids, which could have a major impact on women’s health worldwide.
Sunlight is a principal source of vitamin D for humans. Additional sources include certain food items such as salmon and herrings as well as fortified dairy products. Vitamin D deficiency is more prevalent among African Americans (40%-45%) compared with caucasians (4%). Black pigmentation in African Americans decreases the absorption of ultraviolet rays from the sun. Besides this, along with decreased milk consumption lactose intolerance reduces the levels of vitamin D in African Americans. Vitamin D deficiency is found to be associated not only with metabolic bone disease but also with several other chronic diseases such as type 2 diabetes, schizophrenia, and some type of cancers. We and others have demonstrated that vitamin D3 deficiency is a novel risk factor for the occurrence of human uterine fibroids.19–21 We also recently demonstrated that vitamin D3 treatment can shrink uterine fibroid lesions in a spontaneous Eker rat animal model of uterine fibroids.22 Vitamin D is a hormone produced in the skin through biological reactions that are initiated by the sunlight. It is converted to an active metabolite, 1,25-dihydroxyvitamin D3, in the liver and kidneys.23 Vitamin D3 functions via the nuclear vitamin D receptor (VDR) and acts on VDR target genes. This vitamin D-mediated gene activation requires a VDR/retinoid X receptor heterodimer complex.24,25 We have recently demonstrated that vitamin D3 treatment is safe and can effectively shrink uterine fibroid lesions in an Eker rat-animal model, which suggests that vitamin D3 may be an ideal therapeutic agent for the nonsurgical treatment of uterine fibroids.22 It has also been established that the deregulated vitamin D homeostasis may be of considerable importance for cardiovascular disease. Patients with cardiovascular disease and heart failure often have vitamin D deficiency.10,26 It has also been reported that vitamin D is associated with poor prognosis for heart failure in humans.26,27 Studies have shown that vitamin D deficiency is approximately 10 times more prevalent in African Americans than in caucasians (40% vs 4%).28,29 In our previous observation, we also found that African American women have comparatively lower levels of vitamin D3, and they have higher risk of uterine fibroids than that of caucasian women.19
At present, the available options for uterine fibroid treatment are far from satisfactory, and therefore, the development of a safe, effective, and noninvasive medical treatment method will help too many women with symptomatic uterine fibroids. The initiating factors that lead to the development of fibroids are not well understood. However, ample evidence supports that estrogen and progesterone are the important factors for fibroid growth.13,14 The pivotal role of estrogen in fibroid development and growth has been established through clinical observations as well as laboratory research studies.15,16 The effects of estrogen in uterine fibroid cells are mediated mainly via estrogen receptor α (ER-α).16,17 Several studies have reported that uterine fibroids express ER-α at higher levels compared with adjacent normal myometrium.30–32 The incidence of uterine fibroids and the currently available less effective treatment suggest the need for alternative therapies such as vitamin D3 or its analogue-based therapies as options for uterine fibroid treatment. Vitamin D3 can inhibit growth by cell cycle arrest and by inducing apoptosis33 or reduced tumor metastasis through regulation of proteases.34 Vitamin D receptor is a member of the nuclear receptor superfamily that mediates the antiproliferative effects of bioactive 1,25-dihydroxyvitamin D3. The family includes steroid hormones as well as thyroid hormone and retinoids.35 Analogs of vitamin D3 have been successfully synthesized previously, and its antiproliferative properties and reduced calcemic effect on human cells have been demonstrated previously.25,36 Recently, we demonstrated that vitamin D3 has the ability to reduce uterine fibroid cell proliferation in vitro as well as inhibiting in vivo tumor growth in an Eker rat animal model of uterine fibroids.22,37 Nevertheless, in the case of clinical use, a higher concentration of vitamin D3 is unacceptable because of the side effects of serum and urine calcium level.38 Paricalcitol, an analog of 1,25-dihydroxyvitamin D3, also known as Zemplar, was developed by Abbot Pharmaceutical (Deerfield, Illinois) and is synthesized by Deluca and Colleagues.39 Paricalcitol has less calcemic activity and has been safely used in several vivo studies.25,36 Study showed that paricalcitol treatment effectively reduced cardiac fibrosis in an animal model.39 The beneficial effect of paricalcitol include the potent inhibitory effect on cell proliferation, cardiac fibrosis inhibition in an animal model, and a lower calcemic effect. Thus, based on the data available, we selected paricalcitol as a superior candidate to study its therapeutic utility for fibroid treatment. We characterized the effects of paricalcitol on human uterine fibroid cell (HuLM) proliferation as well as its effects on growth on fibroid tumors in an in vivo mouse model. In addition, we compared the therapeutic efficacy of paricalcitol and 1,25-dihydroxyvitamin D3, and our data support that paricalcitol is slightly more effective than 1,25-dihydroxyvitamin D3 in inhibiting uterine fibroid growth in an animal model. Therefore, our current in vitro (HuLM) and in vivo (subcutaneous tumors created in nude mice with ELT-3 cells) studies serve as excellent preclinical models, and we have evaluated the therapeutic potentials of paricalcitol as a safe nonsurgical treatment option for human uterine fibroids.
Materials and Methods
Reagents and Antibodies
The bioactive form of vitamin D3 (1,25-dihydroxyvitamin D3) was purchased from Sigma Bio chemicals (St Louis, Missouri). The vitamin D3 analog paricalcitol (Zemplar) was purchased from Abbot (Deerfield, Illinois). Miniosmotic pumps were purchased from Alzet osmotic pumps (Cupertino, California). Pellets of estradiol (1.7 mg) were purchased from Innovative Research (Sarasota, Florida). Antibodies for the study of proliferation (Ki67) and apoptosis (caspase 3), ER-α, and progesterone receptors PR-A and PR-B were purchased from Santa Cruz Biotechnology (Santa Cruz, California). We also used immunohistochemistry to evaluate the histological features of uterine fibroids, including antismooth muscle actin, desmin, and vimentin (Santa Cruz, California). The collagen content was evaluated using Masson Trichrome and collagen type 4 staining. For histological evaluation of tumors, ovary, uterus, liver, spleen, and femur samples were formalin fixed, paraffin embedded, and subject to hematoxylin and eosin (H&E) staining.
In Vitro Uterine Fibroid Cell Culture
Immortalized HuLM, which were kindly provided by Dr Darleen Dixon, have been described previously.37 The HuLM cells were cultured and maintained in smooth muscle cell basal medium containing 5% fetal bovine serum (FBS), 0.1% in insulin, 0.2% basic human fibroid growth factor b, 0.1% amphotericin-B (GA-1000) and 0.1% human epidermal growth factor (Lonza Walkersville, Maryland). Once these cells were approximately 70% confluent in culture medium, the media were replaced by Dulbecco Modified Eagle Medium: Nutrient Mixture F-12 (DMEM/F12; phenol free) supplemented with 10% charcoal-stripped FBS and 10 nmol/L estradiol and treated with various concentrations of paricalcitol and 1,25-dihydroxyvitamin D3 in the range of 10 pmol/L to 1000 nmol/L at different time intervals for up to 168 hours. The effects of paricalcitol and 1,25-dihydroxyvitamin D3 on HuLM cell proliferation were assessed using a CyQuant cell proliferation assay kit (Invitrogen, Carlsbad, California).
Experimental Animals
Female athymic nude mice (approximately 5-6 weeks old) were obtained from Taconic Farm Inc (Hudson, New York). These mice were maintained in a specific pathogen germ-free environment. These animals were cared for and handled in accordance with the National Institutes of Health guidelines and were housed in facilities accredited by the Association for the Accreditation of Laboratory Animal Care. All animal experiments were approved by the Institutional Animal Care and Use Committee (IACUC) at Meharry Medical College, Nashville, Tennessee. Regular food and water were available to these animals throughout the experimentation period.
