Abstract
Objective:
To determine a role for endothelin (ET) in progression of uterine fibroids.
Design:
An in vitro model of fibroid and myometrium cultivation.
Patients:
A total of 32 women undergoing hysterectomies for uterine fibroids and 11 women undergoing hysterectomies for abnormal uterine bleeding (control population).
Results:
Women with uterine fibroids were hypertensive and displayed significantly greater circulating ET-1 compared to control patients. Secretion of ET-1 was greater from the fibroids compared to myometrium explants. Endothelin 1 secretion was attenuated with blockade of the angiotensin II type 1 or endothelinA receptors. Hypoxia stimulated ET-1 secretion from both myometrium and fibroid explants. Preproendothelin messenger RNA expression increased with hypoxia from fibroid explants compared to normoxic controls.
Conclusions:
These data support the hypothesis that uterine fibroids are associated with hypertension and increased ET-1, which is exacerbated with hypoxia. These data suggest a possible link between mechanisms of blood pressure regulation and development of uterine leiomyoma.
Keywords: Uterine Leiomyoma, endothelin 1, sFlt-1, hypertension, hypoxia
Introduction
Uterine leiomyomas, or uterine fibroids, are the most common type of benign tumors affecting women during their reproductive years.1–3 Fibroids are associated with health problems such as menorrhagia, infertility, pelvic pain, and are the most frequent indication for hysterectomy in the United States.4,5 There are many risk factors associated with the occurrence of uterine fibroids such as obesity, nulliparity, and untreated high blood pressure.6,7 Despite these observations, the pathophysiology has yet to be fully elucidated, making this problem costly when women’s health is measured worldwide. Importantly, the mechanism identifying the genesis and proliferation of fibroids remains unknown.
Recent reports have identified an epidemiologic link between hypertension and the development of uterine fibroids. In 2 studies, American women8 and Danish women9 who underwent hysterectomy due to uterine leiomyoma also had a greater incidence of hypertension, thereby suggesting a link between cardiovascular disease and development of uterine fibroids in women.9 However, a causal role for fibroids in the development of hypertension in these patients has not been established. Potential peptides linking renal and/or vascular function with fibroid proliferation are angiotensin II (ANGII), soluble fms-like tyrosine kinase 1 (sFlt-1), and endothelin 1 (ET-1). Endothelin 1 is a vasoactive peptide secreted from activated endothelial cells10 and is a marker of endothelial cell activation/dysfunction. Endothelial cell quiescence depends on the balance of circulating inflammatory and angiogenic/antiangiogenic factors such as vascular endothelial growth factor (VEGF), platelet-derived growth factor, and sFlt-1. Soluble fms-like tyrosine kinase 1 is a hypoxia-stimulated tyrosine kinase that is normally produced in the uterus during menses. The sFlt-1 binds VEGF with an affinity 10-fold greater than other receptors, thus acting as a soluble trap of VEGF. Since VEGF has been reported to alter ET-1 production,11 antagonism of VEGF by sFlt-1 could enhance ET-1 synthesis. Furthermore, Murphy et al recently demonstrated that sflt-1 infusion into rats increased blood pressure by stimulating local ET-1 and activation of the ETA receptor.12
Molecular observations on the induction of survival pathways in tumors suggest an important progenitor role for hypoxia. Hypoxia has been shown to play an important role in the pathogenesis of uterine fibroids, as fibroid cells have been found to be severely hypoxic compared to the adjacent myometrium,13–15 and it has been demonstrated that uterine fibroids have an impaired antioxidant cellular enzymatic system, which may make them more susceptible to hypoxia.16 However, the mechanisms by which hypoxia may promote proliferation of fibroids are unclear.13 These mechanisms could lead to activation of pathways altering growth and proliferation of smooth muscle cells that could contribute to the formation of uterine fibroids. Importantly, myometrial stem cells can differentiate into fibroid cells following hypoxia via aberrant estrogen signaling.13,17 Different pathological cases of acute or chronic ischemia have demonstrated a role for hypoxia to induce sFlt-1 which could influence ET-1 expression.18–20
Hypoxia stimulated sFlt-1 induced ET-1 could be one mechanism whereby altered leiomyoma cells enter an antiapoptotic state and may be one potential link between the uterine fibroids and increases in arterial pressure. Therefore, the goal of the current study was to determine whether hypoxia stimulated greater sFlt-1 and/or ET-1 secretion from uterine fibroids compared to the adjacent myometrium. With 36.2% of the population having hypertension, Mississippi is rated as #1 of 50 states for hypertension21 so we also sought to determine whether our patients undergoing hysterectomy for uterine leiomyoma had underlying hypertension.
Methods
Sample Collection
The fibroid and adjacent myometrial tissues were collected from 32 patients undergoing routine hysterectomies for complications associated with uterine leiomyomas at the University of Mississippi Medical Center (UMMC). Myometrial tissues collected from 11 patients undergoing hysterectomies for abnormal uterine bleeding served as a control population. The UMMC Institutional Regulation Board approved the sample collections with patient informed consent prior to their scheduled hysterectomy. Physical parameters such as blood pressure measurement, weight, and height at the time of the preoperative visit were recorded. Patient charts were also reviewed to determine whether hypertension, diabetes, or other medical conditions were diagnosed and whether patients were compliant with medication, if applicable. Fibroids and myometrium tissues were excised from the uterus while in the operation room and immediately placed in sterile media containing phosphate-buffered saline (Sigma Aldrich, Milwaukee, Minnesota) with 5% penicillin/streptomycin (P/S; Invitrogen, Grand Island, New York) before being immediately brought to the laboratory on the UMMC campus.
