Abstract
Neurocysticercosis, the most common helminthic infection of the nervous system, is a major cause of acquired epilepsy worldwide. The disease occurs when humans become intermediate hosts of the tapeworm Taenia solium after ingesting its eggs by contagion from an asymptomatic Taenia carrier. Within the nervous system, parasites may locate in brain parenchyma, subarachnoid space, ventricular system, or spinal cord, causing several pathological changes that are responsible for the clinical pleomorphism of the disease. Seizures are the most common clinical manifestation, but a sizable proportion of patients develop focal deficits, intracranial hypertension, or cognitive decline. Preoperative diagnosis of neurocysticercosis is possible after proper integration of data from neuroimaging studies and immunological tests. Cysticidal drugs (albendazole and praziquantel) have changed the prognosis of most patients with neurocysticercosis. The use of these drugs has shown to reduce the parasite load within the central nervous system and to improve the clinical prognosis of the disease in many cases. Future studies should focus on disease eradication through the implementation of control programs against all the interrelated steps in the life cycle of T solium, including human carriers of the adult tapeworm, infected pigs, and eggs in the environment.
Keywords: cysticercosis, neurocysticercosis, Taenia solium
Introduction
Neurocysticercosis is the most common helminthic infection affecting the central nervous system of humans. It is endemic in most of the developing world, where conditions favoring the transmission of this disease, including swine breeding under poor sanitary conditions, poverty, and illiteracy, are met. In these areas, neurocysticercosis is one of the most common causes of acquired epilepsy and a major public health problem.1–3 Neurocysticercosis is also becoming a health problem in some developed countries due to mass migration of people from areas where the disease is endemic.4–8 Although most patients with neurocysticercosis diagnosed in the United States and Europe are immigrants, the disease has also been diagnosed in native persons with no history of traveling abroad. Human cysticercosis is potentially eradicable. To be effective, eradication programs must be directed to all the targets for control, particularly human carriers of the adult tapeworm, infected pigs, and eggs in the environment.9 Since they represent interrelated steps in the life cycle of Taenia solium, inadequate coverage of one of them may result in a rebound in the prevalence of the disease after the program has been completed.
Etiopathogenesis
Life cycle of T solium
Two hosts are involved in the life cycle of this cestode, humans and pigs. Humans are definitive hosts for the adult tapeworm, whereas both pigs and humans may be intermediate hosts for the larval form (cysticercus). In the usual cycle of transmission, a few gravid proglottids from the adult tapeworm—attached to the intestinal wall of humans—are passed every few days with feces. Each proglottid liberates thousands of infective eggs to the environment. In areas where feces disposal is deficient and swine husbandry is poor, pigs have access to human feces containing T solium eggs. Once in the intestinal tract of the pig, eggs liberate oncospheres that cross the intestinal wall and enter the bloodstream, from where they are carried to the tissues to mature into metacestodes and then into larvaes (cysticercus). The life cycle is completed when humans ingest improperly cooked infected pork and cysticerci—by action of digestive enzymes—are released, their scolices evaginate, attach to the intestinal wall, and proglottids begin to multiply until the adult tapeworm is formed.10
Humans can also become intermediate hosts of T solium after ingesting its eggs. Under this circumstance, human cysticercosis develops. Recent epidemiological evidence showing clustering of patients with cysticercosis around tapeworm carriers has changed previous concepts crediting the environment as the main source of human contamination with T solium eggs. Therefore, human cysticercosis must be seen as a disease mostly transmitted from person to person and the role of infected pigs is to perpetuate the infection only.11
Characteristics of Cysticerci
Cysticerci consist of main portions, the vesicular wall and the scolex. Viable cysticerci have a transparent membrane, a clear vesicular fluid, and an invaginated scolex with a similar structure compared to the adult T solium. This stage, which has been called “vesicular stage,” is seen after the arrival of most cysticerci to the central nervous system. Cysticerci may remain in such stage for years or may—as the result of an immunological attack from the host—enter in a process of degeneration that ends with the death of the parasite and its transformation into a mineralized nodule (“calcified stage”). From the vesicular to the calcified stage, cysticerci often go through 2 intermediate stages of involution that have been called “colloidal” and “granular,” respectively. It seems that this orderly process of degeneration is not followed by all parasites, as some may be destroyed early after entering the central nervous system (even in their early metacestodes phase) and go directly to the granular stage without going first through the vesicular and colloidal stages.12 Arguments favoring this latter hypothesis include the low sensitivity of serological assays in patients with a single cysticercus granuloma and the younger age of patients with single cysticercal granuloma versus that of those with single vesicular cyst.