Treatment of Fibroid Tumors in Nude Mice
Eker rat tumor-derived ELT-3 cells, kindly provided by Dr Cheryl Walker (MD Anderson cancer Center Houston, Texas), were cultured in DMEM/F12 phenol-free medium as described previously.40 After quarantine, the above-mentioned nude mice were randomized into 3 groups such as control, paricalcitol, and vitamin D3 groups. All mice received subcutaneous implantation of 1.7 mg, 90 days sustained-release 17 β estradiol pellets (Innovative Research of America, Sarasota, Florida) as we described previously.16 The pellets were implanted 4 days before the subcutaneous injection of the cells to allow steady state tissue estrogen distribution. The ELT-3 cells (10 million cells/mouse) were injected dorsally using 18-gauge hypodermic needles. Treatment was started when tumors were visible within 2 to 3 weeks postcell implantation. Experimental mice bearing subcutaneous tumors were randomized into 3 groups with 8 mice in each group: (1) the control group was administered 95% propylene glycol (PEG) and 5% ethyl alcohol (vehicle-treated control); (2) the vitamin D3 treatment group was administered 1,25-dihydroxyvitamin D3 delivered by micro-osmotic pumps (Alzet Inc, Cupertino, California) filled with 100 µL of 95% PEG and 5% ethyl alcohol, along with 4.2 µg of 1,25-dihydroxyvitamin D3 implanted into the dorsal subcutaneous space, such that it will automatically release 500 ng/kg/d of 1,25-dihydroxyvitamin D3 to each animal for 28 days; and (3) the final group was the paricalcitol treatment group. Paricalcitol is a selective VDR activator. Propylene glycol-dissolved paricalcitol were injected 3 times a week (300 ng/kg/d) intraperitoneally (ip) on Monday, Wednesday, and Friday for 4 consecutive weeks. The amounts of paricalcitol were calculated for each ip injection to achieve the final dose of 300 ng/kg/d. For example, we calculated 600 ng/kg/mouse for injections on Mondays and Wednesdays. On Friday, we calculated 900 ng/kg/mouse for each ip injection so that it covered for 3 days to achieve the final dose of 300 ng/kg/d. We have chosen 300 ng/kg/d dose for paricalcitol because this is equivalent to currently Food and Drug Administration (FDA)-approved dosage of paricalcitol (for other indications) as well as this dose was previously used in an in vivo murine study without showing evidence of toxicity.39 Animals were observed daily for any postoperative complications or adverse events. At the end of the experiments, all animals were killed, and tissues such as liver, spleen, uterus, ovary, bones, fibroid tumors, and blood samples were collected. Vaginal smears from all animals were collected daily for up to 12 consecutive days to evaluate any differences. The tumor size was measured every week by an electronic slide caliper, and tumor volumes were calculated using the formula: volume = (length × width × depth × 0.52) as described previously.22
Safety and Toxicity Studies in Nude Mice
All experimental animals were carefully examined for any visual sign of toxicity, including reduction in body weight, changes in food and water takings, lethargy, movement, and even unexplained death. Standard autopsies were also performed at the end of the experiment to examine the gross evidence of toxicity. Tissue samples from various organs, including the ovaries, liver, spleen, kidneys, and lungs, were examined macroscopically and microscopically by an animal pathologist who was blinded to the treatment group assignments. In particular, liver tissues were histologically examined after H&E staining to verify any significant toxicity associated with the treatment. In our previous study with vitamin D3 treatment of uterine fibroids in Eker rats, we demonstrated that the dose used in that study had no significant toxicity.22
Immunohistochemical Analyses of Fibroid Tumors
Tumor tissues collected from vehicle-treated control, paricalcitol-treated nude mice, and vitamin D3-treated nude mice were immediately fixed in 10% buffered formalin for 15 to 20 hours, embedded with paraffin, and subjected to immunohistochemistry. Paraffin-embedded tissue sections (4 µm) were deparaffinized and rehydrated by being passed through xylene and graded ethanol solutions as described previously.41 Cell proliferation was analyzed by staining with immunoreactive Ki67 (a cell proliferation marker) using polyclonal anti-Ki67 antibody (Santa Cruz Biotechnology) at a 1:100 dilution. Apoptosis in fibroid tumors was also evaluated using histochemical staining with anticaspase 3 antibody and subsequent staining with peroxidase-conjugated secondary antibodies.
Hormonal Study
To evaluate whether paricalcitol or vitamin D3 can alter steroid hormone secretion in the treated animals, we collected blood samples from all experimental nude mice via cardiac puncture after killing. Blood samples were allowed to clot at room temperature for approximately 15 minutes and then centrifuged at 2000 rpm at 4°C for 15 minutes. Following centrifugation, the serum was separated and stored at −20°C. The serum concentration of estradiol, progesterone, and follicle-stimulating hormone (FSH) were determined by the Virginia University Ligand Assay and Analysis Core facility at the University of Virginia, Charlottesville, Virginia.
Statistical Analysis
All statistical data were analyzed using Student t test. Data are expressed as mean ± standard error of the mean. Student t test was used to assess the significance of differences in the means between vehicle-treated control and paricalcitol-treated or vitamin D3-treated groups. The significance of differences between consecutive points in paricalcitol or 1,25-dihydroxyvitamin D3-treated data were also calculated. Differences were considered statistically significant at the 95% confidence level when P value was less than .05.
Results
Paricalcitol Treatment Effectively Reduced the Proliferation of Cultured HuLM
To test whether paricalcitol can reduce fibroid cell proliferation and that reduction is stronger than that observed with vitamin D3, we performed a cell proliferation assay after treating HuLM cells with different concentrations of either paricalcitol or vitamin D3 for up to 168 hours (Figure 1A and B). We found that after 24 hours of treatment, paricalcitol reduced HuLM cell proliferation up to 9% at a 10 nmol/L concentration (P < .05), whereas vitamin D3 reduced proliferation only by 1% at the same concentration, and the reduction was not significant (Figure 1A and B). Moreover, after 72 hours of treatment, paricalcitol significantly reduced HuLM proliferation to 8% (P < .05) at a lower dose (10 pmol/L), whereas vitamin D3 did not display an effect at that low concentration (Figure 1A and B). Consistently, at comparable conditions (time and concentration), paricalcitol demonstrated slight reduction in proliferation of HuLM than vitamin D3. At 120 and 168 hours, paricalcitol showed a reduction of 45% and 60%, whereas vitamin D3 displayed a reduction of 37% and 56%, respectively. However, in an in vitro cell proliferation experiment, we plan to test whether paricalcitol has more potential to reduce uterine fibroid cell proliferation compared with the bioactive vitamin D3. We used an immortalized HuLM for cell proliferation assay. In this study, we did not verify the myometrial cells for cell proliferation assay. However, we previously showed that myometrial cells are comparatively less responsive to vitamin D3 when compared with HuLM cells.42 In our in vitro proliferation assay, we used a broad range of vitamin D3 or paricalcitol to examine the doses that are more effective to reduce cell proliferation. It is also somewhat difficult to know the amount of vitamin D3 actually absorbed by cells that are treated in culture. The 1 µmol/L concentration is actually much higher than that of the physiological dose. The 100 nmol/L concentration of vitamin D3 is considered to be within the physiological dose. Thus, our results suggest that paricalcitol and vitamin D3 can similarly reduce the proliferation of HuLM in an in vitro culture model.
Figure 1.
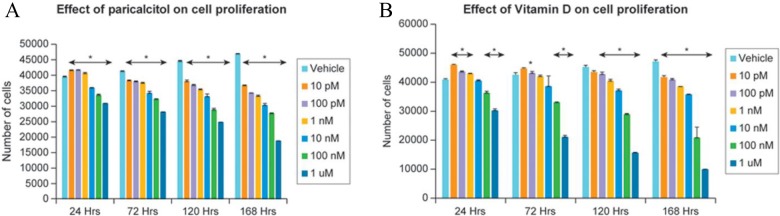
Effect of paricalcitol (A) or vitamin D3 (B) on the proliferation of cultured human uterine fibroid cells (HuLM). HuLM cells (2 × 103 cells/well) were seeded in 96-well plates for overnight and then treated with vehicle and either paricalcitol or vitamin D3 at different concentrations up to 168 hours. Cells were then analyzed for proliferation using CyQuant proliferation assay kit according to the manufacture’s instruction. Individual data points are the mean ± SEM of triplicate determinations (n = 3). *P < .05 when compared with vehicle-treated corresponding control. SEM indicates standard error of the mean.
