Abstract
Our aim was to investigate whether celecoxib, a cyclooxygenase 2 (COX-2) inhibitor, decreases the in vitro proliferation of leiomyoma cells if the inflammatory pathway is blocked. Menstruation is an inflammation of uterus that produces cytokines and prostanoids, but the inflammatory mechanism underlying the growth of leiomyoma remains unexplained. Using in vitro cultures of leiomyoma cells obtained from 5 patients who underwent hysterectomy, cell proliferation, inflammatory signaling, transcription factors, growth factors, and extracellular matrix were examined by (4,5-dimethylthiaxol-2-yi)-2,5-diphenyltetraxolium bromide assay, immunoblotting, and quantitative polymerase chain reaction. Prostaglandin E2 was used to induce menstruation-like condition in the cells. We found that celecoxib inhibited COX-2 through the expression of nuclear factor κB in the cells. Celcoxib also decreased the gene expression of interleukin 6, tumor necrosis factor α, collagen A, fibronectin, platelet-derived growth factor, epidermal growth factor, and transforming growth factor β. In conclusion, the present study indicated that celecoxib could inhibit leiomyoma cell proliferation through blocking the inflammatory pathway that is probably one of the mechanisms underlying its pathogenesis.
Keywords: celecoxib, cyclooxygenase 2 inhibitors, inflammation, NF-kappa B
Introduction
Uterine leiomyomas or fibroids are the most common gynecologic tumors with overall cumulative incidence of 70% to 80% during female reproductive years and are the leading cause of hysterectomy in most countries.1 The most common symptoms of leiomyomas are menorrhagia and dysmenorrhea, and they are also associated with infertility and adverse pregnancy outcomes, such as abortion or preterm labor.2 Because leiomyoma is considered a nonmalignant tumor, it might not be justified to remove the uterus carrying leiomyoma not associated with adverse symptoms developed by enlarged size. Therefore, many leiomyoma studies have focused on the growth mechanisms for managing the clinical symptoms. Although it is well known that leiomyoma growth is mostly dependent on hormonal stimulation by ovarian steroids, it has not been studied whether inflammatory mechanisms influence its growth.3,4
Few studies have shown the potential contribution of cytokines produced by inflammation in the development of leiomyoma so far. According to a study on the relationship between leiomyoma growth and inflammation, levels of serum inflammatory cytokine interleukin (IL) 1α are high in patients with leiomyomas but not those of IL-6 or tumor necrosis factor α (TNF-α).5 By contrast, another study evaluated the ability of the immune cells to produce cytokines and reported that there were no differences between the circulating cytokine levels of women with leiomyomas and those without.6 Therefore, to date, there is no conclusive evidence to clarify the link between leiomyomas and cytokines.
Cyclooxygenase 2 (COX-2) is an enzyme that converts arachidonic acid into prostaglandin (PG) H2, a common substrate for specific PG synthases. Prostaglandin H2 is converted to biologically active PGs, such as PGE2 or thromboxane A2, by specific synthases.7 Among the COX-2-derived prostaglandins, PGE2 is known to induce the expression of COX-2 by positive feedback mechanism, thus leading to the development of malignant tumors.8 COX-2 is rarely expressed in cells and tissues without the upregulation of tumor promoters, which implies that COX-2 is a therapeutic target for cancer.9 In 1994, it was reported for the first time that COX-2 is involved in the development of human cancer, when COX-2 messenger RNA (mRNA) levels were found to be markedly elevated in colorectal carcinomas.10 Since then, substantial evidence has been suggested that COX-2 contributes to the development of colorectal cancer and other human cancers. For example, intestinal polyp formation was inhibited in COX-2 knockout mice with familial adenomatous polyposis, which suggested that COX-2 was involved in adenoma formation.11 Moreover, it has also been reported that other types of malignant cells, including those in the liver and bladder, were inhibited by COX-2 inhibitors.12,13 Recently, it was reported that COX-2 inhibitor is effective to decrease the leiomyoma growth, which were proved by demonstrating higher COX-2 expression in leiomyoma than uterine smooth muscle tissues.14 However, the study did not show whether the inflammatory mechanisms are involved in the action of COX-2 inhibitor.
In the present study, we investigated that COX-2 inhibitor, celecoxib, does not only prevent the proliferation of cells but also attenuates the production of cytokines, extracellular matrix, and growth factors, which play a pivotal role in leiomyoma growth. In addition, the influence of inflammatory mechanism in cellular growth was investigated by probing expression of nuclear factor κB (NF-κB).
Methods
Chemicals and Reagents
Celecoxib, progesterone (P4), PGE2, dimethyl sulfoxide (DMSO), and 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl tetraxolium bromide), MTT, were purchased from Sigma Chemical Co (St Louis, Missouri). The primary antibodies of Erk and phosphorylated Erk (p-Erk) were purchased from Cell Signaling Technology (Danvers, Massachusetts), and COX-2, NF-κB, inhibitor of kB (IkB), platelet-derived growth factor (PDGF), and actin antibodies were purchased from Santa Cruz Biotechnology (Santa Cruz, California). Enhanced chemiluminescence (ECL) detection kit was obtained from ELPIs Biotechnology (ELPIS-BIOTECH, Daejon, Korea). SYBR green was purchased from Agilent technologies (Santa Clara, California), and Tri reagent was purchased from Invitrogen (Carlsbad, California). Dulbecco modified Eagle medium (DMEM), fetal bovine serum (FBS), antibiotics (antimycotics), and trypsin EDTA were purchased from Invitrogen. Hanks Balanced Salt Solution with Ca2+ and Mg2+, collagenase, and DNase were purchased from Invitrogen and 4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid (HEPES) was from Sigma chemical Co.