Extraction of Circulating ET-1
At the time of study consent blood was collected via venipuncture in serum collection tubes for determination of circulating ET-1. The blood was immediately centrifuged for 10 minutes, and the serum stored at −20°C. Extraction of plasma ET was done using Sep-pak Columns (Waters Corporation, Milford, Massachusetts). Briefly each column was activated by washing with buffer A (60% acetonitrile in 0.1% trifluoroacetic acid, 4 × 1 mL) and then with buffer B (0.1% trifluoroacetic acid, 5 × 1 mL). Each plasma sample was acidified by adding an equal volume of buffer B and centrifuged at 3000g for 15 minutes at 4°C. The mixture of plasma and buffer B was loaded onto the columns followed by column washing with buffer B (5 × 1 mL). The absorbed endothelin materials were slowly eluted with buffer A into an Eppendorf (3 × 1 mL). The eluent was completely evaporated with a centrifugal evaporator, and the solid was rehydrated with 200 µL of assay buffer (RnD Systems; Minneapolis, Minnesota).
Measurement of Circulating ET-1 and sFlt-1
Concentrations of ET-1 and sFlt-1 were determined using the ET-1 and the soluble VEGF R1/Flt-1 Quantikine enzyme-linked immunosorbent assay (ELISA) kit from R&D systems (Minneapolis, Minnesota). The ET-1 assay displayed a sensitivity of 13.3 pg/mL, interassay variability of 8.9%, and intra-assay variability of 3.4%. The sFlt-1 ELISA displayed a sensitivity of 3.5 pg/mL, interassay variability of 7.0%, and intra-assay variability of 3.2%. All samples were assayed in duplicate (2 wells per sample) on the same ELISA plate according to the manufacturer’s recommended plate layout.
Tissue Preparation
Tissues were selected from the uterine fibroids and adjacent myometrium randomly and without any preference. The samples were cut into 0.5-mg explants and processed for tissue culture or immediately snap frozen in liquid nitrogen. All frozen tissues were held at −80°C for future analysis.
Protocol 1: Effect of endothelin A, endothelin B, and ANGII Type 1 receptor antagonism on ET-1 secretion
Given the link between hypertension and uterine fibroids, this protocol was designed to determine whether receptor blockade of the endothelin and ANGII pathways could attenuate the release of ET-1 from tissue explants. Patient-matched fibroid and myometrium explants were placed on matrigel (BD Biosciences, San Jose, California)-coated inserts and placed into 6-well plates, where the tissue was allowed to adhere to the inserts for 15 minutes at room temperature under sterile conditions. Tissue culture media of 2.5 mL, Dulbecco modified Eagle medium (Invitrogen, Grand Island, New York) with 5% P/S, was added to each explant and incubated under normoxic conditions (6% O 2) in a humidified atmosphere at 37°C for a period of 24 hours. Endothelin A (ETA) receptor antagonist (kindly provided by Abbott Labs, study #REN-11-0065), endothelin B (ETB) receptor antagonist (BQ-788; Sigma, Milwaukee, Minnesota), or the ANGII type 1 (AT1) receptor (AT1R) antagonist Losartan was added into the cell culture media and cultured for a period of 24 hours. All studies were done in duplicate at each concentration per patient. At the end of the 24-hour incubation, cell culture media were collected for further analysis.
Measurement of ET-1 in Tissue Culture Media
Endothelin 1 concentrations were determined using 100 µL of collected medium and using the ET-1 Quantikine ELISA kit described earlier. Each explant experiment was performed in duplicate, yielding a total of 2 media samples per tissue per drug and oxygen condition. Each sample was then assayed in duplicate (2 wells per sample) on the same ELISA assay plate. Values from both samples (each assayed in duplicate) were averaged together to equal 1 ET-1 value per patient per drug concentration per oxygen condition. Total protein was measured in the cell culture media, using a bicinchoninic acid (BCA) protein quantitation kit from Pierce (Rockford, Illinois).
Protocol 2: Effect of Hypoxia on ET-1
To determine whether hypoxia stimulates ET-1 production from patient-matched fibroid and myometrium tissues, explants were placed on matrigel (BD Biosciences, San Jose, California)-coated inserts as described earlier. Tissue culture media of 2.5 mL without any antagonists was added to each explant and incubated under hypoxic (1% O2) or normoxic (6% O2) conditions in a humidified atmosphere at 37°C for a period of 24 hours utilizing a ProOx P110 oxygen-controlled hypoxia chamber specified for cell and tissue culture (BioSpherix, Lacona, New York) which maintains the set oxygen concentration via infusion of nitrogen. At the end of the 24-hour incubation, cell culture media and the explants were collected for further analysis.
Measurement of ET-1 and sFlt-1 Concentration in Tissue Culture Media
Endothelin 1 and sFlt-1 concentrations were determined using 100 µL of collected media and using the ET-1 and sFlt-1 Quantikine ELISA kits described earlier. Each explant experiment was performed in duplicate, yielding a total of 2 media samples per tissue per drug and oxygen condition. Each sample was then assayed in duplicate (2 wells per sample) on the same ELISA assay plate. Values from both samples (each assayed in duplicate) were averaged together to equal 1 ET-1 or sFlt-1 value per patient per drug concentration per oxygen condition. Total protein was measured in the cell culture media, using a BCA protein quantitation kit from Pierce (Rockford, Illinois.
Determination of preproendothelin messenger RNA levels
Fibroid and myometrium explants were snap frozen in liquid nitrogen and stored at −80°C immediately following the hypoxia experiment. To extract total RNA, the tissues were ground using a Minilys (Precellys, Rockland, Maryland) homogenizer and RNA extracted using the Qiagen RNAeasy Protection Mini-kit (Valencia, California). The isolation procedure was performed as outlined in the instructions provided by the manufacturer. Complimentary DNA (cDNA) was synthesized from 1 µg of RNA with BioRad Iscript cDNA reverse transcriptase (Biorad, Hercules, California), and real-time polymerase chain reaction (PCR) was performed using the BioRad Sybre Green Supermix (Biorad, Hercules, California) and iCycler as described previously.22,23 The following primers for preproendothelin (PPET) were used: forward CTAGGTCTAAGCGATCCTTG and reverse: TCTTTGTCTGCTTGGC, supplied by custom primers from Life technologies (Grand Island, New York).24 Commercially available primers were used for glyceraldehyde 3 phosphate dehyrogenase (SA Biosciences, Valencia, California). The relative messenger RNA (mRNA) expression levels of the target genes in each sample after normalization were calculated using the 2−Δ/ΔCt calculus method of mRNA analysis.25
Statistical Analysis
All data are expressed as mean ± standard error of the mean when applicable. Differences between groups were analyzed via repeated-measures analysis of variance, paired Student t test or Fisher exact test. Post hoc analysis was done with Bonferonni post hoc test. Values of P < .05 were considered significant.