Paricalcitol Treatment Effectively Reduced Fibroid Tumor Lesions in an In Vivo Nude Mice Model
To examine the therapeutic potential of paricalcitol and compare its effect with 1,25-dihydroxyvitamin D3, we performed in vivo treatment of uterine fibroids in athymic nude mice. Female nude mice were used to develop subcutaneous tumors by injecting ELT-3 cells as described earlier. The mice were randomized into control and treatment groups. The mice were then treated with vehicle control and either paricalcitol (300 ng/kg/d) or 1,25-dihydroxyvitamin D3 (500 ng/kg/d) for 4 weeks. Tumor sizes were measured weekly, and then tumor volumes were calculated. The volume graphs are shown in Figure 2. We observed that both paricalcitol and 1,25-dihydroxyvitamin D3 significantly reduced fibroid tumor size in nude mice compared with the vehicle-treated control group (Figure 2). At day 28, fibroid tumors in the vehicle-treated control nude mice were much larger than tumor volumes at day 0, and this difference is statistically significant (Figure 2; P < .05). These results suggest that paricalcitol and 1,25-dihydroxyvitamin D3 can potentially reduce fibroid tumor size in a nude mice model. Moreover, we observed that paricalcitol treatment did not exhibit greater levels of tumor growth inhibition as compared with 1,25-dihydroxyvitamin D3 (Figure 2). These results suggest that paricalcitol can potentially reduce the growth of uterine fibroid tumors in nude mice.
Figure 2.
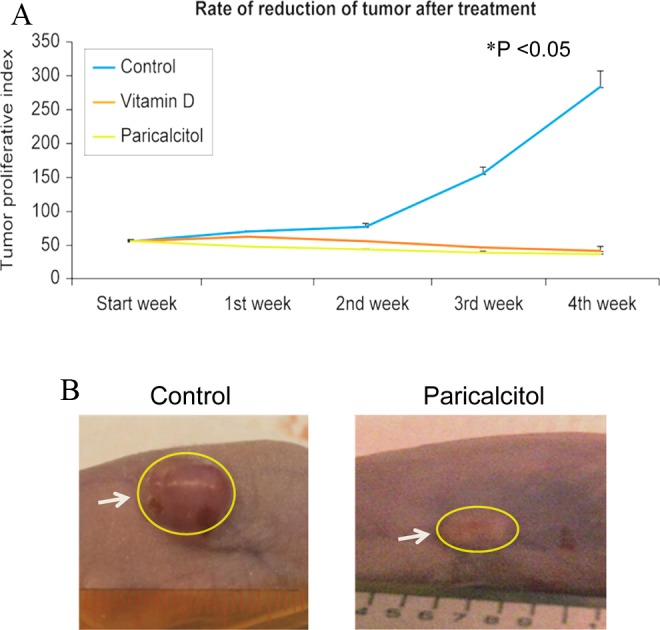
Effects of paricalcitol on growth of fibroid tumors in nude mice model. (A) Female athymic nude mice harboring subcutaneous tumors were randomized into control and treatment groups. The control group of mice was given vehicle only (vehicle-treated control). Paricalcitol or vitamin D3 was given to the treatment groups at the rate of 300 ng/kg/d or 500 ng/kg/d for 4 consecutive weeks, respectively. At the end of the experiments, all animals (n = 8 mice/group) were sacrificed, and fibroid tumors were measured using a slide calipers, and tumor volumes were calculated as described in Materials and Methods section. The reduction in fibroid tumor size was compared between vehicle-treated control and paricalcitol- or vitamin D3-treated mice. *P < .05 when paricalcitol- or vitamin D3-treated tumor compared with vehicle-treated control. Individual data points are the mean ± SEM (n = 8). B and C, Representative pictures of fibroid tumors from vehicle-treated control and paricalcitol-treated mice are shown. White arrows indicate fibroid tumors from control and paricalcitol-treated mice. SEM indicates standard error of the mean.
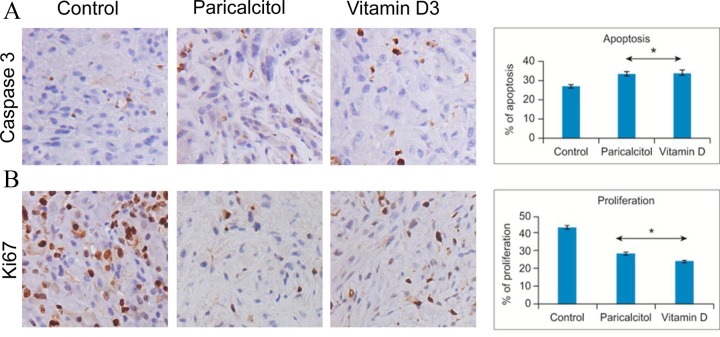
Paricalcitol Treatment Reduced Cell Proliferation and Induced Apoptosis in Nude Mice Fibroid Tumors
To test whether paricalcitol inhibits cell proliferation in nude mice tumors, we performed immunohistochemical analyses using immunoreactive Ki67 (a cell proliferating marker) via polyclonal anti-Ki67 antibodies. Paricalcitol treatment demonstrated a remarkable antiproliferative effect on fibroid tumors in the treated animals. Paricalcitol treatment produced a 66% reduction in cell proliferation, whereas 1,25-dihydroxyvitamin D3 produced a 56% reduction (Figure 3B). Moreover, we also observed that both paricalcitol and 1,25-dihydroxyvitamin D3 treatment induced caspase activity in uterine fibroid tumors in the treatment group compared with vehicle-treated control (Figure 3A), suggesting the activation of the caspase signaling cascade of 123.6% for paricalcitol and 124.9% for 1,25-dihydroxyvitamin D3 treatment. Together, these results suggest that paricalcitol reduces uterine fibroid cell growth through its antiproliferative and proapoptotic functions.
Figure 3.
Immunohistochemical analyses of fibroid tumor tissues derived from nude mice that were treated with vehicle and paricalcitol or vitamin D3. A, The distribution of apoptotic cells was evaluated in fibroid tumors from vehicle-treated control and paricalcitol- or vitamin D3-treated fibroid tumors using immunohistochemical analyses with anticaspase 3 antibody. The apoptosis related marker caspase 3 staining (dark brown nuclei) was significantly higher in fibroid tumors derived from paricalcitol- or vitamin D3-treated mice. B, The cell proliferation marker Ki67 nuclear staining (dark brown nuclei) was also evaluated in vehicle-treated control and paricalcitol- or vitamin D3-treated fibroid tumor tissues. The Ki67 staining was much lower in fibroid tumors that were derived from either paricalcitol- or vitamin D3-treated nude mice. All images were captured at 200× magnification. The degree of apoptosis or proliferation was scored by counting the caspase 3 or Ki67 positive nuclei, respectively. The number of stained cells was counted in a total of 4 random high-power fields in the representative fibroid tumor derived from vehicle-treated control and paricalcitol- or vitamin D3-treated nude mice, and then the average number of cells is presented. The percentage of apoptosis or proliferation is calculated and shown (right panels). *P < .05 when compared with vehicle-treated control.
Paricalcitol Treatment Exhibits No Sign of Damage or Necrosis in Nude Mice Anatomy
Our daily monitoring indicated that all animals tolerated the paricalcitol and 1,25-dihydroxyvitamin D3 dose and survived during the course of the experiment. There were no apparent signs of toxicity such as lethargy, body weight reduction, difficulty in mobility, or change in food and water intake, and no unexplained deaths. In addition, there was no evidence of any gross toxicity, necrosis, or changes in the morphology of the vital organs on macroscopic examination, which included the liver, lungs, spleen, uterus, and ovaries. Histopathological examination of these organs was normal, with no evidence of tissue damage. The H&E staining of tissue sections, particularly liver tissues from vehicle-treated control and paricalcitol or 1,25-dihydroxyvitamin D3-treated nude mice, was examined by an animal pathologist blinded to treatment assignments. Representative histology pictures were shown to compare between vehicle-treated control and paricalcitol or 1,25-dihydroxyvitamin D3-treated tissues in nude mice (Figure 4). We observed that these tissues displayed no sign of obvious damage and necrosis (Figure 4). These results suggest that paricalcitol at the dose of 300 ng/kg/d is therapeutically safe for fibroid treatment.