Cell Culture and Proliferation Assay
Leiomyoma tissues were obtained from patients (n = 5) undergoing hysterectomy who had given written consent in accordance with the guidelines approved by institutional review board of Dong-A University Hospital. This study included premenopausal women, aged 30 to 45 years, undergoing surgery in 2011 to 2013. At the time of surgery, they did not receive any type of hormonal or drug therapy. Tissues were minced and digested in collagenase solution (HEPES 25 mmol/L, 1× antibiotics, collagenase 2 mg/mL, DNase 0.2 mg/mL) for 4 hours at 37°C in a water bath. The digested tissue was passed through gauze, and the cells were collected by centrifugation and washed several times with phosphate-buffered saline (PBS). The isolated cells were seeded in a 100-cm2 dish in culture medium (DMEM/F12) supplemented with 10% FBS and 1% antibiotics (antimycotics) and stored at 37°C in a humidified atmosphere containing 5% CO2 in air. Cells collected between passages 4 and 8 were used for the experiments. Drugs were added from 100× stock in DMSO or PBS.
Cells (1 × 104 cells/mL) seeded into a 96-well plate were incubated with 10 μmol/L PGE2, 1 μmol/L P4, and 10 μmol/L celecoxib and cultured at 37°C under a humidified atmosphere with 5% CO2 for 72 hours. Subsequently, 20 μL MTT solution (5 mg/mL in PBS) was added to each well and placed at room temperature for 4 hours. The absorbance was measured on an ELISA Reader (Bio-Tek instruments, Inc, Winooski, Vermont) at a wave length of 495 nm. Data were expressed in optical density units.
Cyclic Adenosine Monophosphate Assay
Accumulation of cyclic adenosine monophosphate (cAMP) was measured in response to treatment with PGE2, P4, and celecoxib. Cells were seeded in 6-well plates and allowed to attach overnight. The following day the cells were starved for over 4 hours, were treated with serum-free medium containing PGE2, P4, and celecoxib, and were incubated for 15 minutes at 37°C. The cells were lysed in 0.1 mol/L HCl, and cAMP concentration was estimated using enzyme-linked immunosorbent assay (ELISA) with a cAMP direct immunoassay kit (Abcam, Cambridge, Massachusetts) by following the manufacturer protocol. The concentrations were normalized to the protein concentration of lysate measured using the method of Lowry (Bio-rad, Hemel Hempstead, United Kingdom).
Nuclear Protein Extraction
After activation of the cells for the specified duration, they were washed in 1 mL of ice-cold PBS, centrifuged at 3000g for 5 minutes, and resuspended in 100 μL of ice-cold hypotonic buffer (10 mmol/L HEPES/KOH, 2 mmol/L MgCl2, 0.1 mmol/L EDTA, 10 mmol/L KCl, 1× protease inhibitor, pH 7.9). Then, the cells were left on ice for 10 minutes, vortexed, and centrifuged at 15 000g for 30 seconds. Pelleted nuclei were washed in 1 mL PBS 3 times and were gently resuspended in 50 μL of ice-cold saline buffer (50 mmol/L HEPES/KOH, 50 mmol/L KCl, 300 mmol/L NaCl, 0.1 mmol/L EDTA, 10% glycerol, and 1× protease inhibitor, pH 7.9), left on ice for 2 hours, vortexed, sonicated for 30 seconds, and centrifuged at 15 000g for 5 minutes at 4°C. Aliquots of the supernatant that contained nuclear proteins were frozen in liquid nitrogen and stored at −70°C.
Western Blot Analyses
For immunodetection, cells were harvested and lysed in lysis buffer consisting of 20 mmol/L Tris, 137 mmol/L NaCl, 2 mmol/L EDTA, 10% glycerol, 1% Triton X-100, protease inhibitor (Sigma Chemical Co), and phosphatase inhibitor cocktail III (Calbiochem Co., La Jolla, California). The protein lysates were separated by sodium dodecyl sulfate polyacrylamide gel electrophoresis and were transferred electrophoretically onto nitrocellulose membrane. The blots were blocked with 3% nonfat dry milk in Tris-buffered saline and were then incubated with primary antibody. The expression of ERK, NF-κB, IκB, and COX-2 were assayed using ERK (Cell signaling Technology), NF-κB, IκB, and COX-2 (Santa Cruz Biotechnology) antibodies. After washing, the blots were incubated with antigoat horseradish peroxidase-conjugated secondary antibodies for 1 hour and were washed again. Immunodetection was carried out using an ECL peroxidase substrate solution (ELPIs Biotechnology, Daejon, Korea).
