Abstract
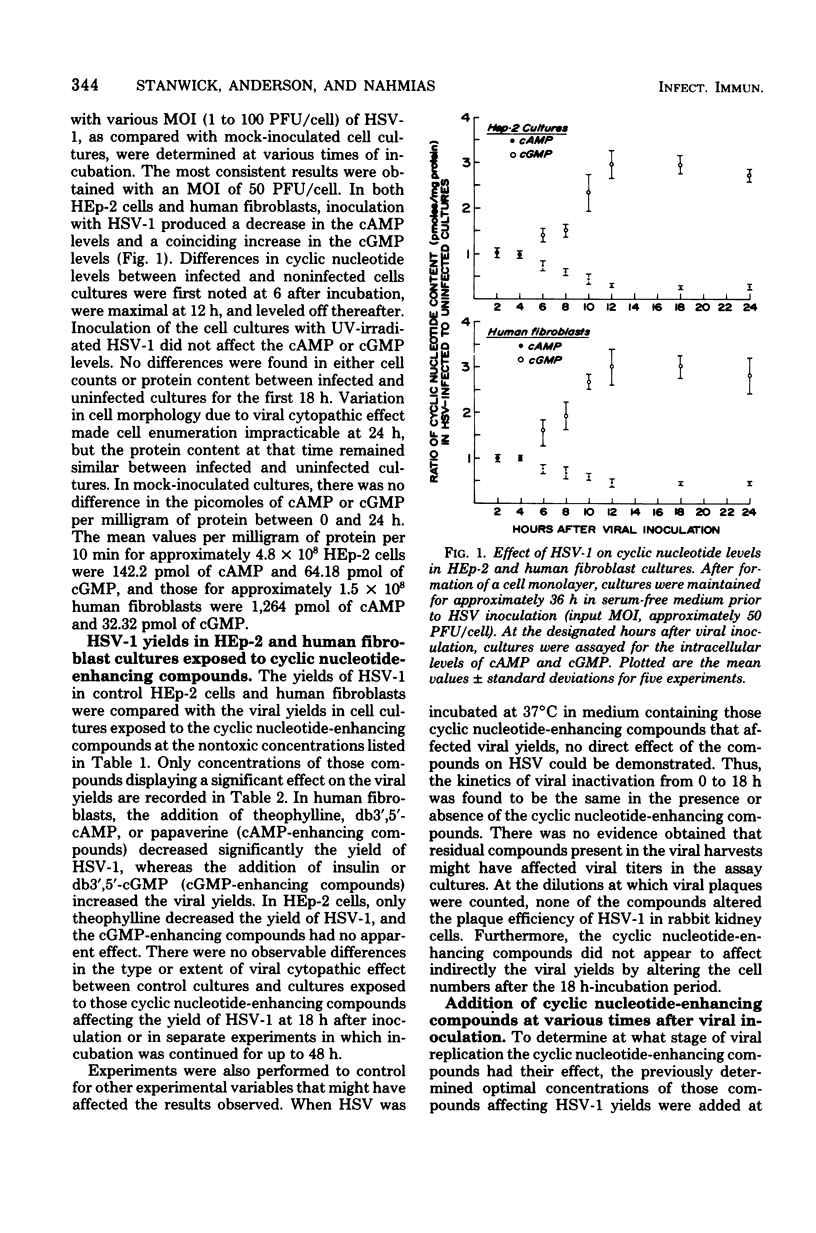
Infection of human fibroblasts and HEp-2 cells with herpes simplex virus type 1 (HSV-1) produced a decrease in the intracellular levels of cyclic adenosine 5'- monophosphate (cAMP) and a concomitant increase in the cyclic guanosine 5'- monophosphate (cGMP) levels. In both cell cultures, changes in cyclic nucleotide levels were first observed at 6 h after viral inoculation and were maximal at 12 h. In human fibroblasts, the addition of theophylline, dibutyryl cAMP, or papaverine (cAMP-enhancing compounds) decreased significantly the yield of HSV-1, whereas the addition of insulin or dibutyryl cGMP (cGMP-enhancing compounds) increased the viral yield. In HEp-2 cells, only theophylline decreased the yield of HSV-1, and the cGMP-enhancing compounds had no apparent effect. Cyclic nucleotide enhancing compounds exhibited their effect only if added to either cell culture within the first 3 h after inoculation with HSV-1.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anderson W. B., Johnson G. S., Pastan I. Transformation of chick-embryo fibroblasts by wild-type and temperature-sensitive Rous sarcoma virus alters adenylate cyclase activity. Proc Natl Acad Sci U S A. 1973 Apr;70(4):1055–1059. doi: 10.1073/pnas.70.4.1055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baringer J. R., Swoveland P. Persistent herpes simplex virus infection in rabbit trigeminal ganglia. Lab Invest. 1974 Feb;30(2):230–240. [PubMed] [Google Scholar]
- Baringer J. R., Swoveland P. Recovery of herpes-simplex virus from human trigeminal ganglions. N Engl J Med. 1973 Mar 29;288(13):648–650. doi: 10.1056/NEJM197303292881303. [DOI] [PubMed] [Google Scholar]
- Bastian F. O., Rabson A. S., Yee C. L., Tralka T. S. Herpesvirus hominis: isolation from human trigeminal ganglion. Science. 1972 Oct 20;178(4058):306–307. doi: 10.1126/science.178.4058.306. [DOI] [PubMed] [Google Scholar]
- Bourne H. R., Lichtenstein L. M., Melmon K. L., Henney C. S., Weinstein Y., Shearer G. M. Modulation of inflammation and immunity by cyclic AMP. Science. 1974 Apr 5;184(4132):19–28. doi: 10.1126/science.184.4132.19. [DOI] [PubMed] [Google Scholar]
- Costa J., Yee C., Troost T., Rabson A. S. Effect of dexamethasone on herpes simplex virus type 2 infection in vitro. Nature. 1974 Dec 20;252(5485):745–746. doi: 10.1038/252745a0. [DOI] [PubMed] [Google Scholar]