Inflammatory Changes Induced by Cysticerci
In general, vesicular cysticerci located in the brain parenchyma elicit little or no inflammatory reaction. On the other hand, cysticerci in the colloidal stage are surrounded by a collagen capsule and a mononuclear inflammatory reaction that includes the parasite itself; astrocytic gliosis, microglial proliferation, edema, neuronal degenerative changes, and perivascular cuffing of lymphocytes are commonly observed in the surrounding brain parenchyma. The edema subsides when parasites enter into the granular and calcified stages, but astrocytic changes in the vicinity of the lesions may become more severe and epithelioid cells appear and coalesce to form multinucleated giant cells.13
Meningeal cysticerci often elicit an intense inflammatory reaction in the subarachnoid space with formation of a dense exudate composed of collagen fibers, lymphocytes, multinucleated giant cells, eosinophils, and hyalinized parasitic membranes that lead to abnormal thickening of the leptomeninges. This inflammatory reaction may be disseminated, damaging the optic chiasm, cranial nerves arising from the brainstem, and penetrating arteries arising from the circle of Willis. The latter may result in the development of a cerebral infarction.14 The foramina of Luschka and Magendie may be occluded by the thickened leptomeninges or by parasitic membranes with the subsequent occurrence of obstructive hydrocephalus. Ventricular cysticerci may also elicit an inflammatory reaction if they are attached to the choroid plexus or to the ventricular wall. In such cases, the disrupted ependymal lining may protrude toward the ventricular cavities blocking cerebrospinal fluid (CSF) transit when the site of protrusion is near the foramina of Monro or the cerebral aqueduct.13
Clinical Manifestations
Diversity of clinical manifestations of neurocysticercosis is related to differences in the number and location of the lesions within the nervous system and to the intensity of the host’s immune response against the parasites. In many cases, the infection is asymptomatic as has been demonstrated in population-based studies in areas where cysticercosis is endemic, where a sizable proportion of persons with neurocysticercosis only have evidence of the disease in neuroimaging studies.15
Seizures are the most common clinical form of presentation of neurocysticercosis, occurring in up to 80% of symptomatic infections.16 Indeed, neurocysticercosis partly explains the increased prevalence of epilepsy in the developing world and has been considered one of the leading causes of epilepsy starting in individuals aged 25 years or older.17
Although it was formerly believed that neurocysticercosis-related seizures occurred almost exclusively when parasites began to degenerate, more recent studies have documented that seizures may occur at any stage of cysticerci involution, from viable cysts to calcifications. Mechanisms of epileptogenesis in these cases vary according to the stage of parasites. Viable cysts most likely induce seizures due to compressive effects on the brain parenchyma, while colloidal and granular cysts induce seizures as a result of the inflammatory reaction associated with the attack of the host immune system to the parasites. In calcified lesions, the gliosis that develops around death parasites, as well as exposure of antigenic material to the brain parenchyma or even the development of hippocampal sclerosis, may account for their epileptogenic activity.18,19
Focal neurological signs have been described in 5% to 15% of patients with neurocysticercosis and vary according to the location of the parasites within the central nervous system.17 Neurocysticercosis-related focal signs often follow a chronic course resembling that of a brain tumor or a focal granulomatous process of other etiology (ie, tuberculomas) and are most often seen in patients with large subarachnoid cysts compressing the brain.20 Acute stroke syndromes have been described in some cases. These are often related to cerebral infarctions located in the posterior limb of the internal capsule, the corona radiate, or the brainstem.14 Some patients with neurocysticercosis develop intracranial hypertension, which may be related to the occurrence of hydrocephalus due to arachnoiditis, granular ependymitis, or ventricular cysts or to cysticercotic encephalitis, a severe form of the disease that occurs as a result of massive cysticerci infection of the brain parenchyma inducing an intense immune reaction from the host.10
Some other patients with neurocysticercosis present psychiatric complaints that may range from poor cognitive performance to severe dementia.21 Patients with intrasellar cysticerci present with ophthalmologic and endocrinologic manifestations.22 Spinal arachnoiditis is characterized by root pain and weakness of subacute onset, and cysts in the spinal cord parenchyma usually present with motor and sensory deficits that vary according to the level of the lesion.23 Intraocular cysticerci cause progressive decrease in visual acuity or visual field defects related to vitritis, uveitis, or endophthalmitis.24
Diagnosis
Introduction of computed tomography (CT) and magnetic resonance imaging (MRI) changed the diagnostic accuracy for neurocysticercosis, as they provide evidence on the number and topography of lesions and their stage of involution.25 Vesicular (viable) cysticerci appear as small and rounded cysts that are well delineated from the brain parenchyma. There is no edema or abnormal enhancement. A sizable proportion of those cysts have in their interior an eccentric hyperdense nodule representing the scolex, giving them “hole-with-dot” appearance, which is pathognomonic of neurocysticercosis (Figure 1). Colloidal and granular (degenerating) cysticerci appear as single or multiple ill-defined lesions surrounded by edema, and most of them present with a ring or a nodular pattern of enhancement after contrast medium administration.26 These lesions are difficult to differentiate from other space-occupying intracranial lesions such as tumors or tuberculous granulomas (Figure 2). Calcified (dead) cysticerci appear on CT as small hyperdense nodules without perilesional edema or abnormal contrast enhancement (Figure 3). These lesions may be not well identified with MRI, although with the use of more powerful machines (1.5 Tesla or superior), calcifications are easily visualized as small hypointense dots.