Quantitative Polymerase Chain Reaction
Quantitative polymerase chain reaction (qPCR) was used to analyze the mRNA expression of proinflammatory cytokines, growth factors, extracellular matrix, and glyceraldehyde 3-phosphate dehydrogenase (GAPDH; internal control) in stimulated leiomyoma cells. The cells were treated with 10 μmol/L PGE2, 1 μmol/L P4, and 10 μmol/L celecoxib for 2 hours or 72 hours, and total RNA was isolated using Tri Reagent. Complimentary DNA (cDNA) was generated from 0.2 μg of total RNA using RevertAid First strand cDNA Synthesis Kit (Fermentas, St Leon-Rot, Germany). The primers used for amplification of each gene are listed in Table 1. The qPCR was performed on Applied Biosystems 7000 real-time PCR system (Life technologies, Carlsbad, California) with SYBR green premix. The expression levels of genes were normalized to those of the GAPDH gene.
Table 1.
Primers for Gene Expression.
| Gene | Accession No. | Primers | bp |
|---|---|---|---|
| IL-1β 1 | NM 002608 | S: TGATGGCTTATTACAGTGGCAATG | 140 |
| AS: GTAGTGGTGGTGGGAGATTCG | |||
| IL-6 2 | NM 000600 | S: GGTACATCCTCGACGGCATCT | 81 |
| AS: GTGCCTCTTTGCTGCTTTCAC | |||
| TNF-α 2 | NM 000594 | S: CCCAGGCAGTCAGATCATCTTC | 85 |
| AS: AGCTGCCCCTCAGCTTGA | |||
| PDGF 3 | NM 002608 | S: AGACCCCGGAGAGGAAGAT | 197 |
| AS: CGTTGGTGCGGTCTATGAG | |||
| EGF 4 | NM 001963 | S: AGATGGGAAAACGTGTCTGG | 246 |
| AS: CACTGACATGTGGCATCCTC | |||
| TGF-β 5 | NM 003239 | S: ATCACC ACAACCCTCA | 66 |
| AS: CCTGGCCCGGGTTGTC | |||
| Fibronectin 6 | NM 212482 | S: CCACCCCCATAAGGCATAGG | 131 |
| AS: GTAGGGGTCAAAGCACGAGTCATC | |||
| Collagen 1A 6 | NM 000088 | S: ATGTCTAGGGTCTAGACATGTTCA | 212 |
| AS: CCTTGCCGTTGTCGCAGACG | |||
| COX-2 7 | NM 000963 | S: TGT GCA ACA CTT GAG TGG CT | 220 |
| AS: ACT TTC TGT ACT GCG GGT GG | |||
| GAPDH 8 | NM 002046 | S: TGA ACGGGA AGCTCACTGG | 288 |
| AS: TCCACCACCCTGTTGCTGTA |
Abbreviations: bp, base pair; COX-2, cyclooxygenase 2; EGF, epidermal growth factor; GADPH, glyceraldehyde 3-phosphate dehydrogenase; IL, interleukin; PDGF, platelet-derived growth factor; TGF-β, transforming growth factor β; TNF-α, tumor necrosis factor α.
1. Naldini A, Leali D, Pucci A, et al. Cutting edge: IL-1beta mediates the proangiogenic activity of osteopontin-activated human monocytes. J Immunol. Oct 1 2006;177(7):4267-4270.
2. Steensberg A, Keller C, Starkie RL, Osada T, Febbraio MA, Pedersen BK. IL-6 and TNF-alpha expression in, and release from, contracting human skeletal muscle. Am J Physiol Endocrinol Metab. Dec 2002;283(6): E1272-1278.
3. van der Meer JJ, de Boer OJ, Teeling P, van der Loos CM, Dessing MC, van der Wal AC. Smooth muscle homeostasis in human atherosclerotic plaques through interleukin 15 signalling. Int J Clin Exp Pathol. Mar 2011;4(3):287-294.
4. Osada H, Yoshitake Y, Ikeda T, et al. Ultraviolet B-induced expression of amphiregulin and growth differentiation factor 15 in human lens epithelial cells. Mol Vis. 2011;17:159-169.
5. Quan T, He T, Kang S, Voorhees JJ, Fisher GJ. Ultraviolet irradiation alters transforming growth factor beta/smad pathway in human skin in vivo. J Invest Dermatol. Aug 2002;119(2):499-506.
6. Tancred TM, Belch AR, Reiman T, Pilarski LM, Kirshner J. Altered expression of fibronectin and collagens I and IV in multiple myeloma and monoclonal gammopathy of undetermined significance. J Histochem Cytochem. Mar 2009;57(3):239-247.
7. Chen L, Sooranna SR, Lei K, et al. Cyclic AMP increases COX-2 expression via mitogen-activated kinase in human myometrial cells. J Cell Mol Med. Jul 2012;16(7):1447-1460.
8. Tokunaga K, Nakamura Y, Sakata K, et al. Enhanced expression of a glyceraldehyde-3-phosphate dehydrogenase gene in human lung cancers. Cancer Res. Nov 1 1987;47(21):5616-5619.
Immunofluorescence
Cells grown on chamber slides were fixed in 2% paraformaldehyde for 20 minutes and then washed 3 times in PBS containing Triton X-10 (PBST). They were blocked in PBS containing 10% serum and Triton X-100 for 1 hour at room temperature. Cells were exposed to mouse anti-PDGF antibody for overnight at 4°C, washed 3 times in PBST, and incubated for 45 minutes at room temperature with secondary antibody diluted in blocking buffer and Alexa Fluor 488 donkey antimouse IgG (Abcam, Cambridge, Massachusetts). Cells were incubated with 4′, 6-diamidino-2-phenylindole (DAPI) (1 μg/mL) staining solution for 5 minutes in the dark and washed in PBST before being mounted in antifade mounting solution (Invitrogen, Carlsbad, California). The imaging was performed on a flexible confocal microscope (Zeiss, Oberkochen, Germany). The staining intensity of PDGF-B was quantified using Image software (AlphaEaseFC 4.0, San Leandro, California).
