Abstract
Background:
Erosion is a common condition which manifests due to consumption of high caloric and low pH acidic food stuffs such as carbonated drinks and fruit juices which cause irreversible damage to dental hard tissues and early deterioration of the dental restorations.
Aim:
The main aim of this study is to evaluate and to compare the erosive potential of carbonated drink (cola) and fruit juice (orange fruit juice) by measuring the surface roughness (Ra) values on two commonly used dental restorative materials.
Materials and Methods:
A total of 36 specimens each were prepared using both testing materials, compomer (Group I) and giomer (Group II). Six specimens in each group were discarded due to wide variation in pre exposed Ra values and the remaining 30 specimens in each group were further sub divided into 10 samples each according to the testing media used. Immersion regime was followed according to Von Fraunhofer and Rogers. The pre and post immersion surface roughness values were recorded using a profilometer.
Results:
Both tested materials showed statistically-significant surface erosion (P < 0.01) when exposed to cola and orange fruit juice than the control group (water).
Discussion:
Compomer showed more surface roughness when compared to giomer when exposed to the three tested media which can be attributed to the variation in filler content, decomposition of resin matrix and fallout of the fillers in composites when exposed to acidic drinks. Other factors responsible for this significant erosion were also discussed.
Conclusions:
Significant surface changes of the dental restorative materials can take place when exposed to low pH drinks for a prolonged period.
Keywords: Compomer, Erosion, Giomer, Orange fruit juice, Profilometer, Soft drink, Surface roughness
Introduction
Over the last few decades, there was a drastic decline in the prevalence of dental caries world-wide which has been accompanied by a remarkable increase in the incidence of non-carious lesions such as dental erosion. Dental erosion is defined as an irreversible loss of dental hard tissue by a chemical process without the involvement of microorganisms and is due to either extrinsic or intrinsic sources.[1,2] Intrinsic causes like recurrent vomiting, which is a part of eating disorders like anorexia or bulimia nervosa result in erosion of teeth. Extrinsic causes include acidic substances, beverages, medication and environmental exposure to acidic agents.[3] With the change in the dietary patterns, prevalence of dental erosion seems to have increased presumably due to an increase in the consumption of soft drinks and fruit juices.[4] According to American dietetic association, it is estimated that 40% of preschool children consume 250 ml of carbonated beverage/day.[1] The erosive effects of fruit juices have been recognized way back in 1892 by Darby. Frequent consumption of these easily and widely available carbonated beverages and fruit juices showed erosion of the enamel both in vitro and in vivo.[5,6,7] Dental erosion is one of the reason for restoring teeth. The replacement of lost tooth structure is usually desired to restore esthetics and function for which various esthetic restorative materials are used.[8] Restorations with smooth surfaces will result in better esthetics, minimal accumulation of dental plaque, reduced marginal deterioration and better longevity, thus emphasizing the importance of surface roughness property of the restorative material.[2]
Amidst different tooth colored restorative materials, glass ionomers are widely used after being introduced to the dental profession in 1972 by Wilson and Kent. In spite of many advantages like chemical bonding and fluoride-releasing property their moisture sensitivity, low mechanical strength and poor wear resistance made them less durable. To overcome these shortcomings resin modified glass ionomer cements and compomers have been introduced for clinical use which are polyacid-modified resin composites, commonly known as “compomers.” This group of esthetic materials was introduced in the early 1990's, claims to have both mechanical and esthetic properties of composites with the added advantage of fluoride-releasing property of conventional glass ionomer cements. Giomer, a true hybrid of glass ionomer and resin composite having properties like fluoride-release and recharge of glass ionomer cements plus excellent esthetic properties, good surface finish and strength characteristics similar to resin composites.[9]
However, in the complex oral environment it can be assumed that both teeth as well as the restorative materials are subjected to low pH values, leading to degradation of their surface integrity. Thus, the aim of this study is to evaluate changes in surface roughness of tooth colored filling materials (compomer and giomer) after immersion in various acidic drinks that represent popular diets and have the potential to cause dental erosion in the oral cavity. This tests the hypothesis that “surface roughness does not change after immersion in acidic food and drinks.”