Figure 4.
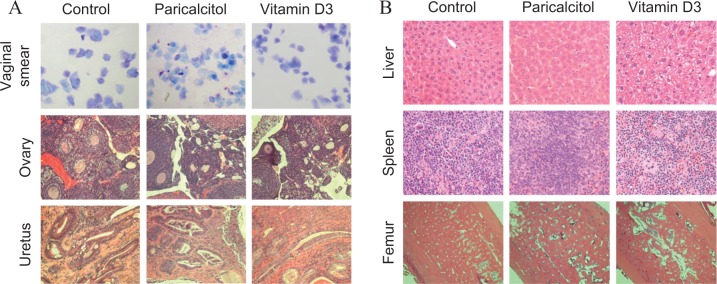
Effect of paricalcitol or vitamin D3 on estrous cycle in experimental nude mice. A, (top panel) we evaluated the effects of paricalcitol or vitamin D3 on vaginal smears (blue) which were stained with toluidine blue solution. The staining patterns were similar between the vehicle-treated control and treated groups of mice. Middle and bottom panels, H and E staining of ovary and uterus from vehicle-treated and paricalcitol- or vitamin D3-treated animals showing no visual structural changes. All images were captured at 100× magnification. B, H and E staining of liver, femur, and kidney tissues from vehicle-treated and paricalcitol or vitamin D3-treated animals also showing similar architecture and no signs of toxicity associated with the treatments. All images were captured at 200× magnification. Representative images are shown from a total of 4 mice/group (n = 4).
Paricalcitol Treatment did not Affect Estrous Cycle in Experimental Nude Mice
To determine whether paricalcitol or vitamin D3 can affect the estrous cycle in mice, we examined the vaginal smears of paricalcitol-, 1,25-dihydroxyvitamin D3-, and vehicle-treated nude mice from the second week of the treatment and continued up to 12 days. These vaginal smears were stained with 2% toluidine blue solution. We observed that treatment with either paricalcitol or 1,25-dihydroxyvitamin D3 did not affect estrous cycle in experimental mice. Similar staining patterns were observed in paricalcitol- or 1,25-dihydroxyvitamin D3-treated mice as well as in vehicle-treated mice, and all mice stayed in the estrous cycle (Figure 4). These results suggest that either paricalcitol or 1,25-dihydroxyvitamin D3 has no effects on estrous cycle in mice given estrogen that are released from the grafted pallets.
Paricalcitol Treatment did not Affect Steroid Hormone Secretion in Nude Mice-Bearing Fibroid Tumors
Prior studies have indicated that steroid hormones such as estrogen, progesterone, and FSH play major roles in uterine fibroid proliferation. To verify whether paricalcitol treatment affects these hormone secretions, we measured these hormones in the serum collected from vehicle-treated control and paricalcitol-treated experimental nude mice. We observed that paricalcitol treatment reduced estradiol secretion in treated mice, whereas the reduction level was not statistically significant (l41.76%; P > .05). Conversely, the secretion of progesterone and FSH was moderately induced by paricalcitol and that induction was also not statistically significant (161%; Figure 5). These results suggest that paricalcitol has no significant effect on the secretion of estradiol, progesterone, and FSH in the experimental nude mice model of uterine fibroids.
Figure 5.
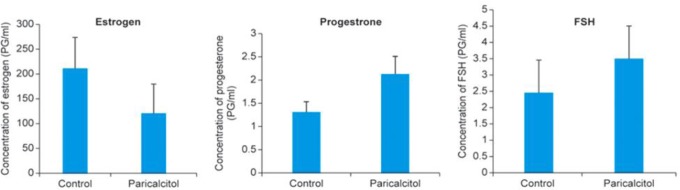
Effect of paricalcitol on secretion of estrogen, progesterone, and FSH in experimental nude mice. Serum levels of estrogen (A), progesterone (B), and FSH (C) in paricalcitol-treated animals were evaluated in experimental mice (n = 4 mice, in each group). Blood samples were collected via cardiac puncture at the end of the experiment. Serum was separated from blood samples from vehicle-treated control and paricalcitol-treated mice as described in Materials and Methods section. The concentrations of estrogen, progesterone, and FSH in each serum sample were determined. No significant differences were observed in the levels of estrogen, progesterone, and FSH between vehicle-treated control and paricalcitol-treated experimental mice. Individual data points are the mean ±SEM (n = 4). FSH indicates follicle stimulating hormone; SEM, standard error of the mean.
Reduced Expression of Steroid Receptors in Fibroid Tumors in the Treated Mice
To examine whether paricalcitol and vitamin D3 can affect the expression levels of ER-α, PR-A, and PR-B in uterine fibroid tumors, we performed immunohistochemical analyses using ER-α, PR-A, and PR-B antibodies. We observed that either paricalcitol or vitamin D3 treatment reduced staining signals of ER-α, PR-A, and PR-B in fibroid tumors when compared to vehicle-treated tumor tissues (Figure 6). We also observed that the staining levels of these proteins were similar in paricalcitol- and vitamin D3-treated fibroid tissues. These fibroid tissue samples were further immunohistochemically analyzed for the expression of various smooth muscle markers such as α-smooth muscle actin (α-SMA), desmin, and vimentin, and the H and E staining to determine the morphological changes. The H and E staining showed reduced cellularity in either paricalcitol- or vitamin D3-treated tumor tissues as compared with vehicle-treated control (Figure 7, top panel). We found that the expression levels of α-SMA, smooth muscle desmin, and smooth muscle vimentin were slightly lower in either paricalcitol- or vitamin D3-treated fibroid tissues as compared to the vehicle-treated control (Figure 7, bottom panels). These results suggest that paricalcitol and vitamin D3 similarly reduced the expression of steroid hormone receptors while without remarkably affecting the expression of structural smooth muscle proteins in uterine fibroids in nude mice model.
Figure 6.
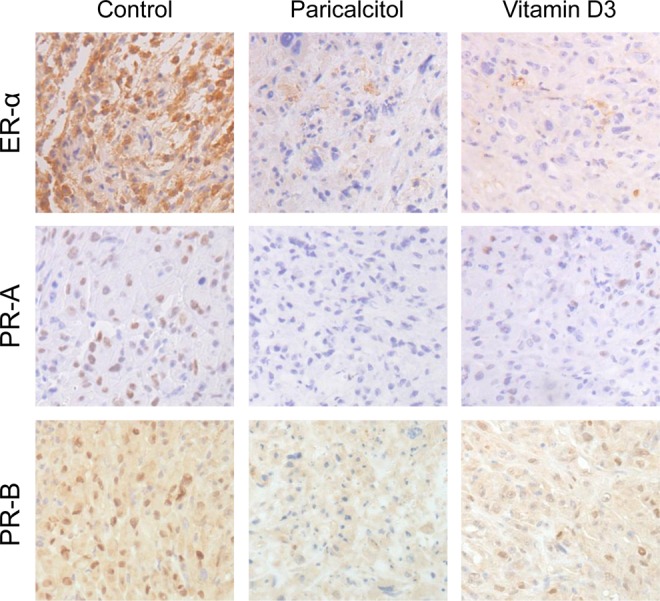
Effect of paricalcitol or vitamin D3 on expression of ER-α, PR-A, and PR-B in fibroid tumors in experimental mice. Immunohistochemical analyses of tumor tissues with anti-ER-α (top panel), anti-PR-A (middle panel), and anti-PR-B (bottom panel) antibodies showing dark brown staining signals in vehicle-treated control as compared with paricalcitol- or vitamin D-treated animals. No significant differences were observed in ER-α, PR-A, and PR-B staining in tumor tissues in mice treated with either paricalcitol or vitamin D3. All images were captured at 200× magnification. Representative images are shown from a total of 4 mice/group (n = 4). ER-α indicates estrogen receptor α; PR-A, progesterone receptor A; PR-B, progesterone receptor B.