Figure 1.
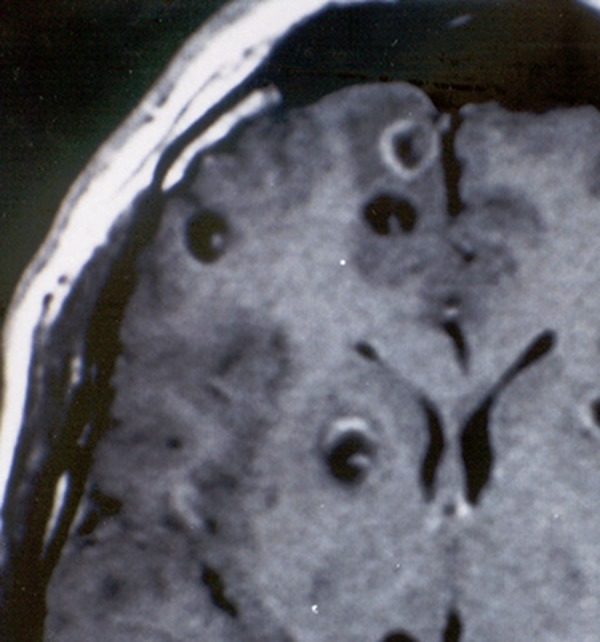
Contrast-enhanced, T1-weighted magnetic resonance imaging (MRI) showing rounded cystic lesions with an eccentric bright dot representing the scolex. This is the single pathognomonic imaging finding of neurocysticercosis.
Figure 2.

Contrast-enhanced computed tomography (CT) showing a degenerating cysticercus in left temporal lobe. The lesion is almost indistinguishable from that seen in patients with brain tumors of focal granulomatous lesions of other etiology.
Figure 3.

Plain computed tomography (CT) showing parenchymal brain calcifications representing death cysticerci.
The most common neuroimaging finding in subarachnoid neurocysticercosis is hydrocephalus caused by occlusion of the foraminae of Luschka and Magendie. The basal arachnoiditis that is responsible for the occurrence of hydrocephalus is visualized as patchy or diffuse areas of abnormal leptomeningeal enhancement. Parasites located within the CSF cisterns at the base of the brain often have a multilobulated appearance and behave as space-occupying lesions.20 Magnetic resonance angiography is of value to demonstrate segmental narrowing or even occlusion of medium-size intracranial arteries in some patients with subarachnoid neurocysticercosis.14 Ventricular cysticerci may not be visualized on CT but can be inferred on the basis of distortion on the shape of the ventricular cavities causing asymmetric hydrocephalus.27 In contrast, most ventricular cysts are visualized on MRI because their signal properties differ from that of the CSF.28 The occurrence of the so-called “ventricular migration sign,” which is related to the mobility of the ventricular cyst within the ventricular cavities in response to movements of the head, facilitates the diagnosis in some cases. Intramedullary cysticerci appear on MRI as rounded lesions that may have an eccentric hyperintense nodule representing the scolex.29 These lesions often enlarge the diameter of the spinal cord. Leptomeningeal cysts may be mobile within the spinal subarachnoid space and may change their position during the examination according to movements of the patient in the exploration table.
Antibodies to species-specific antigens of T solium can be detected by enzyme-linked immunoelectrotransfer blot assay. This finding encouraged researchers to develop highly purified antigens of cysticercus to be used in a reliable immune diagnostic test—the immunoblot—for cysticercosis.30 However, a weakness of the immunoblot is that it may be false negative in about 50% of patients with a single cerebral cyst or in those with calcifications alone.26 Another weakness is that it may be false positive in persons who had been exposed to the adult parasite without developing the disease.26 Detection of circulating antigens of the parasite by the use of monoclonal antibodies has a poor sensitivity as a screening tool for the diagnosis of neurocysticercosis; however, antigen detection may be useful to monitor the response to therapy.31
Despite the important advances in neuroimaging and immune diagnostic tests, the diagnosis of neurocysticercosis may be a challenge. As noted, clinical manifestations are nonspecific, neuroimaging findings are often not pathognomonic, and immune diagnostic tests are faced with problems related to poor specificity and sensitivity. A set of diagnostic criteria based on the evaluation of clinical, radiological, immunological, and epidemiological data have been proposed to provide the elements needed to diagnose patients with suspected neurocysticercosis.32 The set includes 4 categories of diagnostic criteria—absolute, major, minor, and epidemiologic—stratified according to their individual strength. Absolute criteria allow unequivocal diagnosis of neurocysticercosis, major criteria strongly suggest the diagnosis but cannot be used alone to confirm the disease, minor criteria are frequent but nonspecific manifestations of the disease, and epidemiologic criteria refer to circumstantial evidence favoring the diagnosis of cysticercosis. Interpretation of these criteria results in 2 categories of diagnostic certainty—definitive and probable—according to the likelihood that neurocysticercosis is present in a given patient (Table 1).