Materials and Methods
Prior to the study initiation, institutional ethical clearance from Sibar Institute of Dental Scieces, Guntur, Andhra Pradesh has been obtained regarding the study design. A total of 36 specimens each were prepared using the two testing materials-Group I compomer (Dyract extra Dentsply, York, PA, USA) and Group II giomer (Beautifil-II, Shofu, San Marcos, CA, USA). Each material was syringed into a brass mold with an inner diameter of 6 mm and a thickness of 2 mm. The mold, with specimen material, was held between two glass slides and covered with a transparent polyester strip. The glass slide was held firmly during setting to avoid the presence of air bubbles and to obtain a smooth surface. The materials were polymerized for 30 s using a quartz tungsten-halogen light (Astralis 3, Ivoclar Vivadent Inc., Liechtenstein) with an output of 600 mW/cm2 on each surface of the specimen (top and bottom) as per the manufacturer's recommendation [Figure 1]. Specimens were finished and polished with Sof-Lex disks (3M, St. Paul, MN, USA) with a light orange aluminum grit (30-μm slurry; 3M ESPE Dental Products 2385P) while keeping the material surface wet.
Figure 1.
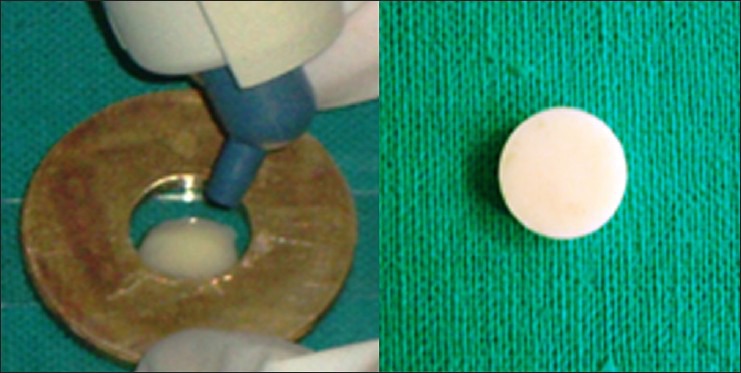
Sample preparation
The specimens in both groups were subdivided into 12 specimens each according to the immersion media used (Media A [cola]; Media B [orange fruit juice]; Media C [water] as control). Six specimens in each group were discarded due to wide variation in pre exposed Ra values and the remaining 30 specimens in each group were further subdivided into 10 samples each according to the testing media used [Figure 2].
Figure 2.
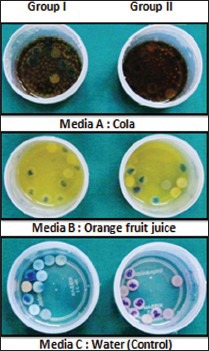
Immersion of specimens in three different media
Base line surface roughness (Ra) values were recorded for all the specimens using a digital profilometer (Mitutoyo Surf Test 202 Analyzer; Mitutoyo Corp, Japan). To measure the roughness profile value, the diamond stylus was moved across the surface under a constant load of 3.9 mm. The instrument was calibrated using a standard reference specimen and then set to travel at a speed of 0.1 mm/s with the range of 600 μm during testing. This procedure was repeated 6 times for each specimen and the average value was considered to be the Ra value.[1,10] The baseline Ra values for Group I specimens ranged from 0.11 to 0.13 μ and for Group II specimens the range was 0.21-0.22 μ [Figure 3].
Figure 3.