Figure 7.
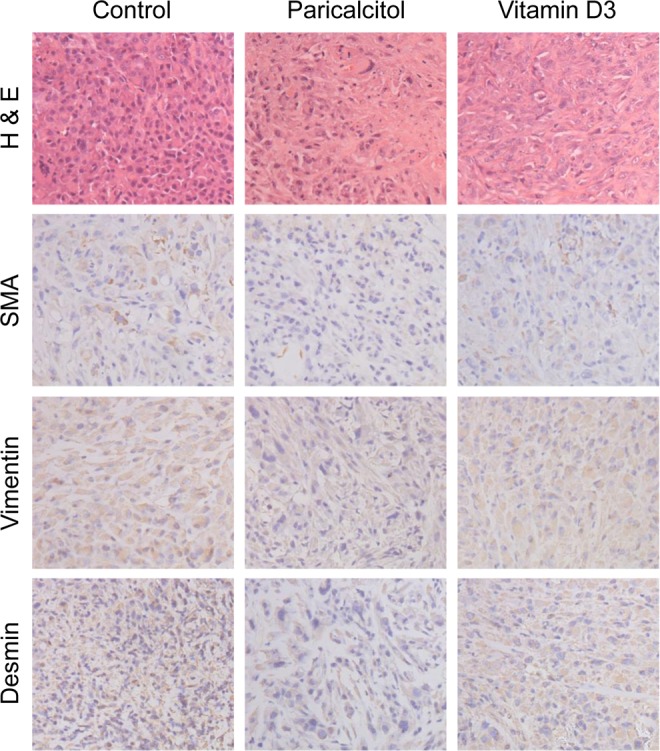
Immunohistochemical analyses of fibroid tumors derived from vehicle-treated control, and paricalcitol or vitamin D3-treated experimental mice. H and E staining showing less cellularity in fibroid tumors derived from paricalcitol- or vitamin D3-treated animals as compared with vehicle-treated control. The immunohistochemical staining signals (dark brown) for α-smooth muscle actin (α-SMA), smooth muscle vimentin, and smooth muscle desmin were slightly lower in fibroid tumors in mice treated with paricalcitol or vitamin D3. All images were captured at 200× magnification. Representative images are shown from a total of 4 mice/group (n = 4).
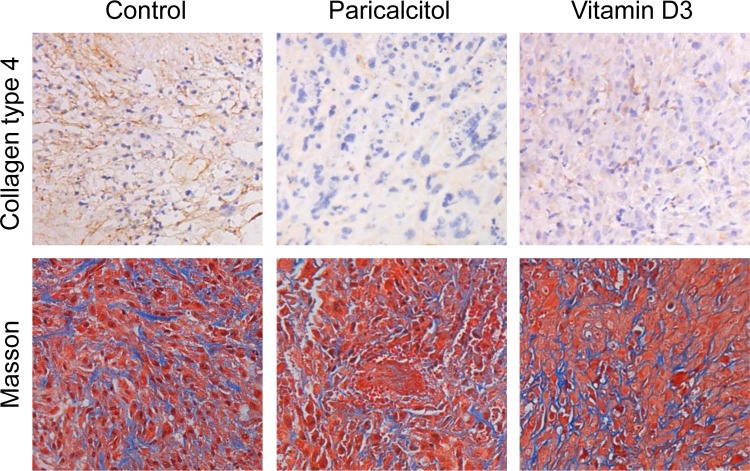
Paricalcitol Reduced Collagen Production in Fibroid Tumors
To determine whether paricalcitol or vitamin D3 treatment can reduce extracellular collagen content in fibroid tumors, we performed immunohistochemical analyses. Masson Trichrome (blue) and collagen type 4 staining (brown) indicated that the extracellular collagen content was lower in either paricalcitol- or vitamin D3-treated tissues as compared with vehicle-treated controls. We also observed that both paricalcitol and vitamin D3 similarly reduced collagen content in fibroid tissues in treated mice (Figure 8, top panel). They displayed a typical staining pattern with tumor cell bundles with moderate volume of well-vascularized connective tissues. In comparison with vehicle-control fibroid tissues, paricalcitol- and vitamin D3-treated tumor tissues displayed decreased cellularity and collagen degradation (Figure 8, bottom panel). These results suggest that paricalcitol reduces the extracellular matrix (ECM)-associated collagen content in uterine fibroid tumors in nude mice.
Figure 8.
Effect of paricalcitol or vitamin D3 on the expression of collagen type 4 in fibroid tumors in experimental nude mice. Top panel, Immunohistochemical analyses with anticollagen type 4 antibody showing higher staining signals (dark brown) in tumor tissues from vehicle-treated control as compared with paricalcitol- or vitamin D- treated animals. Bottom panel, Masson's trichrome staining was performed to differentiate between collagen and smooth muscle cells in fibroid tumors. The presence of collagen content (blue) was lower in paricalcitol- or vitamin D3-treated animals as compared with vehicle-treated control. The staining signals for smooth muscle cells (red) are similar between control and treatment groups. All images were captured at 200× magnification. Representative images are shown from a total of 4 mice/group (n = 4).
Discussion
In this current study, we tested the therapeutic efficacy of paricalcitol, a VDR activator, as a nonsurgical alternative option for the treatment of uterine fibroids and compared its effects with the bioactive 1,25-dihydroxyvitamin D3 using an in vitro and an in vivo animal model. 1,25-Dihydroxyvitamin D3 is a biologically active hormone that is well known to be involved in calcium homeostasis.28 We and others have recently demonstrated that vitamin D deficiency is a common phenomenon observed in patients with uterine fibroids, and deficiency is associated with a higher risk of the development of uterine fibroids.19–21 In addition, our recent observations demonstrated that 1,25-dihydroxyvitamin D3 treatment induced uterine fibroid growth inhibition in an in vivo Eker rat animal model,22 and it induced apoptosis in an in vitro uterine fibroid cell culture system.43 We also demonstrated that 1,25-dihydroxyvitamin D3 has the potential to reduce the ECM proteins and proteoglycan that lead to fibrosis.44 Extracellular matrix-associated proteins such as collagen type 1 and fibronectin were overexpressed and play important roles in the pathophysiology of uterine fibroids.45,46 We demonstrated that 1,25-dihydroxyvitamin D3 can effectively inhibit production of ECM-associated collagen type I and fibronectin proteins and reduced uterine fibroid tumor volume in Eker rat animal model as well as in an in vitro cell culture system.22,42 The ECM consists of fibrillar proteins that provide a structural scaffold for tissue support.47 Fibronectin is an ECM protein that is associated with cell growth, migration, and survival.48 The major component of the ECM is collagen, which provides stability and help in maintaining the structural integrity of tissues.49 In uterine fibroids, fibrillar collagens, such as type I and III collagen messenger RNAs, are elevated.45,50 Excessive deposition of the ECM is due to the imbalance of matrix synthesis versus degradation, which ultimately stimulates fibroid cell proliferation and increase tumor size. Our previous observations demonstrated that vitamin D3 potentially can reduce the expression of fibronectin, collagen, and proteoglycan proteins in HuLM.42 Fibronectin, biglycan, and versican are proteoglycans and are the important components in the ECM. Biglycan, a small proteoglycan in the ECM, also influences differentiation and proliferation processes,51 whereas versican, a large proteoglycan in the ECM, is associated with cell proliferation and apoptosis.52 The RNA and protein levels of versican have been demonstrated to be upregulated in uterine fibroids.53
Matrix metalloproteinases (MMPs) are proteolytic enzymes that play critical roles in ECM degradation, synthesis, and remodeling.54 The activity of MMPs is regulated by the transcriptional and posttranscriptional levels under normal condition and is controlled at the protein level via their activator and inhibitors.55 It has recently been demonstrated that increased activities of MMP2 and MMP9 are associated with fibroid pathogenesis.56 The activation of MMP2 and MMP9 is also associated with collagen deposition and fibrosis in a VDR knockout mice model.57 Our recent published study demonstrated that vitamin D3 can potentially regulate the expression and proteolytic activities of MMP2 and MMP9 in HuLM,58 which eventually leads to the reduction in uterine fibroid pathogenesis. The expression levels of MMPs and their activation processes are also regulated by TIMP2.59 Uterine fibroids are characterized by excessive synthesis and deposition of ECM, and the increased expression and activities of MMP2 and MMP9 in uterine fibroids might be a reflection of the abnormal organization of ECM in this disease.