- Duff R., Rapp F. Properties of hamster embryo fibroblasts transformed in vitro after exposure to ultraviolet-irradiated herpes simplex virus type 2. J Virol. 1971 Oct;8(4):469–477. doi: 10.1128/jvi.8.4.469-477.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill T. J., Blyth W. A. An alternative theory of herpes-simplex recurrence and a possible role for prostaglandins. Lancet. 1976 Feb 21;1(7956):397–399. doi: 10.1016/s0140-6736(76)90220-8. [DOI] [PubMed] [Google Scholar]
- Ida S., Hooks J. J., Siraganian R. P., Notkins A. L. Enhancement of IgE-mediated histamine release from human basophils by viruses: role of interferon. J Exp Med. 1977 Apr 1;145(4):892–906. doi: 10.1084/jem.145.4.892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson G. S., Friedman R. M., Pastan I. Restoration of several morphological characteristics of normal fibroblasts in sarcoma cells treated with adenosine-3':5'-cyclic monphosphate and its derivatives. Proc Natl Acad Sci U S A. 1971 Feb;68(2):425–429. doi: 10.1073/pnas.68.2.425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KLAINER L. M., CHI Y. M., FREIDBERG S. L., RALL T. W., SUTHERLAND E. W. Adenyl cyclase. IV. The effects of neurohormones on the formation of adenosine 3',5'-phosphate by preparations from brain and other tissues. J Biol Chem. 1962 Apr;237:1239–1243. [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Otten J., Bader J., Johnson G. S., Pastan I. A mutation in a rous sarcoma virus gene that controls adenosine 3',5'-monophosphate levels and transformation. J Biol Chem. 1972 Mar 10;247(5):1632–1633. [PubMed] [Google Scholar]
- Pastan I. The effect of dibutyryl cyclic 3',5'-AMP on the thyroid. Biochem Biophys Res Commun. 1966 Oct 5;25(1):14–16. doi: 10.1016/0006-291x(66)90632-2. [DOI] [PubMed] [Google Scholar]
- Peery C. V., Johnson G. S., Pastan I. Adenyl cyclase in normal and transformed fibroblasts in tissue culture. Activation by prostaglandins. J Biol Chem. 1971 Sep 25;246(18):5785–5790. [PubMed] [Google Scholar]
- ROIZMAN B. The programming of herpes virus multiplication in doubly-infected and in puromycin-treated cells. Proc Natl Acad Sci U S A. 1963 Feb 15;49:165–171. doi: 10.1073/pnas.49.2.165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ROIZMAN B. The role of hormones in viral infection. II. Acceleration of viral adsorption and penetration into cells treated with thyroid hormone in vitro. Proc Natl Acad Sci U S A. 1962 Jun 15;48:973–977. doi: 10.1073/pnas.48.6.973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ROIZMAN