Abstract
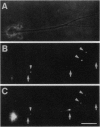
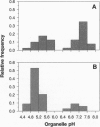
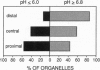
Organelle acidification is an essential element of the endosomal-lysosomal pathway, but our understanding of the mechanisms underlying progression through this pathway has been hindered by the absence of adequate methods for quantifying intraorganelle pH. To address this problem in neurons, we developed a direct quantitative method for accurately determining the pH of endocytic organelles in live cells. In this report, we demonstrate that the ratiometric fluorescent pH indicator 8-hydroxypyrene-1,3,6-trisulfonic acid (HPTS) is the most advantageous available probe for such pH measurements. To measure intraorganelle pH, cells were labeled by endocytic uptake of HPTS, the ratio of fluorescence emission intensities at excitation wavelengths of 450 nm and 405 nm (F450/405) was calculated for each organelle, and ratios were converted to pH values by using standard curves for F450/405 vs. pH. Proper calibration is critical for accurate measurement of pH values: standard curves generated in vitro yielded artifactually low organelle pH values. Calibration was unaffected by the use of culture medium buffered with various buffers or different cell types. By using this technique, we show that both acidic and neutral endocytically derived organelles exist in the axons of sympathetic neurons in different steady-state proportions than in the cell body. Furthermore, we demonstrate that these axonal organelles have a bimodal pH distribution, indicating a rapid acidification step in their maturation that reduces the average pH of a fraction of the organelles by 2 pH units while leaving few organelles of intermediate pH at steady state. Finally, we demonstrate a spatial gradient or organelle pH along axons, with the relative frequency of acidic organelles increasing with proximity to the cell body.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Augenbraun E., Maxfield F. R., St Jules R., Setlik W., Holtzman E. Properties of acidified compartments in hippocampal neurons. Eur J Cell Biol. 1993 Jun;61(1):34–43. [PubMed] [Google Scholar]
- Bassnett S., Reinisch L., Beebe D. C. Intracellular pH measurement using single excitation-dual emission fluorescence ratios. Am J Physiol. 1990 Jan;258(1 Pt 1):C171–C178. doi: 10.1152/ajpcell.1990.258.1.C171. [DOI] [PubMed] [Google Scholar]
- Berthold C. H., Mellström A. Peroxidase activity at consecutive nodes of Ranvier in the nerve to the medial gastrocnemius muscle after intramuscular administration of horseradish peroxidase. Neuroscience. 1986 Dec;19(4):1349–1362. doi: 10.1016/0306-4522(86)90148-x. [DOI] [PubMed] [Google Scholar]
- Bright G. R., Fisher G. W., Rogowska J., Taylor D. L. Fluorescence ratio imaging microscopy: temporal and spatial measurements of cytoplasmic pH. J Cell Biol. 1987 Apr;104(4):1019–1033. doi: 10.1083/jcb.104.4.1019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clement N. R., Gould J. M. Pyranine (8-hydroxy-1,3,6-pyrenetrisulfonate) as a probe of internal aqueous hydrogen ion concentration in phospholipid vesicles. Biochemistry. 1981 Mar 17;20(6):1534–1538. doi: 10.1021/bi00509a019. [DOI] [PubMed] [Google Scholar]
- Daleke D. L., Hong K., Papahadjopoulos D. Endocytosis of liposomes by macrophages: binding, acidification and leakage of liposomes monitored by a new fluorescence assay. Biochim Biophys Acta. 1990 May 24;1024(2):352–366. doi: 10.1016/0005-2736(90)90365-u. [DOI] [PubMed] [Google Scholar]
- Forgac M. Structure and properties of the coated vesicle (H+)-ATPase. J Bioenerg Biomembr. 1992 Aug;24(4):341–350. doi: 10.1007/BF00762527. [DOI] [PubMed] [Google Scholar]