Based on our previous studies, we demonstrated that vitamin D3 is a potent regulator of uterine fibroid proliferation. Nevertheless, vitamin D3 is biologically active, and it is associated with calcium homeostasis. The administration of higher doses of vitamin D3 for a longer time period may cause hypercalcemic side effects. Therefore, to avoid such possible hypercalcemic effects of vitamin D3, in the current study, we have utilized paricalcitol that features less calcemic side effects and is a potent activator of the VDR.39 Vitamin D receptor is a nuclear hormone receptor that particularly binds to vitamin D3 for its biological function in most cell systems, and VDR has been found to be expressed in various human, rat, and murine tissues, including uterine and fibroid tissues.60,61 A recent study demonstrated that the administration of paricalcitol is associated with the reduction in cardiac fibrosis and lower expression of profibrotic genes in the heart and that ultimately improves heart function in a murine model.39 Moreover, paricalcitol may affect the remodeling process, including the changes in cardiomyocytes and nonmyocardiocyte cells such as fibroblasts. Vitamin D deficiency is found in cardiovascular disease, especially with heart failure, and that type of deficiency is associated with a poor heart failure prognosis.10,23
In this current study, we generated subcutaneous fibroid tumor lesions in nude mice using ELT-3 cells that were derived from Eker rat uterine fibroid tumors. The administration of paricalcitol at 300 ng/kg/d resulted in dramatic shrinkage of fibroid tumors compared with the untreated control mice (Figure 2). We used 300 ng/kg/d dose of vitamin D3 which is equivalent to approximately 850 IU for an adult having body weight of 70 kg. This dose of vitamin D3 was calculated using the conversion equation, 1 µg of vitamin D3 is equivalent to 40 IU. In parallel, the administration of 1,25-dihydroxyvitamin D3 at 500 ng/kg/d also significantly reduced the tumor sizes compared with the untreated control mice. We observed that both paricalcitol and vitamin D3 effectively reduced fibroid tumor sizes in nude mice. We also found that paricalcitol-mediated shrinkage of fibroid tumors was very similar to 1,25-dihydroxyvitamin D3 effects (Figure 2). Histochemical analyses of these uterine fibroids clearly demonstrated the maintenance of fibroid tissue morphology, enhanced apoptosis, and reduced proliferation as well as the maintenance of other uterine fibroid key markers such as ER-α, PR-A, and PR-B, and smooth muscles proteins (actin, desmin, and vimentin). We also observed that paricalcitol or 1,25-dihydroxyvitamin D3 treatment can potentially reduce the expression of these marker proteins in comparison with the untreated-control (Figure 8). However, consistently, the effect of paricalcitol was slightly more effective than 1,25-dihydroxyvitamin D3 (Figure 8). These results suggest that paricalcitol is effective in the reduction of fibroid proliferation in vivo animal models likely due to higher VDR affinity and a longer half-life.39
To further evaluate the paricalcitol safety profile, we performed histological examination, particularly of liver tissues from vehicle-treated controls and paricalcitol or 1,25-dihydroxyvitamin D3-treated nude mice. Our observation indicates that treatment with paricalcitol or 1,25-dihydroxyvitamin D3 did not produce any sign of tissue damage and necrosis (Figure 4). These results suggest that paricalcitol is therapeutically nontoxic and safe for fibroid treatment. Moreover, we determined the effect of paricalcitol on ovarian functions, and our findings indicate that paricalcitol treatment did not affect ovarian structure as determined by histology and also no significant effect on secretion of serum estrogen, progesterone, and FSH as well as no effect on vaginal smears as shown in Figure 6. These observations suggest that paricalcitol treatment did not interrupt the folliculogenesis and ovulation process and did not interfere in normal hypothalamic-pituitary-ovarian axis function.
In addition, bone histology in the paricalcitol- or vitamin D3-treated groups did not differ from vehicle-treated controls, again confirming that such treatment did not have deleterious effects on long bones. Osteoporosis is a common challenge during the treatment of uterine fibroids with gonadotropin-releasing hormone (GnRH) agonists because of the ensuing estrogen deficiency. Such major side effect limits GnRHa treatment to short periods and limits its utility as durable treatment of uterine fibroids. However, in this study we observed that paricalcitol treatment did not cause any noticeable changes in the bone structure and histology as shown in Figure 4, which could propose this drug as a reliable long-term medical treatment option for uterine fibroids.
The incidence of uterine fibroids is higher in premenopausal women and because of lack of an effective and safe nonsurgical therapeutic options, the management of uterine fibroids is still challenging. Uterine fibroid is a complicated disease process that exerts an enormous burden on health care resources in the United States as well as around the globe.29,62 Currently, hysterectomy is the only effective treatment option for uterine fibroids. However, the surgical approach is not always a favorable choice, especially in women who desire to preserve their future fertility and those who are not good surgical candidates. Therefore, the development of an effective, safe and nonsurgical therapeutic option for uterine fibroids is a critical need in women’s health care. We have already established enough evidence that the bioactive 1,25-dihydroxyvitamin D3 is a potent regulator of uterine fibroid proliferation in vitro cell culture and in an in vivo animal model.22,37,42,58,63 We and others also recently established the association of lower levels of serum vitamin D3 with an increased risk of symptomatic uterine fibroids.19–21 However, no studies have yet been conducted to evaluate the therapeutic efficacy of paricalcitol on uterine fibroid proliferation in an in vivo fibroid model. Paricalcitol has been recently studied with an in vivo animal model and has been demonstrated to be safe and effective within a wide dosing range.64 A recent study demonstrated that administration of paricalcitol attenuated myocardial fibrogenesis and protected against diastolic dysfunction.39 In addition, paricalcitol have been FDA approved and in standard clinical use (both orally and intravenous) for secondary hyperparathyroidism since 1998 and 2005, respectively, with an excellent safety profile (http://www.medscape.com/viewarticle/538683). This present study was conducted to evaluate the therapeutic efficacy and safety of paricalcitol in the treatment of uterine fibroids in nude mice models. Overall, our data support that paricalcitol is an effective and safe treatment option for uterine fibroids without affecting normal ovary functions, as well as with no histologically visible structural changes in bone.
In conclusion, based on our data, we propose that targeting VDR is a novel approach to treat uterine fibroids. Paricalcitol, the potent VDR activator and an analog of vitamin D3, notably shrinks uterine fibroid tumor size in a nude mice model of uterine fibroids. In this preclinical study, for the first time, we determined that paricalcitol is effective and safe for the treatment of uterine fibroids in a nude mice model. Further evaluation of this compound and other VDR activators in pilot clinical studies is warranted. Thus, paricalcitol could be a novel approach for the oral nonsurgical therapeutic option for the treatment of women with symptomatic uterine fibroids.
Acknowledgments
We would like to thank Dr Carlos Virgous at the Animal Care Facility (ACF) in Meharry Medical College, Nashville, Tennessee for his help in performing all animal experiments. We also thank the Histology core facility at the Vanderbilt University, Nashville, Tennessee for performing all immunohistochenical analyses of tissues samples.
Authors’ Note: Sunil K. Halder and Chakradhari Sharan contributed equally to this manuscript.
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship and/or publication of this article: This work was supported by the National Institute of Health (NIH) /R01 grant (HD046228) to A.A.H.