Results
Women With Uterine Fibroids Have increased Mean Arterial Pressure
A total of 32 women undergoing routine hysterectomies for uterine fibroids and 11 women undergoing hysterectomies for abnormal uterine bleeding were enrolled in the current study. There was no significant difference in the average age between women with fibroids and women in the control group (P = .958) nor in race (P = .201; Table 1) or body mass index (BMI; P = .296; Table 1). Interestingly, women with uterine fibroids (101.6 ± 2.24 mm Hg) had a significantly higher mean arterial pressure (MAP) compared to women without uterine fibroids (90.03 ± 3.6 mm Hg; P = .01; Figure 1A). Only 22% of women with uterine fibroids had normotensive blood pressures (systolic < 120mm Hg as defined by the American Heart Association) which was significantly less when compared to the control population (P = .04, Figure 1B). Although no women with prehypertensive blood pressures in the control group were being treated for hypertension, 40% (n = 6 out of 15) of the women with prehypertensive blood pressures with fibroids were being treated for hypertension with angiotensin-converting enzyme inhibitors (ACEIs) and/or diuretics or with β-blockers (Table 2). Of women with uterine fibroids, 31% had hypertensive (> 140 mm Hg) blood pressures compared to 18% of the control population (P = .04; Figure 1B). All of the women with hypertension having uterine fibroids were currently being treated for hypertension with ACEI and/or diuretics or with calcium channel blockers (Table 2). One woman in the control group with normotensive pressure was being treated for hypertension with an ACEI, while the other women with systolic pressures <120 mm Hg were not treated with antihypertensives. To determine whether MAP decreased after hysterectomy in our fibroid group, blood pressure was measured within 2 to 6 weeks of surgery. Only 19 of 32 patients returned to our hospital for follow-up treatment after hysterectomy for uterine fibroids, at which time their blood pressure was measured. The MAP was significantly decreased in these patients when compared to their presurgery pressures (100.9 ± 3.1 mm Hg vs 93.91 ± 2.1 mm Hg postsurgery; P = .02).
Table 1.
Demographics for Patient Population.
| Fibroid | Control | P Value | |
|---|---|---|---|
| Age, years (range) | 42.75 ± 1.2 (30-59) | 42.91 ± 3.7 (28-65) | .96 |
| Race | .2 | ||
| African American | 27 (84%) | 7 (64%) | |
| Caucasian | 5 (16%) | 4 (36%) | |
| Blood pressure (systolic) | |||
| Normotensive (<120 mm Hg) | 7 (22%) | 4 (36%) | .04* |
| Prehypertensive (120-140 mm Hg) | 15 (47%) | 58 (46%) | 1 |
| Hypertensive (>140 mm Hg) | 10 (31%) | 2 (18%) | .04* |
| Body Mass Index (range) | 34.68 ± 1.53 (22.5-61.3) | 31.63 ± 2.09 (21.9-47.9) | .29 |
* denotes as compared to control population.
Figure 1.
Hypertension and endothelin 1 (ET-1) secretion is associated with uterine fibroids. Mean arterial pressure (MAP) was significantly higher in women with fibroids compared to women without fibroids (A). Panel B demonstrates that women with fibroids were more likely to be hypertensive compared to women without fibroids. ET-1 (C) and sFlt-1 (D) was increased in the circulation of women with fibroids. * denotes P < .05; ** P < .005 compared to the control group.
Table 2.
Uterine Weight, Number of Fibroids, and Fibroid Size.
| Patient # | Blood Pressure | Uterine Weight, g | # Fibroids | Fibroid Size, range |
|---|---|---|---|---|
| 1 | 116/76 | 403 | 3+ | 0.4-5.5 |
| 2 | 116/74 | 1020 | 3+ | 0.7-7.5 |
| 3 | 120/70 | 125 | 1-2 | 3 |
| 4 | 117/70 | 108 | 1-2 | 0.3, 0.5 |
| 5 | 117/81 | 77 | 3+ | 0.3-5.8 |
| 6 | 110/88a,b | 1090 | 3+ | 1-8.3 |
| 7 | 114/74b | 917 | 3+ | 0.5-1.5 |
| 8 | 133/81b | 1250 | 3+ | 7-4.2 |
| 9 | 135/81 | 941 | 1-2 | 6.8, 7.9 |
| 10* | 128/74 | 78 | 3+ | 1.8-2.7 |
| 11 | 121/75 | 696 | 1-2 | 12 |
| 12 | 121/69 | 1449 | 3+ | 0.4-7 |
| 13 | 134/92b | 275 | 1-2 | 5, 6.8 |
| 14 | 123/79 | 303 | 1-2 | 0.3, 4.5 |
| 15 | 121/82b | 1026 | 1-2 | 3.5 |
| 16 | 122/85c | 730 | 3+ | 0.6-12 |
| 17 | 136/85a,b | 180 | 1-2 | 7.1 |
| 18 | 129/83 | 550 | 1-2 | 0.9, 5.5 |
| 19 | 134/97 | 453 | 1-2 | 5.5 |
| 20 | 122/78 | 147 | 1-2 | 4.8-5.2 |
| 21 | 136/87 | 658 | 3+ | 1-3.7 |
| 22 | 122/81a,b | 213 | 1-2 | .9, 2.1 |
| 23 | 143/95a,b | 194 | 3+ | 0.5-1.5 |
| 24* | 155/92a,b | 2900 | 3+ | 0.4-18 |
| 25 | 176/96a | 953 | 3+ | 0.5-7.5 |
| 26 | 147/90a,b | 250 | 3+ | 0.6-4.5 |
| 27 | 170/86a | 137 | 3+ | 0.6-3.1 |
| 28 | 181/96a,b | 329 | 3+ | 1-7.1 |
| 29 | 149/85b | 881 | 3+ | 1.5-9 |
| 30 | 178/115a | 603 | 1-2 | 1.7, 6 |
| 31* | 171/86a,b | 599 | 1-2 | 1.5, 5.4 |
| 32 | 153/101d | 648 | 3+ | 0.5-1.8 |
Classes of anti-hypertensive medication prescribed to patients
aangiotensin converting enzyme inhibitor;
bdiuretic
cbeta blocker
dcalcium channel blocker.