B. The role of hormones in viral infections. I. Suppression of viral adsorption and penetration into cells treated with parathyroid hormone in vitro. Proc Natl Acad Sci U S A. 1962 May 15;48:795–803. doi: 10.1073/pnas.48.5.795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rapp F., Buss E. R. Comparison of herpes simplex virus isolates using a quantitative selection assay for transformation. Intervirology. 1975;6(2):72–82. doi: 10.1159/000149458. [DOI] [PubMed] [Google Scholar]
- Raska K., Jr Cycl AMP in Gl-arrested BHK21 cells infected with adenovirus type 12. Biochem Biophys Res Commun. 1973 Jan 4;50(1):35–41. doi: 10.1016/0006-291x(73)91059-0. [DOI] [PubMed] [Google Scholar]
- Rein A., Carchman R. A., Johnson G. S., Pastan I. Simian virus 40 rapidly lowers cAMP levels in mouse cells. Biochem Biophys Res Commun. 1973 Jun 8;52(3):899–904. doi: 10.1016/0006-291x(73)91022-x. [DOI] [PubMed] [Google Scholar]
- Roizman B. The herpesviruses--a biochemical definition of the group. Curr Top Microbiol Immunol. 1969;49:3–79. [PubMed] [Google Scholar]
- Smith B. J., Defendi V., Wigglesworth N. M. The effect of dibutyryl cyclic AMP on transformation by oncogenic viruses. Virology. 1973 Jan;51(1):230–232. doi: 10.1016/0042-6822(73)90383-8. [DOI] [PubMed] [Google Scholar]
- Stevens J. G. Latent herpes simplex virus and the nervous system,. Curr Top Microbiol Immunol. 1975;70:31–50. doi: 10.1007/978-3-642-66101-3_2. [DOI] [PubMed] [Google Scholar]
- Sydiskis R. J., Roizman B. Polysomes and protein synthesis in cells infected with a DNA virus. Science. 1966 Jul 1;153(3731):76–78. doi: 10.1126/science.153.3731.76. [DOI] [PubMed] [Google Scholar]
- Walz M. A., Price R. W., Notkins A. L. Latent ganglionic infection with herpes simplex virus types 1 and 2: viral reactivation in vivo after neurectomy. Science. 1974 Jun 14;184(4142):1185–1187. doi: 10.1126/science.184.4142.1185. [DOI] [PubMed] [Google Scholar]
- Zimmerman J. E., Jr, Glaser R., Rapp F. Effect of dibutyryl cyclic AMP on the induction of Epstein-Barr virus in hybrid cells. J Virol. 1973 Dec;12(6):1442–1445. doi: 10.1128/jvi.12.6.1442-1445.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zimmerman J. E., Jr, Raska K., Jr Inhibition of adenovirus type 12 induced DNA synthesis in G1-arrested BHK21 cells by dibutyryl adenosine cyclic 3':5'-monophosphate. Nat New Biol. 1972 Oct 4;239(92):145–147. doi: 10.1038/newbio239145a0. [DOI] [PubMed] [Google Scholar]