Statistical Analysis
The data are expressed as mean ± standard error. The statistical significance of the differences in mean values was tested using 1-way analysis of variance and student t test. P values less than .05 were considered as statistically significant.
Results
Cell Proliferation and COX-2 Expression are Inhibited by Celecoxib but not by P4
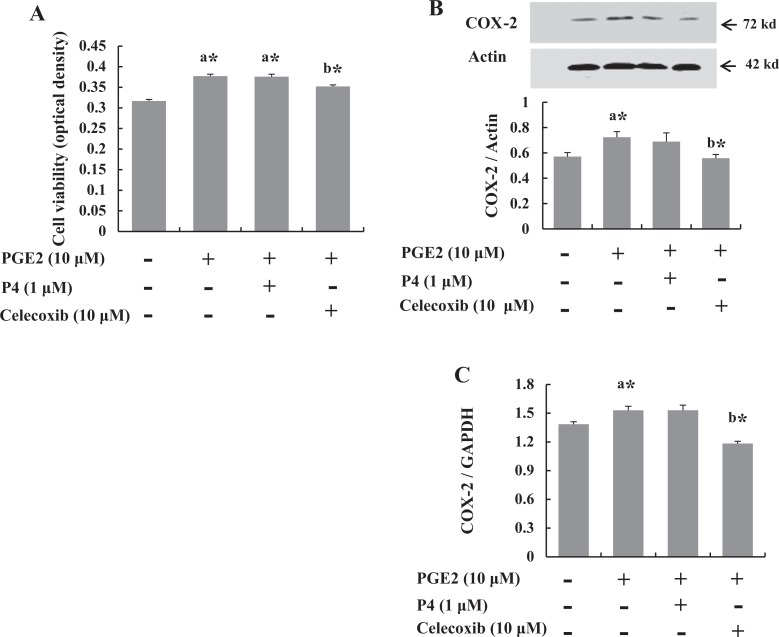
To ascertain whether PGE2 induces the proliferation of the cultured leiomyoma cells and to estimate the duration of culture required to recognize cell population changes, MTT assay was performed at 0, 24, 48, and 72 hours after treatment with 10 μmol/L PGE2. The 72-hour culture duration was considered to be optimal for further experiments (data not shown). As shown in Figure 1A, while the proliferation of leiomyoma cells was significantly induced by PGE2 treatment, the effect was inhibited by treatment with 10 μmol/L celecoxib. The PGE2-induced cell proliferation was not decreased by P4, which suggests that P4 did not have anti-inflammatory action on the cultured leiomyoma cells. In the analysis of COX-2 expression by Western blotting, we found that PGE2 increased the expression, which was decreased by celecoxib, but not by P4 (Figure 1B).
Figure 1.
Celecoxib increased cell proliferation and cyclooxygenase 2 (COX-2) expression. Leiomyoma cells obtained from 5 patients were cultured and treated with prostaglandin E2 (PGE2; 10 μmol/L). A, At 72 hours, cells were subjected to cell proliferation assays by (4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT). PGE2 (10 μmol/L) induced cell proliferation, which was not inhibited by treatment with 1 μmol/L progesterone (P4). However, treatment with 10 μmol/L COX-2 inhibitor, celecoxib, decreased cell proliferation. B, Western blotting after 6 hours treatment. COX-2 expression was decreased by celecoxib treatment, compared with PGE2 and/or P4 treatment. C, Expression of COX-2 messenger RNA in leiomyoma cells. The results are shown as arbitrary densitometric units (COX-2/GAPDH). The MTT assay was performed at 72 hours to compare the viability among the 4 treated groups of cell. The bars represent the mean ± standard error of values from triplicate experiments. a* indicates significant change relative to control and b* indicates significant change relative to PGE2-treated cells. *P < .05.
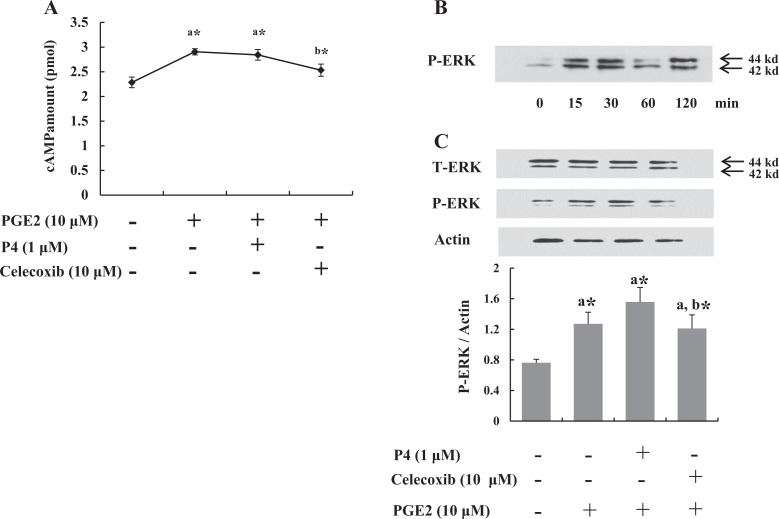
Celecoxib Inhibits cAMP and p-ERK Signaling
Cells were serum starved overnight to remove the effect of cAMP and growth factors on ERK phosphorylation before treatment. Because PGE2 exerts its cellular action through intracellular cAMP, it was examined at 15 minutes after treatment. As shown in Figure 2A, while P4 did not alter the level of intracellular cAMP, celecoxib decreased it significantly.