Table 1.
Diagnostic Criteria for Neurocysticercosis.a
| Diagnostic criteria |
|---|
| Absolute criteria |
| • Histologic demonstration of the parasite from biopsy of a brain or spinal cord lesion |
| • Evidence of cystic lesions showing the scolex on neuroimaging studies |
| • Direct visualization of subretinal parasites by fundoscopic examination |
| Major criteria |
| • Evidence of lesions highly suggestive of neurocysticercosis on neuroimaging studies |
| • Positive serum immunoblot for the detection of anticysticercal antibodies |
| • Resolution of intracranial cystic lesions after therapy with albendazole or praziquantel |
| • Spontaneous resolution of small single enhancing lesions |
| Minor criteria |
| • Evidence of lesions suggestive of neurocysticercosis on neuroimaging studies |
| • Presence of clinical manifestations suggestive of neurocysticercosis |
| • Positive CSF ELISA for detection of anticysticercal antibodies or cysticercal antigens |
| • Evidence of cysticercosis outside the central nervous system |
| Epidemiologic criteria |
| • Individuals coming from or living in an area where cysticercosis is endemic |
| • History of frequent travel to disease-endemic areas |
| • Evidence of a household contact with Taenia solium infection |
| Degrees of diagnostic certainty |
| Definitive diagnosis |
| • Presence of 1 absolute criterion |
| • Presence of 2 major plus 1 minor or 1 epidemiologic criteria |
| Probable diagnosis |
| • Presence of 1 major plus 2 minor criteria |
| • Presence of 1 major plus 1 minor and 1 epidemiologic criteria |
| • Presence of 3 minor plus 1 epidemiologic criteria |
Abbreviations: CSF, cerebrospinal fluid; ELISA, enzyme-linked immunosorbent assay.
a Adapted from Del Brutto et al.32
Treatment
Owing to the clinical and pathological pleomorphism of neurocysticercosis, a single approach is not expected to be useful in all cases. The disease must be characterized in terms of location and number of lesions within the nervous system, cysts’ viability, and severity of the host’s immune response to the parasite.33 Therapy often includes a combination of symptomatic and cysticidal drugs. Surgery still has a role in selected forms of the disease (Table 2).
Table 2.
General Guidelines for Therapy of Neurocysticercosis.a
| Parenchymal neurocysticercosis |
|---|
| Vesicular cysts |
| • Single cyst: albendazole 15 mg/kg/d for 3 days or praziquantel 30 mg/kg in 3 divided doses every 2 hours. Corticosteroids rarely needed. AED for seizures |
| • Mild to moderate infections: albendazole 15 mg/kg/d for 1 week or praziquantel 50 mg/kg/d for 15 days. Corticosteroids may be used when necessary. AED for seizures |
| • Heavy infections: albendazole 15 mg/kg/d for 1 week (repeated cycles of albendazole may be needed). Corticosteroids are mandatory before, during, and after therapy. AED for seizures |
| Colloidal cysts |
| • Single cyst: albendazole 15 mg/kg/d for 3 days or praziquantel 30 mg/kg in 3 divided doses every 2 hours. Corticosteroids may be used when necessary. AED for seizures |
| • Mild to moderate infections: albendazole 15 mg/kg/d for 1 week. Corticosteroids are usually needed before and during therapy. AED for seizures |
| • Cysticercotic encephalitis: cysticidal drugs are contraindicated. Corticosteroids and osmotic diuretics to reduce brain swelling. AED for seizures. Decompressive craniectomies in refractory cases |
| Granular and calcified cysticerci |
| • Single or multiple: no need for cysticidal drug therapy. AED for seizures. Corticosteroids for patients with recurrent seizures and perilesional edema surrounding calcifications |
| Extraparenchymal neurocysticercosis |
| Small cysts over convexity of cerebral hemispheres |
| • Single or multiple: albendazole 15 mg/kg/d for 1 week. Corticosteroids may be used when necessary. AED for seizures |
| Large cysts in Sylvian fissures or basal CSF cisterns |
| • Racemose cysticercus: albendazole, 15 to 30 mg/kg/d for 15 to 30 days (repeated cycles of albendazole may be needed). Corticosteroids are mandatory before, during, and after therapy |
| Other forms of extraparenchymal neurocysticercosis |
| • Hydrocephalus: no need for cysticidal drugs therapy. Ventricular shunt. Continuous corticosteroid administration (50 mg 3 times a week for up to 2 years) may be needed to reduce the rate of shunt dysfunction |
| • Ventricular cysts: endoscopic resection of cysts. Albendazole may be used only in small lesions located in lateral ventricles. Ventricular shunt only needed in patients with associated ependymitis |
| • Angiitis, chronic arachnoiditis: no need for cysticidal drug therapy. Corticosteroids are mandatory |
| • Cysticercosis on the spine: surgical resection of lesions. Anecdotal use of albendazole with good results |
Abbreviations: AED, antiepileptic drug; CSF, cerebrospinal fluid.