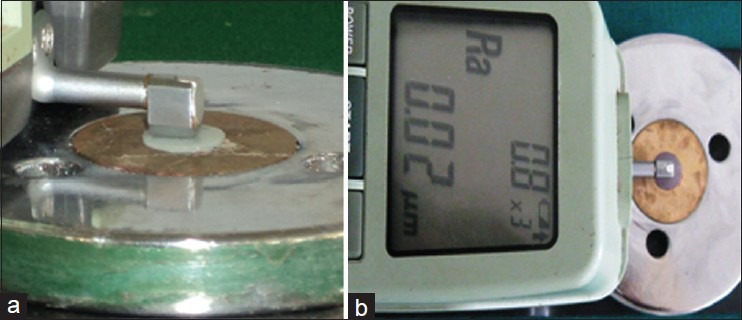
(a) Placement of the stylus. (b) Surface roughness (Ra) readings using profilometer
The immersion regime was followed according to the beverage immersion period protocol adopted by Von Fraunhofer JA and Rogers for dissolution of enamel in beverage solutions.[11] After recording the baseline Ra values, the specimens of both materials were placed separately in 25 ml of testing media (cola, orange fruit juice and water). The total immersion regime is for 7 days with each immersion cycle lasting for 24 h. After every 24 h surface roughness readings were taken for all the samples and were exposed to fresh solution for the next 24 h.
All the readings were recorded by a single observer. To avoid inter examiner variability every fifth sample reading was recorded by a second examiner who was unaware of prior readings. The inter examiner variability error was insignificant (correlation coefficient of 0.98). The data thus obtained was tabulated and subjected to Wilcoxon's signed rank test, one-way ANOVA followed by Mann Whitney test at P < 0.05 level of significance.
Results
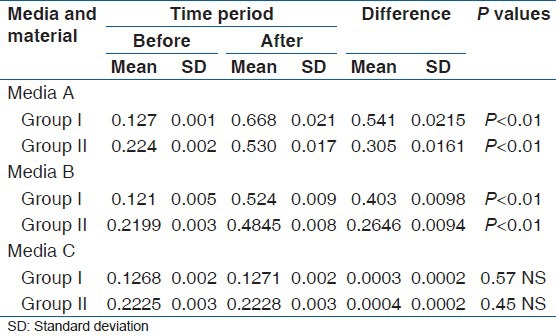
Significantly higher mean Ra values were recorded with both experimental materials. In Group I samples the values noticed were 0.67 for Media A 0.52 for Media B (P < 0.01) and 0.13 for Media C respectively. In case of Group II specimens the values were 0.53, 0.48 (P < 0.01) and 0.22. However, no statistical difference was found in both materials with Media C (water).
Statistically significant Ra values were noticed in specimens of both Groups (I and II) when they are immersed in Media A (0.67, 0.53) when compared with Media B (0.52, 0.48) and Media C (0.13, 0.22).
Out of the two tested materials high surface roughness values were recorded in the Group I samples (0.54, 0.4) when compared with Group II samples (0.3, 0.26) in both tested media [Table 1].
Table 1.
Surface roughness values of two tested materials following immersion in three different media
Discussion
Most people view soft drink/fruit juice consumption as fairly innocuous and its consumption is not as harmless as generally believed; however, there are a number of serious health issues associated with regular consumption of soft drinks. The inherent acids and sugars have both acidogenic and cariogenic potential resulting in dental caries and enamel erosion.[12]
There is a leap up in the prevalence and severity of dental erosion in the last few decades due to changes in the food habits which led to the intake of high calorie and low pH foods/beverages. Most of the carbonated beverages and fruit juices have a pH below 3.5 and scientific studies had shown that enamel dissolution occurs below pH 4 leading to irreversible/irreparable damage.[1]
The overriding goal of dentistry is to improve the quality of life of an individual, which can be accomplished by preventing the disease, relieving pain, improving mastication, enhancing speech and improving appearance. As many of these objectives requires the replacement/alteration of damaged tooth structure, the main challenge for dentists and material scientists have been the development and selection of a biocompatible, long lasting, direct filling esthetic restorative material which serves both preventive and restorative purposes.
One of the most important properties that determine the durability of dental materials in the oral cavity is their resistance to dissolution or disintegration which is affected by common consumable foods and drinks (e.g., water, carbonated soft drinks, alcoholic drinks, food derivatives).[8] Studies have shown that resin-based restorations undergo greater micro morphological damage following an acid challenge for a prolonged time.[10]
This study was designed to simulate the frequent and long term consumption of an individual drinking a carbonated beverage (cola) and fruit juice (orange) by using uninterrupted specimen immersion technique. However, factors such as salivary buffering capacity, acquired pellicle, or remineralization could not be reproduced in this in vitro study.