Figure 2.
Celecoxib decreased intracellular cyclic adenosine monophosphate (cAMP) production by prostaglandin E2 (PGE2) and phosphorylated ERK (p-ERK) expression. A, cAMP level was increased by PGE2 at 15 minutes, which was downregulated by celecoxib. B, Leiomyoma cells were treated with PGE2 for various periods (0, 15, 30, 60, and 120 minutes) to identify time-dependent expression of p-ERK. C, At 30 minutes, expression of p-ERK was decreased compared to PGE2 or P4 treatments. The data are expressed as arbitrary units. The bars represent the mean ± standard error of values from triplicate experiments. a* indicates significant change relative to control, and b* indicates significant change relative to PGE2-treated cells. *P < .05.
Phosphorylation of ERK was analyzed for 15 to 120 minutes to determine optimal time for PGE2-induced ERK activation (Figure 2B). Phosphorylated ERK levels in cells were significantly upregulated within 30 minutes by PGE2 (Figure 2B). Thirty minutes after the treatment, we found that the increase in p-ERK expression induced by PGE2 was attenuated by coadministration of celecoxib (Figure 2C). These results indicate that celecoxib is able to inhibit ERK signaling induced by PGE2, whereas P4 was not able to inhibit ERK signaling.
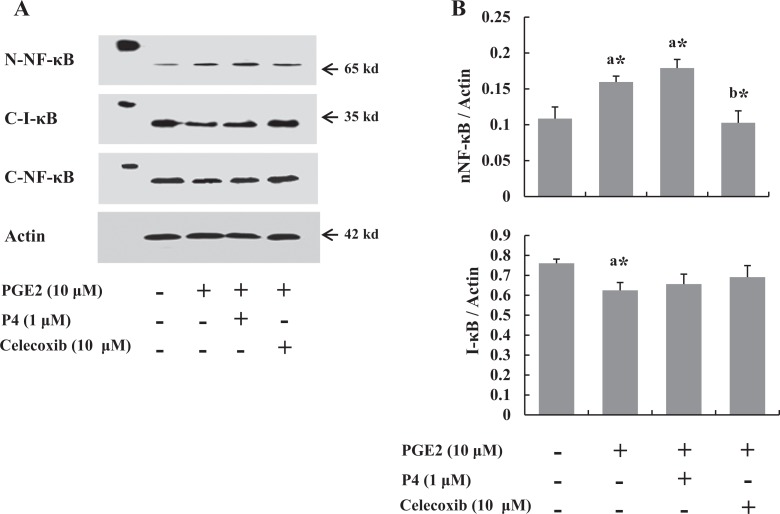
Celecoxib Inhibits NF-κB Expression
To elucidate whether COX-2 inhibitor inhibits the inflammatory pathway, the expressions of nuclear NF-κB and cytosol IκB were evaluated in the cells after P4 or celecoxib treatment. Progesterone did not change the NF-κB expression induced by PGE2, whereas celecoxib remarkably decreased the PGE2-induced changes (Figure 3).
Figure 3.
Leiomyoma cells (n = 5) were treated with prostaglandin E2 (PGE2) for 0, 0.5, 1, 2, and 4 hours, and the levels of nuclear factor κB (NF-κB) were measured by Western blots. A, Western blot after 2 hours treatment. PGE2 induced translocation of NF-κB to nucleus and degradation of I-κB in cytosol, which was inhibited by celecoxib but not P4. B, Graphical representation of nucleic NF-κB and cytosolic I-κB expressions by densitometry. The data are expressed as arbitrary units. The bars represent the mean ± standard error of values from triplicate experiments. a* indicates significant change relative to control and b* indicates significant change relative to PGE2-treated cells. *P < .05.
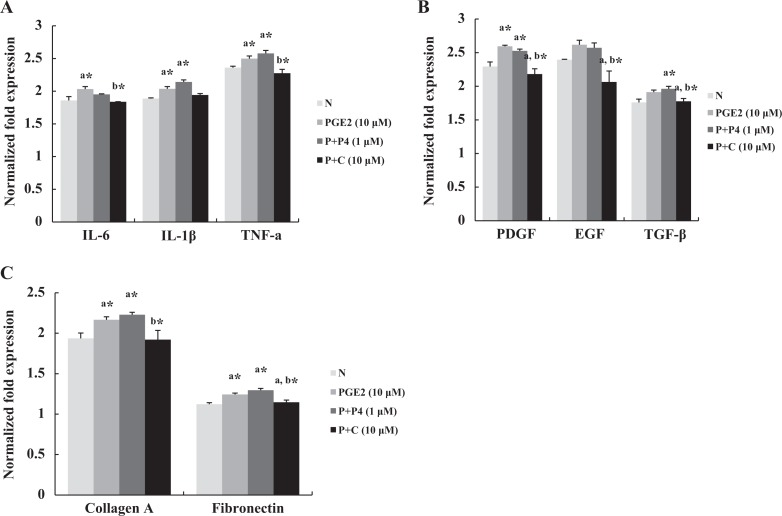
Production of Cytokines, Growth Factors, and Extracellular Matrix is Inhibited by Celecoxib
Cytokines, growth factors, and extracellular matrix were measured by qPCR to evaluate the effect of celecoxib on their expression in leiomyoma cells. We found that celecoxib decreased IL-6 and TNF-α expression significantly but not that of IL-1β (Figure 4A). In the case of growth factors, the expression of PDGF, epidermal growth factor, and TGF-β were decreased in the cells treated with celecoxib (Figure 4B). Analysis of the expression of collagen A and fibronectin indicated that fibronectin expression was decreased significantly (Figure 4C). Immunofluorescent expression of PDGF, one of well-known growth factors related to leiomyoma growth, was increased in the cells treated with PGE2 significantly, which were inhibited by celecoxib treatment (Figure 5).