*Denotes menopausal patient.
There was no significant correlation between the number of uterine fibroids a patient had and her preoperative MAP (r = .133, P = .469; Table 2) or between uterine weight and preoperative MAP (r = .089, P = .630; Table 2). There was no significant correlation between fibroid size and preoperative MAP (r = .135, P = .460, Table 2); however, as expected, there was a significant positive correlation between uterine weight and fibroid size (r = .447, P = .01, Table 2). In the current study, only 3 patients with fibroid (Table 2) and 1 control patient (Supplemental Data Table 1) were menopausal or postmenopausal. Supplemental Data Table 2 presents confounding variables outside hypertension such as diabetes, abnormal thyroid diagnoses, and neurological problems in patients with uterine fibroids.
Circulating ET-1 and sFlt-1 are Increased in Women With Uterine Fibroids
To determine whether circulating levels of ET-1 were higher in women with uterine fibroids, we extracted and measured endothelin from plasma. Women with uterine fibroids (n = 10) had significantly more circulating ET-1compared to women without uterine fibroids (n = 6; P = .005; Figure 1C). As obesity (classified as BMI > 30), diabetes, and hypertension can increase circulating ET-1 levels,26–28 we restratified data based on the presence or absence of these factors in women with uterine fibroids. There was no positive correlation between any of these factors and increased ET-1 or sFlt-1 levels (Supplemental Data Table 3). Similar to circulating ET-1, levels of sFlt-1 were significantly increased (P = .02, Figure 1D) in women with uterine fibroids (n = 6) compared to women without (n = 6).
Protocol 1: Blockade of the AT1R Decreases ET-1 Secretion
Previous studies have demonstrated a role for activation of the AT1R in stimulating proliferation of Eker rat leiomyoma cells (ELT-3).11,29 To determine whether activation of the AT1R induced ET-1 secretion, we added Losartan (0.1 or 1 µmol/L), an AT1R antagonist into tissue culture media with myometrium control explants (n = 5) and into tissue culture media with patient-matched myometrium and fibroid explants (n = 5). Endothelin 1 secretion was significantly decreased in the myometrium at both the 0.1 µmol/L (P = .007) and the 1.0 µmol/L doses (P = .003; Figure 2A) compared to untreated explants. Explants of fibroid tissue also secreted less ET-1 at both 0.1 µmol/L (P = .07) and 1.0 µmol/L (P = .03) doses when compared to the untreated explants (Figure 2A). There were no differences in ET-1 secretion from myometrium collected from our control population (P = .301; Supplemental Figure 1A) and treated with Losartan compared to untreated explants (0 µmol/L Losartan).
Figure 2.

Endothelin 1 (ET-1) secretion is attenuated by blockade of the angiotensin II Type 1 (AT1) and endothelin-A (ETA) receptors. ET-1 secretion from fibroid explants incubated under normoxic conditions was decreased through blockade of the AT1 receptor (A), the ETA (B), or the endothelin-B (ETB) receptor (C). * denotes P < .05; ** P < .005; *** P < .0005 compared to the 0 µmol/L (untreated) tissue control.
Blockade of the ET-1 Receptor Decreases ET-1 Secretion
To determine which ET receptor is most likely activated in fibroids, we added an ETA or ETB receptor antagonist (0.1 or 1 µmol/L) into the tissue culture media with myometrium control explants (n = 5) and into the tissue culture media of patient-matched myometrium and fibroid explants (n = 5). Of these 5 patients, 1 was menopausal; however, this patient’s data points were in line with the other 4 nonmenopausal patients. Endothelin 1 secretion was significantly decreased from the myometrium at the 0.1 µmol/L (P = .0008) and 1.0 µmol/L doses (P = .01; Figure 2B) of the ETA antagonist compared to untreated explants. Explants from the fibroid tissue also secreted significantly less ET-1 at the 0.1 µmol/L (P = .01) and 1.0 µmol/L doses (P = .01) when compared to the untreated explants (Figure 2B). Blockade of the ETA receptor at the 0.1 µmol/L dose significantly increased ET-1 secretion from myometrium collected from our control population when compared to ET-1 secretion from the untreated explants (P = .003; Supplemental Figure 1B), whereas treatment with 1 µmol/L did not significantly change ET-1 secretion when compared to the untreated explants (P = .139) or the explants treated with 0.1 µmol/L of the ETA antagonist (P = .06).
Myometrium tissue treated with the ETB antagonist had a significant decrease in ET-1 secretion at the 0.1 µmol/L (P = .01) and 1.0 µmol/L doses (P = .008) when compared to the untreated explants (Figure 2C). The ETB receptor antagonism did not significantly decrease ET-1 production at the 0.1 µmol/L (1.12 ± .42 pg/mg/mL; P = .18) or the 1.0 µmol/L dose (1.01 ± .36 pg/mg/mL; P = .11) when compared to the untreated fibroid explants (2.03 ± .45 pg/mg/mL; Figure 2C). There were no differences in ET-1 secretion from myometrium collected from our control population when treated with ETB antagonists (P = .377; Supplemental Figure 1C).
