To the Editor
The excellent article by Inamoto et al.(1), provides for the first time a careful and comprehensive description of joint and fascia (JF) manifestations as a prominent component of disability in patients with chronic graft-versus-host disease (cGVHD). They also provide needed data on the validity of used practical scales for joint assessments in cGVHD clinical trials: NIH joint/fascia (JF) scale(2) and Photographic Range of Motion (P-ROM) (3). However, since JF involvement is significantly associated with skin changes, particularly superficial and deep skin sclerosis, it remains unclear if isolated joint involvement exists in patients with cGVHD. To address this question we performed an analysis of patients (n=283) enrolled in the National Cancer Institute cross-sectional natural history study of cGVHD (NCT 00092235). Patients' characteristics can be seen in supplemental table 1. Overall, the prevalence of JF involvement in our cohort was much higher than reported by Inamoto et al. (60% vs. 29%); however when patients with clinical evidence of sclerotic cGVHD (scl JF) involvement were excluded, the prevalence of JF decreased to 15% (n=43). The distribution of severity for non-sclerosis JF cohort (NIH 0-3) was mild in 74% (n=32), moderate in 21% (n=9) and 5% (n=2) severe (figure 1). The most commonly affected joints in patients with non-scl JF were ankles (93%), followed by shoulders (19%), wrists and fingers (16%) and elbows (9%). Median ROM (% predicted) was significantly greater in non-scl JF vs scl JF, (67 vs. 25%, p<0.0001). In addition, non-scl JF patients had significantly shorter median time from HSCT to study enrollment and duration of cGVHD when compared to scl JF patients (29 vs. 45 months, p<0.0001 and 19 vs. 33 months, p=0.0005, respectively). Patients with non-scl JF performed significantly better on the Lee cGVHD Symptom Scale in the “Muscles and Joints 0-4” subscale than those with scl-JF (6 vs. 9, p=0.01) and significantly more patients with scores 3-4 were seen in scl JF group (57 vs. 27%, p=0.0004). Further, patients with scl JF had a history of more prior therapies for cGVHD than non-scl JF patients median (5.0 vs. 3.5, p<0.0001). Patients with scl-JF also had significantly elevated CRP (median 2.32 vs. 0.83 mg/L, p=0.006), total protein (6.5 vs. 6.2 g/dL, p=0.02), complement (147 vs. 127 g/L, p=0.008), C3 (146 vs. 127 g/L, p<0.0001) and C4 (29 vs. 24 g/L, p=0.0009) compared to non-scl JF patients. However, more patients with non-scl JF had anti-cardiolipin M antibodies compared to scl-JF (14 vs. 2.4%, p=0.009). In a stepwise multiple logistic regression analysis, shorter duration of cGVHD, lower maximum score (defined as maximum score, ranging from 0 to 3 in any organ) and lower loss of ROM classified 77% of patients as having isolated joint involvement without sclerotic changes.
Figure 1. Distribution of sclerotic and non-sclerotic skin in patients with joints and fascia cGVHD (n=169) according to NIH score.
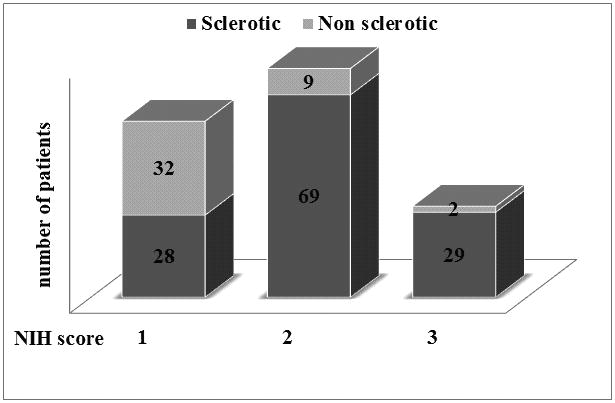
Distribution of sclerotic (dark grey) with non sclerotic (light grey) skin involvement in patients with joints and fascia involvement according to NIH score (1-3)
The NIH consensus criteria JF score does not distinguish the contributions to cGVHD severity made by isolated joint involvement vs. joint restriction associated with skin sclerosis (2). Although JF involvement is common in cGVHD, the incidence of isolated joint involvement in the absence of detectable superficial or subcutaneous skin sclerosis is low. In comparison to patients with overt sclerotic skin changes, patients with isolated joint involvement appear to have less functional disability, present earlier after cGVHD diagnosis and demonstrate lower levels of systemic markers of inflammation. Taken together these data suggest that isolated joint involvement in cGVHD is uncommon. Additional studies are needed to determine if joint involvement in the absence of sclerotic skin changes represents involvement of the deep tissues below the limit of clinical detection or represents a separate clinical process. Future refinements of the NIH criteria may need to recognize joint restrictions that occur in the absence of sclerotic skin involvement.
Supplementary Material
Footnotes
Preliminary data of this manuscript were presented at the Annual Tandem Meeting of ASBMT and CIBMT in February 2014 in Dallas Tx.
References
- 1.Inamoto Y, Pidala J, Chai X, Kurland BF, Weisdorf D, Flowers ME, et al. Joint and fascia manifestations in chronic graft-versus-host disease and their assessment. Arthritis Rheum. 2013:1–31. doi: 10.1002/art.38293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Filipovich AH, Weisdorf D, Pavletic S, Socie G, Wingard JR, Lee SJ, et al. National Institutes of Health Consensus Development Project on criteria for clinical trials in chronic graft-versus-host disease: I. diagnosis and staging working group report. Biology of Blood and Marrow Transplantation. 2005;11(12):945–56. doi: 10.1016/j.bbmt.2005.09.004. [DOI] [PubMed] [Google Scholar]
- 3.Carpenter PA. How I conduct a comprehensive chronic graft-versus-host disease assessment. 2011;118(10):2679–87. doi: 10.1182/blood-2011-04-314815. [DOI] [PubMed] [Google Scholar]
- 4.Lee S, Cook EF, Soiffer R, Antin JH. Development and validation of a scale to measure symptoms of chronic graft-versus-host disease. Biol Blood Marrow Transplant. 2002;8(8):444–52. doi: 10.1053/bbmt.2002.v8.pm12234170. [DOI] [PubMed] [Google Scholar]
- 5.Grkovic L, et al. Clinical laboratory markers of inflammation as determinants of chronic graft-versus-host disease activity and NIH global severity. Leukemia. 2012;26(4):633–643. doi: 10.1038/leu.2011.254. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