Figure 4.
The messenger RNA (mRNA) levels of proinflammatory cytokines, growth factors, and extracellular matrix were measured by quantitative polymerase chain reaction from leiomyoma cells (n = 5). A, Interleukin 6 and tumor necrosis factor α mRNA expressions were decreased in the cells treated with P + C for 2 hours. B, On the third day of treatment, expressions of platelet-derived growth factor, epidermal growth factor, and transforming growth factor β were decreased by celecoxib, compared to prostaglandin E2 (PGE2) or progesterone. C, Increased expressions of collagen A and fibronectin induced by PGE2 and/or P4 were reduced by celecoxib treatment. The data are expressed as arbitrary units. The bars represent the mean ± standard error of values from triplicate experiments. N indicates non-treated; P + P4, prostaglandin E2 plus progesterone; P + C, prostaglandin E2 plus celecoxib. a* indicates significant change relative to control and b* indicates significant change relative to PGE2-treated cells. *P < .05.
Figure 5.
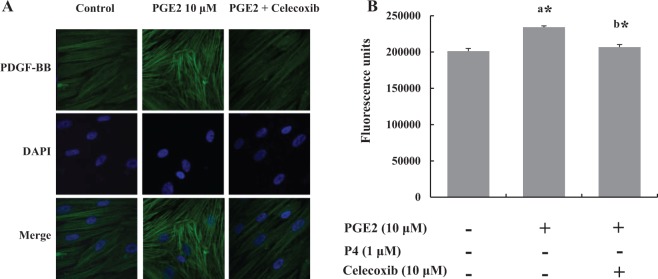
Leiomyoma cells (n = 5) were treated with prostaglandin E2 (PGE2) or PGE2 plus celecoxib for 3 days. A, Representative fluorescence microscopy visualization showed the effect of celecoxib on PGE2-stimulated platelet-derived growth factor (PDGF) expression. The top panel shows representative images of the expression of PDGF, the middle panel shows corresponding DAPI nuclear staining, and the bottom panel shows merged images. B, Quantification of PGE2-stimulated fluorescence. The bars represent the mean ± standard error of values from triplicate experiments. a* indicates significant change relative to control and b* indicates significant change relative to PGE2-treated cells. *P < .05.
Discussion
The results of the present study on primary cultures of leiomyoma cells are supportive of the potential relationship between leiomyoma growth and inflammation. We found that the inflammatory mechanism is probably one of factors affecting the leiomyoma size and it could be controlled by COX-2 inhibitors. Although the uterus is a specialized organ usually exposed to inflammation due to menstruation, it has not been clarified whether inflammatory mechanisms influence the growth of leiomyoma during menstrual period. In fact, it has long been known that PGF2α and PGE2, well-known inflammatory mediators, are not only secreted normally during menstrual period but PGE2 levels are also highest during menstruation.15 Therefore, we added PGE2 to leiomyoma cells in order to simulate menstruation-like environment. In our previous study on the effect of aromatase inhibitor on leiomyoma cell proliferation, PGE2 was used to mimic the conditions similar to that of menstruation, in the cultured cells. Prostaglandin E2 was treated as a kind of stimulant to induce aromatase in the cells.16 In the present study, PGE2 increased leiomyoma cell viability and COX-2 expression compared to the nontreated cells, as illustrated in Figure 1. Therefore, this study indicates that prostanoids secreted during menstruation could promote the leiomyoma size.
Progesterone has been considered to play a major role in the growth of leiomyoma by binding with P4 receptor.17,18 On the other hand, it has also been reported that P4 exerts its anti-inflammatory action in the myometrial and breast cancer cell line by antagonizing NF-κB activation and induction of COX-2, a crucial and rate-determining enzyme in PG biosynthesis.19 In this experiment, P4 was used to clarify whether the inflammatory pathway induced by PGE2 could be blocked by P4 or not. As a result, declining of intracellular cAMP level was not observed significantly after adding P4 into PGE2-treated cells (Figure 2A). Furthermore, despite the previous reports indicating the growth-promoting effect of P4 on leiomyoma cells, NF-κB expression was not inhibited by P4 as much as celecoxib (Figure 3). These data suggest that P4 does not inhibit the intracellular inflammatory signal induced by PGE2, but celecoxib inhibits it. Although these results might imply that P4 had less anti-inflammatory activity than celecoxib on leiomyoma cells treated with PGE2, additional studies are required to verify this notion because the dose of celecoxib (10 μmol/L) was higher than that of P4 (1 μmol/L) in this experiment.