Protocol 2: Fibroid Explants Secrete Significantly More ET-1
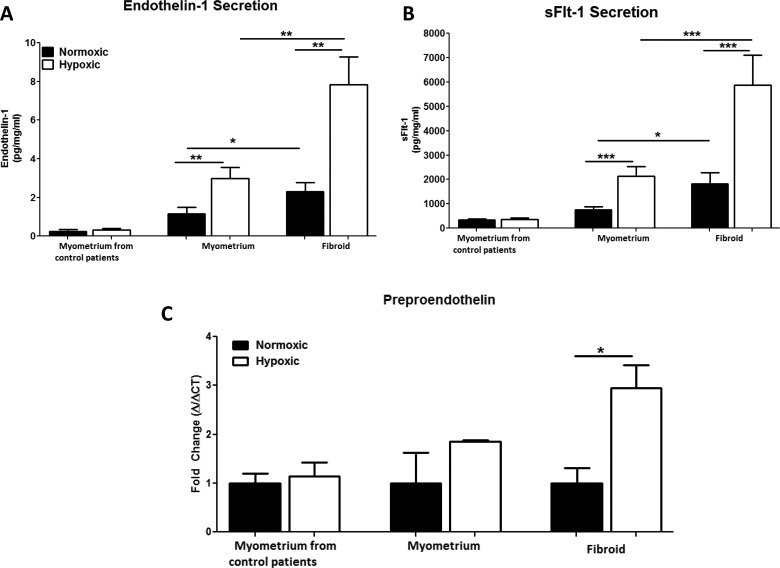
Endothelin 1 secretion was significantly increased in fibroid explants compared to myometrium explants (n = 32, P = .025; Figure 3A). To determine whether hypoxia exacerbates ET-1 secretion in uterine fibroids, patient-matched myometrium and fibroid explants (n = 32) and myometrium control explants (n = 6) were cultured under normoxic or hypoxic conditions for 24 hours. Endothelin 1 secretion from myometrial explants increased in response to hypoxia compared to normoxic myometrial explants (P = .004; Figure 3A). Endothelin 1 secretion from fibroid explants also increased significantly in response to hypoxia compared to normoxic fibroid explants (P = .001; Figure 3A). Endothelin 1 secretion from fibroid explants cultured under normoxia was significantly higher compared to patient-matched myometrial explants (P = .02, Figure 3A). As expected ET-1 secretion was significantly higher in fibroid explants exposed to 24 hours of hypoxia (P = .005; Figure 3A) compared to myometrium explants exposed to hypoxia. Importantly, after 24 hours of hypoxia, myometrial ET-1 explant secretion (2.99 ± .56 pg/mg/mL) was comparable to normoxic fibroid ET-1 explant secretion (2.30 ± .46 pg/mg/ml; P = .351). Hypoxia did not significantly increase ET-1 secretion (0.318 ± 0.07 pg/mg/mL) in myometrium explants from our control population compared to patient-matched normoxic controls (0.226 ± 0.118 pg/mg/mL; P = .348, Figure 3A). These results indicate that hypoxia stimulates ET-1 secretion from myometrial explants of women with uterine fibroids and exacerbates ET-1 response in fibroid explants.
Figure 3.
Hypoxia increases endothelin 1 (ET-1) and soluble fms-like tyrosine kinase 1 (sFlt-1) secretion. ET-1 secretion from fibroid explants incubated in hypoxic conditions is significantly higher compared to the myometrium and normoxic controlled fibroid explants (A). Hypoxia significantly increases sFlt-1 secretion compared to normoxic controls. sFlt-1 secretion from hypoxia-exposed fibroid explants is significantly higher compared to hypoxia-exposed myometrium (B). ET-1 transcript was quantitated by real-time polymerase chain reaction in uterine fibroid and myometrial explants from normoxic and hypoxic cultures. Preproendothelin expression in the fibroid is significantly increased compared to the myometrium (C). Hypoxia increased preproendothelin (PPET) expression in the myometrium and in the fibroid. N = 7 patients per group. * denotes P < .05; ** P < .005; *** P < .0005 between 2 groups being compared as indicated by the solid black line.
Soluble fms-like tyrosine kinase 1 was measured in cell culture media collected from normoxic and hypoxic tissue explants after 24 hours of culture. The sFlt-1 secretion from myometrial explants increased in response to hypoxia compared to normoxic myometrial explants (P = .0007; Figure 3B). The sFlt-1 secretion from fibroid explants increased significantly, in response to hypoxia compared to normoxic fibroid explants (P = .003; Figure 3B). The sFlt-1 secretion was significantly increased in fibroid explants incubated under normoxic conditions compared to patient-matched myometrial explants (P = .018; Figure 3B) incubated under normoxia. Despite the hypoxia-stimulated sFlt-1 secretion from myometrial explants, sFlt-1 secretion was still significantly higher in fibroid explants exposed to 24 hours of hypoxia (P = .002; Figure 3B). Hypoxia did not significantly increase sFlt-1 secretion (358.3 ± 66.99 pg/mg/mL) in myometrium explants from our control population compared to patient-matched normoxic controls (335.2 ± 35.4 pg/mg/mL; P = .762, Figure 3B). After 24 hours of hypoxia, myometrial sFlt-1 explant secretion (2144 ± 381.7 pg/mg/mL) was comparable to normoxic fibroid sFlt-1 explant secretion (1819 ± 1226 pg/mg/ml; P = .585). These results indicate that hypoxia stimulates sFlt-1 secretion from myometrial explants of women with uterine fibroids and exacerbates sFlt-1 response in fibroid explants.