- Furukawa R., Wampler J. E., Fechheimer M. Cytoplasmic pH of Dictyostelium discoideum amebae during early development: identification of two cell subpopulations before the aggregation stage. J Cell Biol. 1990 Jun;110(6):1947–1954. doi: 10.1083/jcb.110.6.1947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furukawa R., Wampler J. E., Fechheimer M. Measurement of the cytoplasmic pH of Dictyostelium discoideum using a low light level microspectrofluorometer. J Cell Biol. 1988 Dec;107(6 Pt 2):2541–2549. doi: 10.1083/jcb.107.6.2541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ganz M. B., Boyarsky G., Sterzel R. B., Boron W. F. Arginine vasopressin enhances pHi regulation in the presence of HCO3- by stimulating three acid-base transport systems. Nature. 1989 Feb 16;337(6208):648–651. doi: 10.1038/337648a0. [DOI] [PubMed] [Google Scholar]
- Gatzinsky K. P., Berthold C. H., Corneliuson O. Acid phosphatase activity at nodes of Ranvier in alpha-motor and dorsal root ganglion neurons of the cat. J Neurocytol. 1988 Aug;17(4):531–544. doi: 10.1007/BF01189808. [DOI] [PubMed] [Google Scholar]
- Gatzinsky K. P., Berthold C. H. Lysosomal activity at nodes of Ranvier during retrograde axonal transport of horseradish peroxidase in alpha-motor neurons of the cat. J Neurocytol. 1990 Dec;19(6):989–1002. doi: 10.1007/BF01186826. [DOI] [PubMed] [Google Scholar]
- Giuliano K. A., Gillies R. J. Determination of intracellular pH of BALB/c-3T3 cells using the fluorescence of pyranine. Anal Biochem. 1987 Dec;167(2):362–371. doi: 10.1016/0003-2697(87)90178-3. [DOI] [PubMed] [Google Scholar]
- Hollenbeck P. J., Bray D. Rapidly transported organelles containing membrane and cytoskeletal components: their relation to axonal growth. J Cell Biol. 1987 Dec;105(6 Pt 1):2827–2835. doi: 10.1083/jcb.105.6.2827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hollenbeck P. J. Products of endocytosis and autophagy are retrieved from axons by regulated retrograde organelle transport. J Cell Biol. 1993 Apr;121(2):305–315. doi: 10.1083/jcb.121.2.305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hollenbeck P. J. The distribution, abundance and subcellular localization of kinesin. J Cell Biol. 1989 Jun;108(6):2335–2342. doi: 10.1083/jcb.108.6.2335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kano K., Fendler J. H. Pyranine as a sensitive pH probe for liposome interiors and surfaces. pH gradients across phospholipid vesicles. Biochim Biophys Acta. 1978 May 18;509(2):289–299. doi: 10.1016/0005-2736(78)90048-2. [DOI] [PubMed] [Google Scholar]
- Lee K. D., Nir S., Papahadjopoulos D. Quantitative analysis of liposome-cell interactions in vitro: rate constants of binding and endocytosis with suspension and adherent J774 cells and human monocytes. Biochemistry. 1993 Jan 26;32(3):889–899. doi: 10.1021/bi00054a021. [DOI] [PubMed] [Google Scholar]
- Martinez R., Gillies R. J., Giuliano K. A. Effect of serum on the intracellular pH of BALB/c-3T3 cells: serum deprivation causes changes in sensitivity of cells to serum. J Cell Physiol. 1988 Jul;136(1):154–160. doi: 10.1002/jcp.1041360120. [DOI] [PubMed] [Google Scholar]
- Matteoli M., Takei K., Perin M. S., Südhof T. C., De Camilli P. Exo-endocytotic recycling of synaptic vesicles in developing processes of cultured hippocampal neurons. J Cell Biol. 1992 May;117(4):849–861. doi: 10.1083/jcb.117.4.849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mellman I., Fuchs R., Helenius A. Acidification of the endocytic and exocytic pathways. Annu Rev Biochem. 1986;55:663–700. doi: 10.1146/annurev.bi.55.070186.003311. [DOI] [PubMed] [Google Scholar]