Many techniques are available such as visual examination, photogrammetric, profilometry, focus variation 3D scanning microscopy and scanning electron microscope (SEM) to determine the surface characteristics of restorative materials, which are qualitative and quantitative. In the present study, quantitative measurements were taken which can be done by using stylus profilometry as it is an established technique to define their surface characteristics and it is also economical compared to focus variation 3D scanning microscopy and SEM.[13]
In general, the surface roughness values of the restorative materials used in the study were higher following immersion than the pre immersion values. This observed difference in Ra values for various materials after immersion in different acidic drinks is due to their diverse chemical composition, porosity and the effect of these drinks on different chemical components.[14] As the restorative materials used in this study were not exposed to mechanical forces, any changes that are noticed can be attributed to chemical dissolution only.
Phosphoric acid is a common constituent of most of the soft drinks. This acid content gives a peculiar tangy taste and has a preservative property, is known to play a well-established role in erosive property.[15]
Citrus fruits are rich source of vitamins, minerals and dietary fibers that are essential for normal growth and development and overall nutritional well-being. These citrus fruits along with their nutritional value have erosive property due to the presence of citric, malic, tartaric, benzoic, oxalic and succinic acids.[16] The main organic acid found in citric juices is citric acid (2-hydroxyl-1,2,3-propanetricar-boxylic acid) which is a weak tricarboxylic acid. In the present study, fresh orange juice was selected due to its ready availability and its acidity is said to be comparable to that of soft drinks.[17]
Studies have reported that acidic condition can degrade glass ionomer cement, polyacid-modified resin composites and restorative composite.[18] The immersion solutions used in this study have a pH value of 2.74 (cola) and 3.72 (orange fruit juice) respectively which are highly acidic and have the potential to cause erosion of the restorative materials. The greatest increase in Ra values was noticed in both tested materials when they are immersed in Media A followed by Media B and Media C (water as control). The difference in Ra values of the two tested materials in the present study were in accordance with the studies conducted by Abu-Bakr et al.,[10] Wongkhantee et al.,[18] EI-Korashy and Mobarak,[9] Han et al.[8]
Surface roughness of resin composites are influenced by the filler content, volume, matrix type, coupling agent disintegration between resin filler interface in composites.[8,9,18] A study by Han et al.,[8] has shown the linear relationship of wear resistance to filler volume. In this study, giomer had the highest filler content (68.6%) when compared with compomer (47%), the presence of lesser filler content in compomer could be the reason for the higher erosion of Group I specimens.
Apart from the filler volume, its properties, distribution and surface treatment (by silane) are also important factors for resin materials on their erosion resistance to acidic and/or alcoholic solution. In giomer, instead of applying purely glass or quartz as the typical fillers of size (0.01-5 μm), incorporated inorganic fillers that are derived from the complete fully pre-reacted glass or partial reaction surface pre-reacted glass (S-PRG) type of ion-leachable glasses (e.g., fluoroboroaluminosilicate glasses) with polyalkenoic acids in water before being interfaced with the organic matrix are incorporated.[14,19,20,21] Fujimoto et al.,[19] found that S-PRG fillers altered the pH values of acidic solution closer to neutral and S-PRG filler, like the conventional glass ionomer cements, had a modulating effect on acidic solutions and were relatively stable under acidic conditions. Due to the S-PRG fillers, giomer was found to be less susceptible to erosion than compomer when exposed to carbonated beverage and fruit juice.
Studies have shown that fillers tend to fall out from resin materials and the matrix component decomposes when exposed to low pH environments.[1,8] This means that drinking acidic drinks over a long period and with continuous sipping can erode the tooth enamel and the resin material as well.[8]
The titratable acidity is the amount of alkali needed to be added to an acid to bring it up to a neutral pH. It therefore represents the amount of available acid and is an indicative of its erosive potential.[1] Study by Edwards et al., in 1999 and Owens 2007 showed least buffering capacity with non-fruit based carbonated drinks followed by fruit based carbonated drinks and highest with fruit juices.[12] This could be the probable reason for the difference in the surface roughness values of testing materials when compared between Group I and II.