Preproendothelin Expression is Increased in Uterine Fibroids Compared to the Adjacent Myometrium
Utilizing real-time PCR, we measured PPET in fibroid and myometrium tissue explants from cultured explants to determine whether hypoxia exacerbates PPET mRNA expression. Under normoxic conditions, PPET expression was increased 6.5-fold (P = .01; data not shown) in fibroid explants compared to myometrial explants and PPET expression was increased 10-fold (P = .002; data not shown) in hypoxic fibroid explants when compared to hypoxic myometrial explants. Hypoxia did not significantly increase PPET expression in myometrial explants (P = .150, Figure 3C) compared to normoxic explants. Hypoxia increased PPET expression in fibroid explants almost 3-fold (P = .028, Figure 3C) compared to normoxic controls. Hypoxia did not significantly increase PPET expression in myometrium collected from the control population (P = .724, Figure 3C).
Discussion
Recent reports have identified an epidemiologic link between hypertension and the development of uterine fibroids in women with fibroids having an approximate 2-fold increase in the incidence of hypertension.9,10 These studies suggested that this association increased the risk of cardiovascular disease in women with uterine fibroids. In the present study, we report that women with uterine fibroids have increased circulating sFlt-1 and ET-1(2 peptides that are linked to renal and vascular function) and that these women are hypertensive compared to the control population. Furthermore, myometrial ET-1 secretion is much greater from patients with fibroids compared to myometrium ET-1 secretion from patients without fibroids. Importantly, expression and secretion of ET-1 were exacerbated with chronic hypoxia from either patient-matched myometrium or fibroid explants. In addition, ET-1 secretion from fibroid explants was attenuated through blockade of the ANGII or ET-1 pathway. In the current study, we also report a significant decrease in MAP after hysterectomy. As such, the results from the current study support a link between fibroid secretion of vasoactive factors and the development of hypertension in the leiomyoma patient.
A reduction in oxygen in the uterus is believed to play an important role in the development of uterine fibroids. Over the past few years, there have been several studies demonstrating that fibroids are indeed hypoxic compared to the adjacent myometrium.13,15,30 It has also been suggested that dysregulation of the estrogen pathway during hypoxia activates myometrial stem cells, thus contributing to the development of uterine fibroids.13 Moreover, we found that hypoxia increases secretion of ET-1 and sFlt-1 in the myometrium and fibroid tissue. These findings suggest that hypoxia may also play an important role in regulating the increases in endothelin and some of the dysregulation in angiogenesis seen in women with uterine fibroids.
As ET-1 is a powerful vasoconstrictor in which only nanogram changes in concentration are required to elicit a powerful physiological response,31 its dysregulation has been linked to hypertensive disorders such as chronic kidney disease as well as a number of female reproductive disorders including preeclampsia, endometriosis, and ovarian and cervical cancers.10,18,20,32–36 Additionally, ET-1 has been implicated as a promoter for myometrial and uterine tumor cell growth, its mRNA is upregulated in uterine fibroids, and it has been found to mediate cell proliferation in the tumor-derived cell line from Eker rat.10,36,37 In the current study, we found that circulating ET-1 was increased in women with uterine fibroids compared to women without uterine fibroids. In addition, when cultured under normoxic conditions, fibroid explants secreted significantly more ET-1 compared to the adjacent myometrium. Furthermore, blockade of the ET-1 receptors decreased ET-1 secretion.
Components of the renin–angiotensin–aldosterone system have been identified as important regulators of fibrosis. Angiotensin II is an important blood pressure control pathway that has been linked to growth and proliferation of uterine leiomyoma. Studies utilizing the Eker rat model indicate that ANGII induced ELT3 cell proliferation via upregulation of the AT1R11,29 and that proliferation was inhibited by AT1R blockade.11 In the current study, in vitro blockade of the AT1R with Losartan decreased ET-1 secretion in uterine fibroids which supports a role for the renin–angiotensin system in ET-1 secretion from uterine fibroids. Previously Dohi et al demonstrated that ANGII stimulated endothelial production of endothelin in vitro in mesenteric resistance arteries of spontaneously hypertensive rats.38 These authors suggest that vascular endothelin production acts as an amplifier of the pressor effects of the renin–angiotensin system. This may serve as one potential physiological system whereby hypertension, as evidenced in the patients with uterine fibroids in this study, could be associated with the increased proliferation of uterine fibroids.
Overexpression of sFlt-1 has been shown to play a crucial role in the development of hypertension and immune activation, and placental hypoxia has also been shown to induce sFlt-1 expression during pregnancy, indicating that hypoxia may play a role in regulating expression of sFlt-1.39 In the present study, sFlt-1 was increased in fibroid explants compared to the myometrium, indicating that hypoxia regulates sFlt-1 expression in uterine fibroids. While sFlt-1 has not been reported in literature concerning uterine fibroids, we believe that both the significant increase in circulating sFlt-1 in women with uterine fibroids and the data indicating that hypoxia exacerbates its secretion from uterine fibroids suggest that sFlt-1 contributes to endothelial dysfunction and the development of high blood pressure in the leiomyoma patient.
The results from our study demonstrating that women with uterine fibroids were more likely to be hypertensive are in agreement with earlier work by Luoto et al who reported that 42% of hypertensive women had uterine fibroids compared to 37% of normotensive women.40 Although only 32% of the women in the current study were hypertensive, the larger sample size (n = 543 patients) in Luoto study could well attribute to these differences. Unexpectedly, the number or size of uterine fibroids was not correlated with preoperative MAP (Table 2). A study published earlier this year by Moorman et al found similar results in that neither BMI or hypertension was statistically associated with uterus size or the number of fibroids.41 These results when taken together with ours suggest that maybe another factor such as ET, sFlt-1, or local activation of the renin–angiotensin system influence hypertension associated fibroid growth.