a Level 1 of evidence favors the use of cysticidal drugs in patients with parenchymal brain vesicular and colloidal cysts. For other forms of the disease, guidelines are based on levels 2 and 3 of evidence). Adapted with permission from Del Brutto. Neurocysticercosis. Continuum (Minneap, Minn). 2012;18:1392-1416.
The prognosis of a sizable proportion of neurocysticercosis patients improved after the introduction—more than 30 years ago—of cysticidal drugs. Praziquantel came first and it was soon followed by albendazole. The initial regimen of praziquantel therapy, at doses of 50 mg/kg/d given at 3 divided doses (every 8 hours) for 15 days, was arbitrarily chosen.34 Thereafter, it was suggested that if cysticerci are exposed to high concentrations of praziquantel for up to 6 hours by giving 3 individual doses of 25 to 30 mg/kg at 2-hour intervals, this might be sufficient to destroy cysticerci. Although initial results with this single-day regimen were promising,35 it seems that this works better for patients with a single parenchymal brain cyst and that the traditional 15-day trial should be used for those with more than 1 cyst.36 Albendazole was initially administered at doses of 15 mg/kg/d during 1 month.37 However, further studies showed that similar results may be obtained when the length of therapy is shortened to 1 week38 and even to 3 days if the patient has a single parenchymal brain cyst.39 Albendazole has been shown to be superior to praziquantel in some trials comparing the efficacy of these drugs.40,41 Another advantage of albendazole is that it also destroys subarachnoid and ventricular cysts.42 In some patients with these forms of the disease, higher doses (up to 30 mg/kg/d) or more prolonged, or even repeated, courses of the drug may be necessary.43,44
Despite the favorable results observed in several clinical trials, the use of cysticidal drugs has been questioned, leading to confusion and incorrect management decisions. For example, it has been suggested that cysticidals destroy the cysts but do not modify the clinical course of the disease.45 A more recent placebo-controlled trial showed that albendazole was effective for therapy of viable parenchymal brain cysticerci,46 and other well-designed studies have shown that the prognosis of patients with colloidal parenchymal brain cysts was better after therapy than when the disease was left untreated, providing evidence that favors the use of cysticidal drugs in patients with parenchymal brain cysticerci.47–49 Moreover, some meta-analysis of randomized trials evaluated the clinical outcome and the results on neuroimaging studies in patients with parenchymal neurocysticercosis, confirming that published evidence indicates that cysticidal drug therapy results in better resolution of both colloidal and vesicular cysticerci, in a lower risk of seizure recurrence in patients with colloidal cysticerci, and in a reduction in the rate of generalized seizures in patients with vesicular cysticerci.50–52 More recently, a panel from the American Academy of Neurology revised data to issue a guideline document and concluded that albendazole plus corticosteroids should be considered for patients with neurocysticercosis, as the use of these drugs reduce the number of viable cysts on control neuroimaging studies (level B of evidence) and the long-term risk of seizure recurrence (level B evidence).53
On the other hand, it is important to remember that some forms of neurocysticercosis should not be treated with cysticidal drugs, as their use may exacerbate the syndrome of intracranial hypertension observed in patients with cysticercotic encephalitis or in those with hydrocephalus. In the latter, cysticidal drugs may be used only after a ventricular shunt has been placed (only if the patient has associated viable cysts) to avoid further increases in the intracranial pressure as a result of therapy. Cysticidal drugs must be used with caution in patients with giant subarachnoid cysticerci because the inflammation developed by the host in response to destruction of parasites may occlude leptomeningeal vessels surrounding the cyst. In such cases, concomitant steroid administration will avoid the hazard of an ischemic stroke. In patients with ventricular cysts, the use of cysticidal drugs should be restricted for 2 main reasons: (1) the inflammatory reaction that often surrounds dying cysts may cause acute hydrocephalus if those lesions are located within the fourth ventricle or near the foraminae of Monro or the cerebral aqueduct and (2) new neuroendoscopic techniques allow resection of parasites at minimal risk of the patient.54
The use of antiepileptic drug (AED) often results in the control of seizures in patients with neurocysticercosis-related epilepsy.55 However, the optimal length of AED therapy in patients with neurocysticercosis has not been established. It has been suggested that short-term (3-6 months) use of AEDs—while inflammation around a cyst still exists—may be enough. No controlled evidence exists to support this claim and standard guidelines on the use of AEDs must be followed. After seizure remission and appropriate tapering of AEDs, a sizable proportion of patients will present further seizure episodes and require reinstalling medication for longer time.56 Prognostic factors associated with seizure recurrence include the development of calcifications and the presence of both recurrent seizures and multiple brain cysts before the start of therapy.57
Footnotes
Declaration of Conflicting Interests: The author declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author received no financial support for the research, authorship, and/or publication of this article.