References
- 1. Maruo T, Matsuo H, Samoto T, et al. Effects of progesterone on uterine leiomyoma growth and apoptosis. Steroids. 2000;65 (10-11):585–592 [DOI] [PubMed] [Google Scholar]
- 2. Walker CL, Burroughs KD, Davis B, Sowell K, Everitt JI, Fuchs-Young R. Preclinical evidence for therapeutic efficacy of selective estrogen receptor modulators for uterine leiomyoma. J Soc Gynecol Investig. 2000;7 (4):249–256 [PubMed] [Google Scholar]
- 3. Stewart EA. Uterine fibroids. Lancet. 2001;357 (9252):293–298 [DOI] [PubMed] [Google Scholar]
- 4. Farhi J, Ashkenazi J, Feldberg D, Dicker D, Orvieto R, Ben Rafael Z. Effect of uterine leiomyomata on the results of in-vitro fertilization treatment. Hum Reprod. 1995;10 (10):2576–2578 [DOI] [PubMed] [Google Scholar]
- 5. Eldar-Geva T, Meagher S, Healy DL, MacLachlan V, Breheny S, Wood C. Effect of intramural, subserosal, and submucosal uterine fibroids on the outcome of assisted reproductive technology treatment. Fertil Steril. 1998;70 (4):687–691 [DOI] [PubMed] [Google Scholar]
- 6. Surrey ES, Lietz AK, Schoolcraft WB. Impact of intramural leiomyomata in patients with a normal endometrial cavity on in vitro fertilization-embryo transfer cycle outcome. Fertil Steril. 2001;75 (2):405–410 [DOI] [PubMed] [Google Scholar]
- 7. Hart R, Khalaf Y, Yeong CT, Seed P, Taylor A, Braude P. A prospective controlled study of the effect of intramural uterine fibroids on the outcome of assisted conception. Hum Reprod. 2001;16 (11):2411–2417 [DOI] [PubMed] [Google Scholar]
- 8. Wilcox LS, Koonin LM, Pokras R, Strauss LT, Xia Z, Peterson HB. Hysterectomy in the United States, 1988-1990. Obstet Gynecol. 1994;83 (4):549–555 [DOI] [PubMed] [Google Scholar]
- 9. Rein MS, Barbieri RL, Friedman AJ. Progesterone: a critical role in the pathogenesis of uterine myomas. Am J Obstet Gynecol. 1995;172 (1 pt 1):14–18 [DOI] [PubMed] [Google Scholar]
- 10. Zittermann A, Schleithoff SS, Tenderich G, Berthold HK, Korfer R, Stehle P. Low vitamin D status: a contributing factor in the pathogenesis of congestive heart failure? J Am Coll Cardiol. 2003;41 (1):105–112 [DOI] [PubMed] [Google Scholar]
- 11. Cardozo ER, Clark AD, Banks NK, Henne MB, Stegmann BJ, Segars JH. The estimated annual cost of uterine leiomyomata in the United States. Am J Obstet Gynecol. 2012;206 (3):211. e211–e219 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Shen SH, Fennessy F, McDannold N, Jolesz F, Tempany C. Image-guided thermal therapy of uterine fibroids. Semin Ultrasound CT MR. 2009;30 (2):91–104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Al-Hendy A, Salama S. Gene therapy and uterine leiomyoma: a review. Hum Reprod Update. 2006;12 (4):385–400 [DOI] [PubMed] [Google Scholar]
- 14. Ezzati M, Norian JM, Segars JH. Management of uterine fibroids in the patient pursuing assisted reproductive technologies. Womens Health (Lond Engl). 2009;5 (4):413–421 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Hassan MH, Othman EE, Hornung D, Al-Hendy A. Gene therapy of benign gynecological diseases. Adv Drug Deliv Rev. 2009;61 (10):822–835 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Zhang D, Al-Hendy M, Richard-Davis G, et al. Green tea extract inhibits proliferation of uterine leiomyoma cells in vitro and in nude mice. Am J Obstet Gynecol. 2010;202 (3):289. e281–289 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Hassan MH, Salama SA, Zhang D, et al. Gene therapy targeting leiomyoma: adenovirus-mediated delivery of dominant-negative estrogen receptor gene shrinks uterine tumors in Eker rat model. Fertil Steril. 2010;93 (1):239–250 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Norman AW, Bouillon R. Vitamin D nutritional policy needs a vision for the future. Exp Biol Med (Maywood). 2010;235 (9):1034–1045 [DOI] [PubMed] [Google Scholar]
- 19. Sabry M, Halder SK, Allah AS, Roshdy E, Rajaratnam V, Al-Hendy A. Serum vitamin D3 level inversely correlates with uterine fibroid volume in different ethnic groups: a cross-sectional observational study. Int J Womens Health. 2013;5:93–100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Baird DD, Hill MC, Schectman JM, Hollis BW. Vitamin d and the risk of uterine fibroids. Epidemiology. 2013;24 (3):447–453 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Paffoni A, Somigliana E, Vigano P, et al. Vitamin D status in women with uterine leiomyomas. J Clin Endocrinol Metab. 2013;98 (8):E1374–E1378 [DOI] [PubMed] [Google Scholar]
- 22. Halder SK, Sharan C, Al-Hendy A. 1,25-dihydroxyvitamin D3 treatment shrinks uterine leiomyoma tumors in the Eker rat model. Biol Reprod. 2012;86 (4):116, 1–10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Liu LC, Voors AA, van Veldhuisen DJ, et al. Vitamin D status and outcomes in heart failure patients. Eur J Heart Fail. 2011;13 (6):619–625 [DOI] [PubMed] [Google Scholar]
- 24. Freedman LP. Multimeric Coactivator Complexes for Steroid/Nuclear Receptors. Trends Endocrinol Metab. 1999;10 (10):403–407 [DOI] [PubMed] [Google Scholar]
- 25. Guyton KZ, Kensler TW, Posner GH. Cancer chemoprevention using natural vitamin D and synthetic analogs. Annu Rev Pharmacol Toxicol. 2001;41:421–442 [DOI] [PubMed] [Google Scholar]
- 26. Meems LM, van der Harst P, van Gilst WH, de Boer RA. Vitamin D biology in heart failure: molecular mechanisms and systematic review. Curr Drug Targets. 2011;12 (1):29–41 [DOI] [PubMed] [Google Scholar]
- 27. Nesby-O'Dell S, Scanlon KS, Cogswell ME, et al. Hypovitaminosis D prevalence and determinants among African American and white women of reproductive age: third National health and nutrition examination survey, 1988-1994. Am J Clin Nutr. 2002;76 (1):187–192 [DOI] [PubMed] [Google Scholar]
- 28. Holick MF. Too little vitamin D in premenopausal women: why should we care? Am J Clin Nutr. 2002;76 (1):3–4 [DOI] [PubMed] [Google Scholar]
- 29. Downes E, Sikirica V, Gilabert-Estelles J, et al. The burden of uterine fibroids in five European countries. Eur J Obstet Gynecol Reprod Biol. 2010;152 (1):96–102 [DOI] [PubMed] [Google Scholar]
- 30. Al-Hendy A, Salama SA. Ethnic distribution of estrogen receptor-alpha polymorphism is associated with a higher prevalence of uterine leiomyomas in black Americans. Fertil Steril. 2006;86 (3):686–693 [DOI] [PubMed] [Google Scholar]
- 31. Brandon DD, Bethea CL, Strawn EY, et al. Progesterone receptor messenger ribonucleic acid and protein are overexpressed in human uterine leiomyomas. Am J Obstet Gynecol. 1993;169 (1):78–85 [DOI] [PubMed] [Google Scholar]
- 32. Brandon DD, Erickson TE, Keenan EJ, et al. Estrogen receptor gene expression in human uterine leiomyomata. J Clin Endocrinol Metab. 1995;80 (6):1876–1881 [DOI] [PubMed] [Google Scholar]
- 33. Chen TC, Holick MF. Vitamin D and prostate cancer prevention and treatment. Trends Endocrinol Metab. 2003;14 (9):423–430 [DOI] [PubMed] [Google Scholar]