The current study supports an association between antiangiogenic factors, endothelial dysfunction, and hypertension in women with uterine fibroids. While this association has been commonly known to be true in the pregnancy-related illness, preeclampsia, and chronic kidney disease, this is the first study to suggest a possible association in women with uterine fibroids. All of the women in the current study underwent hysterectomy after failure of oral contraceptives and/or Depo-Provera. Gonadotrophin-releasing hormone antagonists (GnRHa) have been shown to be effective in decreasing fibroid size prior to hysterectomy, due to the decrease in estrogen. While none of the patients in the current study were treated with GnRHa, it is possible that administration of GnRHa or possibly analogs of Vitamin D, both of which have been shown to reduced fibroid size, might possibly have a positive effect on either ET-1 or sFlt-1 production and secretion.42,43 Although the present data demonstrate hypoxia induced endothelin 1 and sFlt-1 are stimulated in fibroids and the myometrium from the leiomyoma patient, it remains unknown by which mechanisms these factors may contribute to the development and proliferation of uterine fibroids. Additionally, it is unknown what cell type, smooth muscle cells, endothelial cells, or fibroblasts are the potential sources of ET-1 and sFlt-1 in the uterine fibroids. The current study highlights the importance of future research needed to determine a role for hypoxia altered vasoactive pathways to mediate the development of uterine leiomyoma and hypertension in this unique patient population and to determine what cell types are mediated by these pathways.
Acknowledgments
We are grateful to Lillian Ray and Evan Turnage in their assistance in collecting, preparing, and culturing specimens for this study. Importantly, we acknowledge the contribution of Bryan D. Cowan, MD, in the development and funding for a portion of this project. His memory and dedication in the area of uterine leiomyoma research is an inspiration for us to continue his passion.
Footnotes
Declaration of Conflicting Interests: The author(s) declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: The Endothelin A receptor antagonist was kindly provided by Abbott Labs (study #REN-11-0065) to Babbette LaMarca.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This research was in part supported by a grant from the American Society of Reproductive Medicine to Kedra Wallace and Intramural Research Support from the University of Mississippi Medical Center to Babbette LaMarca.
Supplemental Material: The supplemental material is available at http://rs.sagepub.com/supplemental.
References
- 1. Parazzini F, Chiaffarino F, Polverino G, Chiantera V, Surace M, La Vecchia C. Uterine fibroids risk and history of selected medical conditions linked with female hormones. Eur J Epidemiol. 2004;19(3):249–253 [DOI] [PubMed] [Google Scholar]
- 2. Mauskopf J, Flynn M, Thieda P, Spalding J, Duchane J. The economic impact of uterine fibroids in the United States: a summary of published estimates. J Womens Health (Larchmt) 2005;14(8):692–703 [DOI] [PubMed] [Google Scholar]
- 3. Zimmerman A, Bernuit D, Gerlinger C, Schaefers M, Geppert K. Prevalence, symptoms and management of uterine fibroids: an international internet-based survey of 21,746 women. BMC Womens Health. 2012;12:1–11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Farquhar C, Steiner C. Hysterectomy rates in the United States 1990-1997. Obstet Gynecol. 2002;99(2):229–234 [DOI] [PubMed] [Google Scholar]
- 5. Wilcox L, Koonin L, Pokras R, Strauss L, Xia Z, Petersion H. Hysterectomy in the United States, 1988-1990. Obstet Gynecol. 1994;83(4):549–555 [DOI] [PubMed] [Google Scholar]
- 6. Flake G, Andersen J, Dixon D. Etiology and pathogenesis of uterine leiomyomas: a review. Environ Health Perspect. 2003;111(8):1037–1054 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Boynton-Jarrett R, Rich-Edwards J, Malspeis S, Missmer S, Wright R. A prospective study of hypertension and risk of uterine leiomyomata. Am J Epidemiol. 2005;161(7):628–638 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Silver A, Raghuvir R, Fedirko B, Elser D. Systemic hypertension among women with uterine leiomyomata: Potential final common pathways of target end-organ remodeling. J Clin Hypertens (Greenwich). 2005;7(11):664–668 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Settnes A, Andreasen A, Jorgensen T. Hypertension is associated with an increased risk for hysterectomy: a Danish cohort study. Eur J Obstet Gynecol Reprod Biol. 2005;122(2):218–224 [DOI] [PubMed] [Google Scholar]
- 10. Tanfin Z, Ieiber D, Robin P, Oyeniran C, Breuiller-Fouche M. Endothelin-1: Physiological and pathological roles in myometrium. Int J Biochem Cell Biol. 2011;43(3):299–302 [DOI] [PubMed] [Google Scholar]
- 11. Isobe A, Takeda T, Sakata M, et al. Dual repressive effect of angiotensin II-type 1 receptor blocker telmisartan on angiotensin II-induced and estradiol-induced uterine leiomyoma cell proliferation. Hum Reprod. 2008;23(2):440–446 [DOI] [PubMed] [Google Scholar]
- 12. Murphy S, LaMarca B, Cockrell K, Granger J. Role of endothelin in mediating soluble fms-like tyrosine kinase 1-induced hypertension in pregnant rats. Hypertension. 2010;55(2):394–398 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Zhou S, Yi T, Shen K, Zhang B, Huang F, Zhao X. Hypoxia: The driving force of uterine myometrial stem cells differentiation into leiomyoma cells. Med Hypotheses. 2011;77(6):985–986 [DOI] [PubMed] [Google Scholar]
- 14. Mayer A, Hockel M, von Wallbrunn A, Horn LC, Wree A, Vaupel P. HIF-mediated hypoxic response is missing in severely hypoxic uterine leiomyomas. Adv Exp Med Biol. 2010;662:399–405 [DOI] [PubMed] [Google Scholar]