- Mundigl O., Matteoli M., Daniell L., Thomas-Reetz A., Metcalf A., Jahn R., De Camilli P. Synaptic vesicle proteins and early endosomes in cultured hippocampal neurons: differential effects of Brefeldin A in axon and dendrites. J Cell Biol. 1993 Sep;122(6):1207–1221. doi: 10.1083/jcb.122.6.1207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nedergaard M., Desai S., Pulsinelli W. Dicarboxy-dichlorofluorescein: a new fluorescent probe for measuring acidic intracellular pH. Anal Biochem. 1990 May 15;187(1):109–114. doi: 10.1016/0003-2697(90)90425-9. [DOI] [PubMed] [Google Scholar]
- Nelson N. The vacuolar H(+)-ATPase--one of the most fundamental ion pumps in nature. J Exp Biol. 1992 Nov;172:19–27. doi: 10.1242/jeb.172.1.19. [DOI] [PubMed] [Google Scholar]
- Ohkuma S., Poole B. Fluorescence probe measurement of the intralysosomal pH in living cells and the perturbation of pH by various agents. Proc Natl Acad Sci U S A. 1978 Jul;75(7):3327–3331. doi: 10.1073/pnas.75.7.3327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Owen C. S. Comparison of spectrum-shifting intracellular pH probes 5'(and 6')-carboxy-10-dimethylamino-3-hydroxyspiro[7H-benzo[c]xanthene-7, 1'(3'H)-isobenzofuran]-3'-one and 2',7'-biscarboxyethyl-5(and 6)-carboxyfluorescein. Anal Biochem. 1992 Jul;204(1):65–71. doi: 10.1016/0003-2697(92)90140-3. [DOI] [PubMed] [Google Scholar]
- Padh H., Ha J., Lavasa M., Steck T. L. A post-lysosomal compartment in Dictyostelium discoideum. J Biol Chem. 1993 Mar 25;268(9):6742–6747. [PubMed] [Google Scholar]
- Palmgren M. G. Acridine orange as a probe for measuring pH gradients across membranes: mechanism and limitations. Anal Biochem. 1991 Feb 1;192(2):316–321. doi: 10.1016/0003-2697(91)90542-2. [DOI] [PubMed] [Google Scholar]
- Parton R. G., Dotti C. G. Cell biology of neuronal endocytosis. J Neurosci Res. 1993 Sep 1;36(1):1–9. doi: 10.1002/jnr.490360102. [DOI] [PubMed] [Google Scholar]
- Parton R. G., Simons K., Dotti C. G. Axonal and dendritic endocytic pathways in cultured neurons. J Cell Biol. 1992 Oct;119(1):123–137. doi: 10.1083/jcb.119.1.123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sasaki S., Ishibashi K., Nagai T., Marumo F. Regulation mechanisms of intracellular pH of Xenopus laevis oocyte. Biochim Biophys Acta. 1992 Oct 6;1137(1):45–51. doi: 10.1016/0167-4889(92)90098-v. [DOI] [PubMed] [Google Scholar]
- Schuldiner S., Rottenberg H., Avron M. Determination of pH in chloroplasts. 2. Fluorescent amines as a probe for the determination of pH in chloroplasts. Eur J Biochem. 1972 Jan 31;25(1):64–70. doi: 10.1111/j.1432-1033.1972.tb01667.x. [DOI] [PubMed] [Google Scholar]
- Straubinger R. M., Papahadjopoulos D., Hong K. L. Endocytosis and intracellular fate of liposomes using pyranine as a probe. Biochemistry. 1990 May 22;29(20):4929–4939. doi: 10.1021/bi00472a025. [DOI] [PubMed] [Google Scholar]
- Sulzer D., Holtzman E. Acidification and endosome-like compartments in the presynaptic terminals of frog retinal photoreceptors. J Neurocytol. 1989 Aug;18(4):529–540. doi: 10.1007/BF01474548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swanson J. Fluorescent labeling of endocytic compartments. Methods Cell Biol. 1989;29:137–151. doi: 10.1016/s0091-679x(08)60192-2. [DOI] [PubMed] [Google Scholar]
- Thomas J. A., Buchsbaum R. N., Zimniak A., Racker E. Intracellular pH measurements in Ehrlich ascites tumor cells utilizing spectroscopic probes generated in situ. Biochemistry. 1979 May 29;18(11):2210–2218. doi: 10.1021/bi00578a012. [DOI] [PubMed] [Google Scholar]
- Tsien R. Y. Fluorescent indicators of ion concentrations. Methods Cell Biol. 1989;30:127–156. doi: 10.1016/s0091-679x(08)60978-4. [DOI] [PubMed] [Google Scholar]