References
- 1. Del Brutto OH, Santibañez R, Idrovo L, et al. Epilepsy and neurocysticercosis in Atahualpa: a door-to-door survey in rural coastal Ecuador. Epilepsia. 2005;46(4):583–587 [DOI] [PubMed] [Google Scholar]
- 2. Medina MT, Duron RM, Martinez L. Prevalence, incidence, and etiology of epilepsies in rural Honduras: the Salama study. Epilepsia. 2005;46(1):124–131 [DOI] [PubMed] [Google Scholar]
- 3. Montano SM, Villaran MV, Ylquimiche L, et al. Neurocysticercosis. Association between seizures, serology, and brain CT in rural Peru. Neurology. 2005;65(2):229–234 [DOI] [PubMed] [Google Scholar]
- 4. Del Brutto OH, Garcia HH. Neurocysticercosis in nonendemic countries: time for a reappraisal. Neuroepidemiology. 2012;39(2):145–146 [DOI] [PubMed] [Google Scholar]
- 5. Del Brutto OH. A review of cases of human cysticercosis in Canada. Can J Neurol Sci. 2012;39(3):319–322 [DOI] [PubMed] [Google Scholar]
- 6. Del Brutto OH. Neurocysticercosis in Western Europe. A re-emerging disease? Acta Neurol Belg. 2012;112(4):335–343 [DOI] [PubMed] [Google Scholar]
- 7. Serpa JA, Graviss EA, Kass JS, White AC., Jr. Neurocysticercosis in Houston, Texas: an update. Medicine (Baltimore). 2011;90(1):81–86 [DOI] [PubMed] [Google Scholar]
- 8. Sorvillo F, Wilkins P, Shafir S, Eberhard M. Public health implications of cysticercosis acquired in the United States. Emer Infect Dis. 2011;17(1):1–6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Garcia HH, Gonzalez AE, Del Brutto OH, et al. Strategies for elimination of taeniasis/cysticercosis. Am J Trop Med Hyg. 2007;262(1-2):153–157 [DOI] [PubMed] [Google Scholar]
- 10. García HH, Del Brutto OH. Neurocysticercosis: updated concepts about an old disease. Lancet Neurol. 2005;4(10):653–661 [DOI] [PubMed] [Google Scholar]
- 11. Lescano AG, Garcia HH, Gilman RH, et al. Taenia solium cysticercosis hotspots surrounding tapeworm carriers: clustering on human seroprevalence but not on seizures. PLoS Negl Trop Dis. 2009;3(1):e371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Garcia HH, Gonzalez AE, Rodriguez S, et al. Neurocysticercosis. Unraveling the nature of the single cysticercal granuloma. Neurology. 2010;75(7):654–658 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Pittella JE. Neurocysticercosis. Brain Pathol. 1997;7(1):681–693 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Marquez JM, Arauz A. Cerebrovascular complications of neurocysticercosis. Neurologist. 2012;18(1):17–22 [DOI] [PubMed] [Google Scholar]
- 15. Winkler AS. Neurocysticercosis in sub-Saharan Africa: a review of prevalence, clinical characteristics, diagnosis, and management. Pathog Glob Health. 2012;106(5):261–274 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Ndimubanzi PC, Carabin H, Budke CM, et al. A systematic review of the frequency of neurocysticercosis with a focus on people with epilepsy. PLoS Negl Trop Dis. 2010;4(11):e870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Carabin H, Ndimubanzi PC, Budke CM, et al. Clinical manifestations associated with neurocysticercosis: a systematic review. PLoS Negl Trop Dis. 2011;5(5):e1152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Nash TE, Del Brutto OH, Butman JA, et al. Calcified neurocysticercosis and epileptoenesis. Neurology. 2004;62(11):1934–1938 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Rathore C, Radhakrishnan K. What causes seizures in patients with calcified neurocysticercal lesions. Neurology. 2012;78(9):612–613 [DOI] [PubMed] [Google Scholar]
- 20. Fleury A, Carrillo-Mezo R, Flisser A, Sciutto E, Corona T. Subarachnoid basal neurocysticercosis: a focus on the most severe form of the disease. Expert Rev Anti-Infect Ther. 2011;9(1):123–133 [DOI] [PubMed] [Google Scholar]