- 15. Mayer A, Hockel M, Wree A, Leo C, Horn LC, Vaupel P. Lack of hypoxic response in uterine leiomyomas despite severe tissue hypoxia. Cancer Res. 2008;68(12):4719–4726 [DOI] [PubMed] [Google Scholar]
- 16. Fletcher N, Saed M, Abu-Soud H, Al-Hendy A, Diamond M, Saed G. Uterine fibroids are characterized by an impaired antioxidant cellular system: potential role of hypoxia in the pathophysiology of uterine fibroids. J Assist Reprod Genet. 2013;30(7):969–974 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Chang H, Senaratnea T, Zhang L, et al. Uterine leiomyomas exhibit fewer stem/progenitor cell characteristics when compared with corresponding normal myometrium. Reprod Sci. 2010;17(2):158–167 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. LaMarca B, Alexander B, Gilbert J, Ryan M, Sedeek M, Granger J. Pathophysiology of hypertension in response to placental ischemia during pregnancy: a central role for endothelin? Gender Med. 2008;5(suppl A):S133–S138 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Lerman A, Kubo S, Tschumperlin L, Burnett JJ. Plasma endothelin concentrations in humans with end-stage heart failure and after heart-transplantation. J Am Coll Cardiol. 1992;20(4):849–853 [DOI] [PubMed] [Google Scholar]
- 20. Pekonen F, Nyman T, Rutanen R. Differential expression of mRNAs for endothelin-related proteins in human endometrium, myometrium and leiomyoma. Mol Cell Endocrinol. 1994;103(1-2):165–170 [DOI] [PubMed] [Google Scholar]
- 21. CDC. Data Trends & Maps Web site. US Department of Health and Human Services, Centers for Diseases Control and Prevention, National Center for Chronic Disease Prevention and Health Promotion. Division for Heart Disease and Stroke Prevention. 2009;2009. [Google Scholar]
- 22. Wallace K, Novotny S, Heath J, et al. Hypertension in response to CD4+ T cells from reduced uterine perfusion pregnant rats is associated with activation of the endothelin-1 system. Am J Physiol Regul Integr Comp Physiol. 2012;303(2):R144–R149 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Lamarca B, Wallace K, Herse F, et al. Hypertension in response to placental ischemia during pregnancy: role of B lymphocytes. Hypertension. 2011;57(4):864–871 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. LaMarca B, Cockrell K, Sullivan E, Bennett W, Granger J. Role of endothelin in mediating tumor necrosis factor-induced hypertension in pregnant rats. Hypertension. 2005;46(1):82–86 [DOI] [PubMed] [Google Scholar]
- 25. Livak K, Schmittgen T. Analysis of relative gene expression data using real-time quantitative PCR and the 2-DeltaDeltaCt Method. Methods. 2001;25(4):402–408 [DOI] [PubMed] [Google Scholar]
- 26. Mauricio M, Aldasoro M, Ortega J, Vila J. Endothelial dysfunction in morbid obesity. Curr Pharm Des. 2013;19(32):5718–5729 [DOI] [PubMed] [Google Scholar]
- 27. Kalani M. The importance of endothelin-1 for microvascular dysfunction in diabetes. Vasc Health Risk Manag. 2008;4(5):1061–1068 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Vanhoutte P. Is endothelin involved in the pathogenesis of hypertension? Hypertension. 1993;21(6 pt 1):747–751 [DOI] [PubMed] [Google Scholar]
- 29. Isobe A, Takeda T, Wakabayashi A, et al. Aldosterone stimulates the proliferation of uterine leiomyoma cells. Gynecol Endocrinol. 2010;26(5):372–377 [DOI] [PubMed] [Google Scholar]
- 30. Keith B, Simon M. Hypoxia-inducible factors, stem cells and cancer. Cell. 2007;129(3):465–472 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Guyton A, Hall J. Textbook of Medical Physiology. Philadelphia: WB Saunders Company; 2000 [Google Scholar]
- 32. Cameron I, Bacon C, Collett G, Davenport A. Endothelin expression in the uterus. J Steriod Biochem Mol Biol. 1995;53(1-6):209–214 [DOI] [PubMed] [Google Scholar]
- 33. Granger J, Abram S, Stec D, Chandler D, LaMarca B. Endothelin, the kidney and hypertension. Curr Hypertens Rep. 2006;8(4):298–303 [DOI] [PubMed] [Google Scholar]
- 34. Smollich M, Wulfing P. The endothelin axis: A novel target for pharmacotherapy of female malignancies. Curr Vasc Pharmacol. 2007;5(3):239–248 [DOI] [PubMed] [Google Scholar]
- 35. Bagnato A, Rosano L. The endothelin axis in cancer. Int J Biochem Cell Biol. 2008;40(8):1443–1451 [DOI] [PubMed] [Google Scholar]
- 36. Tanfin Z, Breuiller-Fouche M. The endothelin axis in uterine leiomyomas: new insights. Biol Reprod. 2012;87(1):5. [DOI] [PubMed] [Google Scholar]
- 37. Raymond M, Robin P, De Zen F, Vilain G, Tanfin Z. Differential endothelin receptor expression and function in rat myometrial cells and leiomyoma ELT3 cells. Endocrinology. 2009;150(10):4766–4776 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Dohi Y, Kojima M, Sato K. Endothelial modulation of contractile responses in arteries from hypertensive rats. Hypertension. 1996;28(5):732–737 [DOI] [PubMed] [Google Scholar]
- 39. Karumanchi A, Bdolah Y. Hypoxia and sFlt-1 in Preeclampsia: The “Chicken-and-Egg” question. Endocrinology. 2004;145(11):4835–4837 [DOI] [PubMed] [Google Scholar]
- 40. Luoto R, Rutanen E, Auvinen A. Fibroids and hypertension. A cross-sectional study of women undergoing hysterectomy. J Reprod Med. 2001;46(4):359–364 [PubMed] [Google Scholar]
- 41. Moorman P, Leppert P, Myers E, Wang F. Comparision of characteristics of fibroids in African American and white women undergoing premenopausal hysterectomy. Fertil Steril. 2013;99(3):768–776 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Lethaby A, Vollenhoven B, Sowter M. Efficacy of pre-operative gonadotrophin hormone releasing analogues for women with uterine fibroids undergoing hysterectomy or myomectomy: a systematic review. BJOG. 2003;109(10):1097–1108 [DOI] [PubMed] [Google Scholar]
- 43. Halder S, Osteen K, Al-Hendy A. Vitamin D3 inhibits expression and activities of matric metalloproteinase-2 and -9 in human uterine fibroid cells. Human Rep. 2013;28(9):2407–2416 [DOI] [PMC free article] [PubMed] [Google Scholar]