Cyclooxygenase 2 inhibitor, celecoxib, has been well recognized to suppress the growth of a few malignant cells, especially in colorectal cancer.7,20 However, there have been no trials to demonstrate whether COX-2 inhibitor shows antigrowth effect on leiomyoma cells. Moreover, it has not been clear until now whether the inflammatory mechanism would be involved in leiomyoma growth. In this context, this study investigates another mechanism on leiomyoma growth, which is different from the role of sex-steroid hormone. Celecoxib attenuated the gene expression of proinflammatory mediators, IL-6, IL-1β, and TNF-α as well as nuclear NF-κB (Figures 3A and 4).
Nuclear factor κB belongs to a family of dimeric transcription factors (usually RelA/p65-p50) that participate in immune and inflammatory reactions. The activity of NF-κB is mainly facilitated by its interaction with inhibitory IκB proteins in most species. Generally, the activation of NF-κB by several stimuli induces phosphorylation of IκB, followed by its ubiquitination and subsequent degradation. This process releases the NF-κB dimer and allows it to translocate from the cytoplasm into the nucleus to express target genes.21 Inflammatory mediators including IL-8, TNF, and COX-2 are related to menstruation along with major targets of NF-κB.22–24 In this study, we found that celecoxib decreased nuclear NF-κB expression in the cells but P4 did not decrease it (Figure 3). These results suggest that celecoxib is more likely to repress the activation of NF-κB pathway than P4. However, according to previous studies on the effect of P4 in term or preterm labor, P4 exerts anti-inflammatory action on myometrium by blocking the production of cytokines or activation of NF-κB.25,26 However, these studies were performed without the involvement of PGE2. Additionally, it has been recently reported that NF-κB activity induces more downstream target genes under P4 withdrawal situations such as menstruation or following treatment with P4 receptor inhibitors such as RU-486.27 This suggests that celecoxib is able to effectively control the inflammatory activity during menstruation and associated enhancement of inflammatory mediators, such as PGs and cytokines. Therefore, further clinical studies are necessary to evaluate whether celecoxib or other COX-2 inhibitors could reduce or prevent the growth of leiomyoma.
According to a recent morphometric study on leiomyoma growth, excessive production of collagenous matrix is accumulated as the tumor volume progresses.28 In this study, we found that celecoxib inhibits the production of extracellular matrix, such as collagen A and fibronectin (Figure 4C). Moreover, celecoxib also reduced the expression of several cytokines as well as growth factors that are related to leiomyoma growth through qPCR or immunofluorescence imaging study (Figures 4 and 5).
In conclusion, the present study suggests that the inflammatory mechanism during menstruation may play an important role in the growth of leiomyoma, and celecoxib could inhibit the growth by blocking the inflammatory pathway. However, further studies with relevant experimental and clinical data are needed to confirm the beneficial effects of celecoxib in controlling leiomyoma growth.
Footnotes
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was supported by grant no. A120043 from the Korea Health Care Technology R&D Project, Ministry of Health and Welfare, Korea.
References
- 1. Baird DD, Dunson DB, Hill MC, Cousins D, Schectman JM. High cumulative incidence of uterine leiomyoma in black and white women: ultrasound evidence. Am J Obstet Gynecol. 2003;188 (1):100–107 [DOI] [PubMed] [Google Scholar]
- 2. Wegienka G, Baird DD, Hertz-Picciotto I, et al. Self-reported heavy bleeding associated with uterine leiomyomata. Obstet Gynecol. 2003;101 (3):431–437 [DOI] [PubMed] [Google Scholar]
- 3. Walker CL, Stewart EA. Uterine fibroids: the elephant in the room. Science. 2005;308 (5728):1589–1592 [DOI] [PubMed] [Google Scholar]
- 4. Wegienka G. Are uterine leiomyoma a consequence of a chronically inflammatory immune system? Med Hypotheses. 2012;79 (2):226–231 [DOI] [PubMed] [Google Scholar]
- 5. Sikorski R, Kapec E, Zaleska W. Serum levels of proinflammatory cytokines in women with uterine myomas[in Polish]. Ginekol Pol. 2001;72 (12A):1485–1488 [PubMed] [Google Scholar]
- 6. Gmyrek GB, Sozanski R, Jerzak M, et al. Evaluation of monocyte chemotactic protein-1 levels in peripheral blood of infertile women with endometriosis. Eur J Obstet Gynecol Reprod Biol. 2005;122 (2):199–205 [DOI] [PubMed] [Google Scholar]
- 7. Subbaramaiah K, Dannenberg AJ. Cyclooxygenase 2: a molecular target for cancer prevention and treatment. Trends Pharmacol Sci. 2003;24 (2):96–102 [DOI] [PubMed] [Google Scholar]
- 8. Hull MA, Ko SC, Hawcroft G. Prostaglandin EP receptors: targets for treatment and prevention of colorectal cancer? Mol Cancer Ther. 2004;3 (8):1031–1039 [PubMed] [Google Scholar]