- 21. Forlenza O, Vieira AH, Nobrea JPS, et al. Psychiatric manifestations in neurocysticercosis: a study of 38 patients from a neurologic clinics in Brazil. J Neurol Neurosurg Psychiatry. 1997;62(6):612–616 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Del Brutto OH, Del Brutto VJ. Intrasellar cysticercosis: a systematic review. Acta Neurol Belg. 2013;113(3):225–227 [DOI] [PubMed] [Google Scholar]
- 23. Chhiber SS, Singh B, Bansal P, Pandita KK, Razdan S, Singh J. Intramedullary spinal cysticercosis cured with medical therapy: case report and review of the literature. Surg Neurol. 2009;72(6):765–769 [DOI] [PubMed] [Google Scholar]
- 24. Madiubba S, Vishwanath K, Reddy G, Vemuganti GK. Changing trends in ocular cysticercosis over two decades: an analysis of 118 surgically excised cysts. Indian J Med Microbiol. 2007;25(3):214–219 [DOI] [PubMed] [Google Scholar]
- 25. Garcia HH, Del Brutto OH, Nash TE, et al. New concepts in the diagnosis and management of neurocysticercosis (Taenia solium). Am J Trop Med Hyg. 2005;72(1):3–9 [PubMed] [Google Scholar]
- 26. Singh G, Rajshekhar V, Murthy JM, et al. A diagnostic and therapeutic scheme for a solitary cysticercus granuloma. Neurology. 2010;75(24):2236–2245 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Madrazo I, García-Rentería JA, Sandoval M, López Vega FJ. Intraventricular cysticercosis. Neurosurgery. 1983;12(2):148–152 [DOI] [PubMed] [Google Scholar]
- 28. Do Amaral LLF, Ferreira RM, da Rocha AJ, Ferreira NP. Neurocysticercosis. Evaluation with advances magnetic resonance techniques and atypical forms. Top Mag Reson Imaging. 2005;16(2):127–144 [DOI] [PubMed] [Google Scholar]
- 29. Del Brutto OH, Garcia HH. Intramedullary cysticercosis of the spinal cord: a review of patients evaluated with MRI. J Neurol Sci. 2013;331(1-2):114–117 [DOI] [PubMed] [Google Scholar]
- 30. Tsang VC, Brand JA, Boyer AE. An enzyme-linked immunoelectrotransfer blot assay and glycoprotein antigens for diagnosing human cysticercosis (Taenia solium). J Infect Dis. 1989;159(1):50–59 [DOI] [PubMed] [Google Scholar]
- 31. Mahanty S, Garcia HH. Cysticercosis and neurocysticercosis as pathogens affecting the nervous system. Progress Neurobiol. 2010;91(2):172–184 [DOI] [PubMed] [Google Scholar]
- 32. Del Brutto OH, Rajshekhar V, White AC, Jr, et al. Proposed diagnostic criteria for neurocysticercosis. Neurology. 2001;57(2):177–183 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Garcia HH, Evans CA, Nash TE, et al. Current consensus guidelines for treatment of neurocysticercosis. Clin Microbiol Rev. 2002;15(4):747–756 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Sotelo J, Escobedo F, Rodriguez Carbajal J, Torres B, Rubio-Donnadieu F. Therapy of parenchymal brain cysticercosis with praziquantel. N Engl J Med. 1984;310(16):1001–1007 [DOI] [PubMed] [Google Scholar]
- 35. Del Brutto OH, Campos X, Sánchez J, Mosquera A. Single-day praziquantel versus 1-week albendazole for neurocysticercosis. Neurology. 1999;52(5):1079–1081 [DOI] [PubMed] [Google Scholar]
- 36. Pretell EJ, Garcia HH, Gilman RH, Saavedra H, Martinez M; Cysticercosis Working Group in Peru. Failure of one-day praziquantel treatment in patients with multiple neurocysticercosis lesions. Clin Neurol Neurosurg. 2001;103(3):175–177 [DOI] [PubMed] [Google Scholar]
- 37. Escobedo F, Penagos P, Rodriguez J, Sotelo J. Albendazole therapy for neurocysticercosis. Arch Intern Med. 1987;147(4):738–741 [PubMed] [Google Scholar]
- 38. Garcia HH, Gilman RH, Horton J, et al. Albendazole therapy for neurocysticercosis: a prospective double-blind trial comparing 7 versus 14 days of treatment. Neurology. 1997;48(5):1421–1427 [DOI] [PubMed] [Google Scholar]