It has been reported that hardness, initial surface roughness, filler content, filler size and water absorption of the substrate affect wear resistance. Water absorption gives rise to resin matrix swelling and stress formation. Therefore complete debonding of the fillers in the surface layer can result in surface roughening.[10] The results obtained in our study correlates with the previous research conducted by Abu-Bakr et al.,[10] showing that low pH affects the chemical erosion of the hybrid restorative materials by acid etching the surface and leaching the principal matrix forming cations (Na, Ca, Al, Sr). As a result, individual particles dissociate from each other.[19]
Research has shown drinks are extremely acidic, even when exceedingly diluted.[19] Wongkhantee et al.,[18] showed no statistical differences in the surface characteristics of tooth colored restorative materials when it is exposed to acidic drinks for a short period of time. This represents the dilution and duration of contact with beverages plays a major role in altering the surface characteristics of restorative materials.
In the present study, the micro morphological differences in filler type, content, surface microstructure of materials and low pH, high titratable acidity, duration of exposure of carbonated beverage, fruit juice correlated with significant differences in the tested property of compomer and giomer. Although, this study could not absolutely reproduce the complex oral environment, it confirms the erosive potential of certain acidic food and drinks that the children should be aware of.
However, we recommend further studies combining both qualitative and quantitative evaluations which will indicate more precisely the effect of non-alcoholic and fruit beverages on the clinical integrity of the restorative materials in the oral environment.
Conclusions
Both compomer and giomer showed significant change in the surface roughness after exposing to cola drink and fruit juice
Compomer eroded more than giomer when exposed to cola drink and fruit juice
Cola drink showed more erosive potential than fruit juice and water on both restorative materials (compomer and giomer).
Footnotes
Source of Support: Nil.
Conflict of Interest: None declared.
References
- 1.Kitchens M, Owens BM. Effect of carbonated beverages, coffee, sports and high energy drinks, and bottled water on the in vitro erosion characteristics of dental enamel. J Clin Pediatr Dent. 2007;31:153–9. doi: 10.17796/jcpd.31.3.1157l653t8206100. [DOI] [PubMed] [Google Scholar]
- 2.Pedrini D, Candido MS, Rodrigues AL. Analysis of surface roughness of glass-ionomer cements and compomer. J Oral Rehabil. 2003;30:714–9. doi: 10.1046/j.1365-2842.2003.01133.x. [DOI] [PubMed] [Google Scholar]
- 3.May J, Waterhouse PJ. Dental erosion and soft drinks: A qualitative assessment of knowledge, attitude and behaviour using focus groups of schoolchildren. A preliminary study. Int J Paediatr Dent. 2003;13:425–33. doi: 10.1046/j.1365-263x.2003.00500.x. [DOI] [PubMed] [Google Scholar]
- 4.Shaw L, O’sullivan E. UK National Clinical Guidelines in Paediatric Dentistry. Diagnosis and prevention of dental erosion in children. Int J Paediatr Dent. 2000;10:356–65. doi: 10.1046/j.1365-263x.2000.010004356.x. [DOI] [PubMed] [Google Scholar]
- 5.Larsen MJ, Nyvad B. Enamel erosion by some soft drinks and orange juices relative to their pH, buffering effect and contents of calcium phosphate. Caries Res. 1999;33:81–7. doi: 10.1159/000016499. [DOI] [PubMed] [Google Scholar]