- 34. Koli K, Keski-Oja J. 1alpha,25-dihydroxyvitamin D3 and its analogues down-regulate cell invasion-associated proteases in cultured malignant cells. Cell Growth Differ. 2000;11 (4):221–229 [PubMed] [Google Scholar]
- 35. Mangelsdorf DJ, Thummel C, Beato M, Herrlich P, Schutz G, Umesono K, Blumberg B, Kastner P, Mark M, Chambon P. et al. The nuclear receptor superfamily: the second decade. Cell. 1995, 83 (6):835–839 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Bouillon R, Verstuyf A, Verlinden L, et al. Non-hypercalcemic pharmacological aspects of vitamin D analogs. Biochem Pharmacol. 1995;50 (5):577–583 [DOI] [PubMed] [Google Scholar]
- 37. Sharan C, Halder SK, Thota C, Jaleel T, Nair S, Al-Hendy A. Vitamin D inhibits proliferation of human uterine leiomyoma cells via catechol-O-methyltransferase. Fertil Steril. 2011;95 (1):247–253 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Miller GJ. Vitamin D and prostate cancer: biologic interactions and clinical potentials. Cancer Metastasis Rev. 1998;17 (4):353–360 [DOI] [PubMed] [Google Scholar]
- 39. Meems LM, Cannon MV, Mahmud H, et al. The vitamin D receptor activator paricalcitol prevents fibrosis and diastolic dysfunction in a murine model of pressure overload. J Steroid Biochem Mol Biol. 2012;132 (3-5):282–289 [DOI] [PubMed] [Google Scholar]
- 40. Howe SR, Gottardis MM, Everitt JI, Goldsworthy TL, Wolf DC, Walker C. Rodent model of reproductive tract leiomyomata. Establishment and characterization of tumor-derived cell lines. Am J Pathol. 1995;146 (6):1568–1579 [PMC free article] [PubMed] [Google Scholar]
- 41. Al-Hendy A, Auersperg N. Applying the herpes simplex virus thymidine kinase/ganciclovir approach to ovarian cancer: an effective in vitro drug-sensitization system. Gynecol Obstet Invest. 1997;43 (4):268–275 [DOI] [PubMed] [Google Scholar]
- 42. Halder SK, Osteen KG, Al-Hendy A. 1,25-dihydroxyvitamin d3 reduces extracellular matrix-associated protein expression in human uterine fibroid cells. Biol Reprod. 2013;89 (6):150, 1–13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Mathiasen IS, Lademann U, Jaattela M. Apoptosis induced by vitamin D compounds in breast cancer cells is inhibited by Bcl-2 but does not involve known caspases or p53. Cancer Res. 1999;59 (19):4848–4856 [PubMed] [Google Scholar]
- 44. Walker CL, Stewart EA. Uterine fibroids: the elephant in the room. Science. 2005;308 (5728):1589–1592 [DOI] [PubMed] [Google Scholar]
- 45. Stewart EA, Friedman AJ, Peck K, Nowak RA. Relative overexpression of collagen type I and collagen type III messenger ribonucleic acids by uterine leiomyomas during the proliferative phase of the menstrual cycle. J Clin Endocrinol Metab. 1994;79 (3):900–906 [DOI] [PubMed] [Google Scholar]
- 46. Ding L, Xu J, Luo X, Chegini N. Gonadotropin releasing hormone and transforming growth factor beta activate mitogen-activated protein kinase/extracellularly regulated kinase and differentially regulate fibronectin, type I collagen, and plasminogen activator inhibitor-1 expression in leiomyoma and myometrial smooth muscle cells. J Clin Endocrinol Metab. 2004;89 (11):5549–5557 [DOI] [PubMed] [Google Scholar]
- 47. Chirco R, Liu XW, Jung KK, Kim HR. Novel functions of TIMPs in cell signaling. Cancer Metastasis Rev. 2006;25 (1):99–113 [DOI] [PubMed] [Google Scholar]
- 48. Limper AH, Roman J. Fibronectin. A versatile matrix protein with roles in thoracic development, repair and infection. Chest. 1992, 101 (6):1663–1673 [DOI] [PubMed] [Google Scholar]
- 49. Gelse K, Poschl E, Aigner T. Collagens--structure, function, and biosynthesis. Adv Drug Deliv Rev. 2003;55 (12):1531–1546 [DOI] [PubMed] [Google Scholar]
- 50. Byers PH. Collagens: building blocks at the end of the development line. Clin Genet. 2000;58 (4):270–279 [DOI] [PubMed] [Google Scholar]
- 51. Polgar A, Falus A, Koo E, et al. Elevated levels of synovial fluid antibodies reactive with the small proteoglycans biglycan and decorin in patients with rheumatoid arthritis or other joint diseases. Rheumatology (Oxford). 2003;42 (4):522–527 [DOI] [PubMed] [Google Scholar]
- 52. Ohara N. A putative role of versican in uterine leiomyomas. Clin Exp Obstet Gynecol. 2009;36 (2):74–75 [PubMed] [Google Scholar]
- 53. Norian JM, Malik M, Parker CY, Joseph D, Leppert PC, Segars JH, Catherino WH. Transforming growth factor beta3 regulates the versican variants in the extracellular matrix-rich uterine leiomyomas. Reprod Sci. 2009;16 (12):1153–1164 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Mujumdar VS, Tyagi SC. Temporal regulation of extracellular matrix components in transition from compensatory hypertrophy to decompensatory heart failure. J Hypertens. 1999;17 (2):261–270 [DOI] [PubMed] [Google Scholar]
- 55. Sternlicht MD, Werb Z. How matrix metalloproteinases regulate cell behavior. Annu Rev Cell Dev Biol. 2001;17:463–516 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Bogusiewicz M, Stryjecka-Zimmer M, Postawski K, Jakimiuk AJ, Rechberger T. Activity of matrix metalloproteinase-2 and -9 and contents of their tissue inhibitors in uterine leiomyoma and corresponding myometrium. Gynecol Endocrinol. 2007;23 (9):541–546 [DOI] [PubMed] [Google Scholar]
- 57. Rahman A, Hershey S, Ahmed S, Nibbelink K, Simpson RU. Heart extracellular matrix gene expression profile in the vitamin D receptor knockout mice. J Steroid Biochem Mol Biol. 2007;103 (3-5):416–419 [DOI] [PubMed] [Google Scholar]
- 58. Halder SK, Osteen KG, Al-Hendy A. Vitamin D3 inhibits expression and activities of matrix metalloproteinase-2 and -9 in human uterine fibroid cells. Hum Reprod. 2013;28 (9):2407–2416 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Nagase H: Cell surface activation of progelatinase A (proMMP-2) and cell migration. Cell Res. 1998;8 (3):179–186 [DOI] [PubMed] [Google Scholar]
- 60. Wu J, Garami M, Cheng T, Gardner DG. 1,25(OH)2 vitamin D3, and retinoic acid antagonize endothelin-stimulated hypertrophy of neonatal rat cardiac myocytes. J Clin Invest. 1996;97 (7):1577–1588 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Zanello SB, Collins ED, Marinissen MJ, Norman AW, Boland RL. Vitamin D receptor expression in chicken muscle tissue and cultured myoblasts. Horm Metab Res. 1997;29 (5):231–236 [DOI] [PubMed] [Google Scholar]
- 62. Mauskopf J, Flynn M, Thieda P, Spalding J, Duchane J. The economic impact of uterine fibroids in the United States: a summary of published estimates. J Womens Health (Larchmt). 2005;14 (8):692–703 [DOI] [PubMed] [Google Scholar]
- 63. Halder SK, Goodwin JS, Al-Hendy A. 1,25-Dihydroxyvitamin D3 reduces TGF-beta3-induced fibrosis-related gene expression in human uterine leiomyoma cells. J Clin Endocrinol Metab. 2011;96 (4):E754–E762 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Flanagan JN, Zheng S, Chiang KC, et al. Evaluation of 19-nor-2alpha-(3-hydroxypropyl)-1alpha,25-dihydroxyvitamin D3 as a therapeutic agent for androgen-dependent prostate cancer. Anticancer Res. 2009;29 (9):3547–3553 [PubMed] [Google Scholar]