- 9. Lai TY, Chen LM, Lin JY, et al. 17beta-estradiol inhibits prostaglandin E2-induced COX-2 expressions and cell migration by suppressing Akt and ERK1/2 signaling pathways in human LoVo colon cancer cells. Mol Cell Chem. 2010;342 (1-2):63–70 [DOI] [PubMed] [Google Scholar]
- 10. Eberhart CE, Coffey RJ, Radhika A, Giardiello FM, Ferrenbach S, DuBois RN. Up-regulation of cyclooxygenase 2 gene expression in human colorectal adenomas and adenocarcinomas. Gastroenterology. 1994;107 (4):1183–1188 [DOI] [PubMed] [Google Scholar]
- 11. Oshima M, Dinchuk JE, Kargman SL, et al. Suppression of intestinal polyposis in Apc delta716 knockout mice by inhibition of cyclooxygenase 2 (COX-2). Cell. 1996;87 (5):803–809 [DOI] [PubMed] [Google Scholar]
- 12. Gee J, Lee IL, Jendiroba D, Fischer SM, Grossman HB, Sabichi AL. Selective cyclooxygenase-2 inhibitors inhibit growth and induce apoptosis of bladder cancer. Oncol Rep. 2006;15 (2):471–477 [PubMed] [Google Scholar]
- 13. Cui W, Yu CH, Hu KQ. In vitro and in vivo effects and mechanisms of celecoxib-induced growth inhibition of human hepatocellular carcinoma cells. Clin Cancer Res. 2005;11(22):8213–8221 [DOI] [PubMed] [Google Scholar]
- 14. Ke X, Dou F, Cheng Z, et al. High expression of cyclooxygenase-2 in uterine fibroids and its correlation with cell proliferation. Eur J Obstet Gynecol Reprod Biol. 2013;168 (2):199–203 [DOI] [PubMed] [Google Scholar]
- 15. Downie J, Poyser NL, Wunderlich M. Levels of prostaglandins in human endometrium during the normal menstrual cycle. J Physiol. 1974;236 (2):465–472 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Han M, Kim JY, Park JE, Kim JM, Lee KS. Effects of letrozole on proliferation and apoptosis in cultured leiomyoma cells treated with prostaglandin E(2). Eur J Obstet Gynecol Reprod Biol. 2008;138 (1):83–88 [DOI] [PubMed] [Google Scholar]
- 17. Maruo T, Ohara N, Wang J, Matsuo H. Sex steroidal regulation of uterine leiomyoma growth and apoptosis. Hum Reprod Update. 2004;10 (3):207–220 [DOI] [PubMed] [Google Scholar]
- 18. Hoekstra AV, Sefton EC, Berry E, et al. Progestins activate the AKT pathway in leiomyoma cells and promote survival. J Clin Endocrinol Metab. 2009;94 (5):1768–1774 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Hardy DB, Janowski BA, Corey DR, Mendelson CR. Progesterone receptor plays a major antiinflammatory role in human myometrial cells by antagonism of nuclear factor-kappaB activation of cyclooxygenase 2 expression. Mol Endocrinol. 2006;20 (11):2724–2733 [DOI] [PubMed] [Google Scholar]
- 20. Ninomiya I, Nagai N, Oyama K, et al. Antitumor and anti-metastatic effects of cyclooxygenase-2 inhibition by celecoxib on human colorectal carcinoma xenografts in nude mouse rectum. Oncol Rep. 2012;28 (3):777–784 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Karin M, Ben-Neriah Y. Phosphorylation meets ubiquitination: the control of NF-[kappa]B activity. Annu Rev Immunol. 2000;18:621–663 [DOI] [PubMed] [Google Scholar]
- 22. Catalano RD, Wilson MR, Boddy SC, Jabbour HN. Comprehensive expression analysis of prostanoid enzymes and receptors in the human endometrium across the menstrual cycle. Mol Hum Reprod. 2011;17 (3):182–192 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Maybin JA, Hirani N, Jabbour HN, Critchley HO. Novel roles for hypoxia and prostaglandin E2 in the regulation of IL-8 during endometrial repair. Am J Pathol. 2011;178 (3):1245–1256 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Peluffo MC, Young KA, Hennebold JD, Stouffer RL. Expression and regulation of tumor necrosis factor (TNF) and TNF-receptor family members in the macaque corpus luteum during the menstrual cycle. Mol Reprod Dev. 2009;76 (4):367–378 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Sadowsky DW, Adams KM, Gravett MG, Witkin SS, Novy MJ. Preterm labor is induced by intraamniotic infusions of interleukin-1beta and tumor necrosis factor-alpha but not by interleukin-6 or interleukin-8 in a nonhuman primate model. Am J Obstet Gynecol. 2006;195 (6):1578–1589 [DOI] [PubMed] [Google Scholar]
- 26. Condon JC, Hardy DB, Kovaric K, Mendelson CR. Up-regulation of the progesterone receptor (PR)-C isoform in laboring myometrium by activation of nuclear factor-kappaB may contribute to the onset of labor through inhibition of PR function. Mol Endocrinol. 2006;20 (4):764–775 [DOI] [PubMed] [Google Scholar]
- 27. Li YF, Xu XB, Chen XH, Wei G, He B, Wang JD. The nuclear factor-kappaB pathway is involved in matrix metalloproteinase-9 expression in RU486-induced endometrium breakdown in mice. Hum Reprod. 2012;27 (7):2096–2106 [DOI] [PubMed] [Google Scholar]
- 28. Flake GP, Moore AB, Flagler N, et al. The natural history of uterine leiomyomas: morphometric concordance with concepts of interstitial ischemia and inanosis. Obstet Gynecol Int. 2013;2013:285103. [DOI] [PMC free article] [PubMed] [Google Scholar]