- 6.West NX, Maxwell A, Hughes JA, Parker DM, Newcombe RG, Addy M. A method to measure clinical erosion: The effect of orange juice consumption on erosion of enamel. J Dent. 1998;26:329–35. doi: 10.1016/s0300-5712(97)00025-0. [DOI] [PubMed] [Google Scholar]
- 7.Meurman JH, Frank RM. Scanning electron microscopic study of the effect of salivary pellicle on enamel erosion. Caries Res. 1991;25:1–6. doi: 10.1159/000261335. [DOI] [PubMed] [Google Scholar]
- 8.Han L, Okamoto A, Fukushima M, Okiji T. Evaluation of flowable resin composite surfaces eroded by acidic and alcoholic drinks. Dent Mater J. 2008;27:455–65. doi: 10.4012/dmj.27.455. [DOI] [PubMed] [Google Scholar]
- 9.EI-Korashy DI, Mobarak EH. Effect of colas on surface roughness of some contemporary tooth-colored restorative materials: A non-contact interferometric approach. Egypt Dent J. 2006;52:895–9. [Google Scholar]
- 10.Abu-Bakr N, Han L, Okamoto A, Iwaku M. Changes in the mechanical properties and surface texture of compomer immersed in various media. J Prosthet Dent. 2000;84:444–52. doi: 10.1067/mpr.2000.109635. [DOI] [PubMed] [Google Scholar]
- 11.Von Fraunhofer JA, Rogers MN. Dissolution of dental enamel in soft drinks. Gen Dent. 2005;53:28–31. [PubMed] [Google Scholar]
- 12.Cheng R, Yang H, Shao MY, Hu T, Zhou XD. Dental erosion and severe tooth decay related to soft drinks: A case report and literature review. J Zhejiang Univ Sci B. 2009;10:395–9. doi: 10.1631/jzus.B0820245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kimyai S, Savadi-Oskoee S, Ajami AA, Sadr A, Asdagh S. Effect of three prophylaxis methods on surface roughness of giomer. Med Oral Patol Oral Cir Bucal. 2011;16:e110–4. doi: 10.4317/medoral.16.e110. [DOI] [PubMed] [Google Scholar]
- 14.Badra VV, Faraoni JJ, Ramos RP, Palma-Dibb RG. Influence of different beverages on the microhardness and surface roughness of resin composites. Oper Dent. 2005;30:213–9. [PubMed] [Google Scholar]
- 15.Maganur PC, Prabhakar AR, Satish V, Namineni S, Kurthukoti A. Erosive effect of soft drink and fresh fruit juice on restorative materials. World J Dent. 2013;4:32–40. [Google Scholar]
- 16.Bamise CT, Oziegbe EO. Laboratory analysis of pH and neutralizable acidity of commercial citrus fruits in Nigeria. Adv Biol Res. 2013;7:72–6. [Google Scholar]
- 17.Edwards M, Creanor SL, Foye RH, Gilmour WH. Buffering capacities of soft drinks: The potential influence on dental erosion. J Oral Rehabil. 1999;26:923–7. doi: 10.1046/j.1365-2842.1999.00494.x. [DOI] [PubMed] [Google Scholar]
- 18.Wongkhantee S, Patanapiradej V, Maneenut C, Tantbirojn D. Effect of acidic food and drinks on surface hardness of enamel, dentine, and tooth-coloured filling materials. J Dent. 2006;34:214–20. doi: 10.1016/j.jdent.2005.06.003. [DOI] [PubMed] [Google Scholar]
- 19.Fujimoto Y, Iwasa M, Murayama R, Miyazaki M, Nagafuji A, Nakatsuka T. Detection of ions released from S-PRG fillers and their modulation effect. Dent Mater J. 2010;29:392–7. doi: 10.4012/dmj.2010-015. [DOI] [PubMed] [Google Scholar]
- 20.Ikemura K, Tay FR, Endo T, Pashley DH. A review of chemical-approach and ultramorphological studies on the development of fluoride-releasing dental adhesives comprising new pre-reacted glass ionomer (PRG) fillers. Dent Mater J. 2008;27:315–39. doi: 10.4012/dmj.27.315. [DOI] [PubMed] [Google Scholar]
- 21.Avşar A, Tuloglu N. Effect of different topical fluoride applications on the surface roughness of a colored compomer. J Appl Oral Sci. 2010;18:171–7. doi: 10.1590/S1678-77572010000200012. [DOI] [PMC free article] [PubMed] [Google Scholar]