- 39. Bustos JA, Pretell EJ, Llanos-Zavalaga F, et al. Efficacy of a 3-day course of albendazole treatment in patients with a single neurocysticercosis cyst. Clin Neurol Neurosurg. 2006;108(2):193–194 [DOI] [PubMed] [Google Scholar]
- 40. Sotelo J, Del Brutto OH, Penagos P, et al. Comparison of therapeutic regimen of anticysticercal drugs for parenchymal brain cysticercosis. J Neurol. 1990;237(2):69–72 [DOI] [PubMed] [Google Scholar]
- 41. Takayanagui OM, Jardim E. Therapy for neurocysticercosis: comparison between albendazole and praziquantel. Arch Neurol. 1992;49(3):290–294 [DOI] [PubMed] [Google Scholar]
- 42. Del Brutto OH. Albendazole therapy for subarachnoid cysticerci: clinical and neuroimaging findings of 17 patients. J Neurol Neurosurg Psychiatry. 1997;62(6):659–661 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Gongora-Rivera F, Soto-Hernandez JL, Gonzalez-Esquivel D, et al. Albendazole trial at 15 or 30 mg/kg/day for subarachnoid and intraventricular cysticercosis. Neurology. 2006;66(3):436–438 [DOI] [PubMed] [Google Scholar]
- 44. Proaño JV, Madrazo I, Avelar F, López-Félix B, Díaz G, Grijalva I. Medical treatment for neurocysticercosis characterized by giant subarachnoid cysts. N Engl J Med. 2001;345(12):879–885 [DOI] [PubMed] [Google Scholar]
- 45. Salinas R, Counsell C, Prasad K, Gelband H, Garner P. Treating neurocysticercosis medically: a systematic review of randomized, controlled trials. Trop Med Int Health. 1999;4(11):713–718 [DOI] [PubMed] [Google Scholar]
- 46. Garcia HH, Pretell EJ, Gilman RH, et al. A trial of antiparasitic treatment to reduce the rate of seizures due to cerebral cysticercosis. N Engl J Med. 2004;350(3):249–258 [DOI] [PubMed] [Google Scholar]
- 47. Baranwal AK, Singhi PD, Khandelwal N, Singhi SC. Albendazole therapy in children with focal seizures and single small enhancing computerized tomoraphic lesions: a randomized, placebo-controlled, double-blind trial. Pediatr Infect Dis J. 1998;17(8):696–700 [DOI] [PubMed] [Google Scholar]
- 48. Gogia S, Talikdar B, Choudhury V, Arora BS. Neurocysticercosis in children: clinical findings and response to albendazole therapy in a randomized, double-blind, placebo-controlled trial in newly diagnosed cases. Trans R Soc Trop Med Hyg. 2003;97(4):416–421 [DOI] [PubMed] [Google Scholar]
- 49. Kalra V, Dua T, Kumar V. Efficacy of albendazole and short-course dexamethasone treatment in children with 1 or 2 ring-enhancing lesions of neurocysticercosis: a randomized controlled trial. J Pediatr. 2003;143(1):111–114 [DOI] [PubMed] [Google Scholar]
- 50. Del Brutto OH, Roos K, Coffey CS, García HH. Meta-analysis: cysticidal drugs for neurocysticercosis: albendazole and praziquantel. Ann Intern Med. 2006;145(1):43–51 [DOI] [PubMed] [Google Scholar]
- 51. Mazumdar M, Pandharipande P, Poduri A. Does albendazole affect seizure remission and computed tomography response in children with neurocysticercosis? A systematic review and meta-analysis. J Child Neurol. 2007;22(2):135–142 [DOI] [PubMed] [Google Scholar]
- 52. Abba K, Ramaratnam S, Ranganathan LN. Anthelminthics for people with neurocysticercosis. Cochrane Database Syst Rev. 2010;(1):CD000215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Baird RA, Zunt JR, Halperin JJ, Gronseth G, Roos KL. Evidence-based guideline: treatment of parenchymal neurocysticercosis. Report of the guideline development subcommittee of the American academy of neurology. Neurology. 2013;80(15):1424–1429 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Sinha S, Sharma BS. Intraventricular neurocysticercosis: a review of current status and management issues. Br J Neurosurg. 2012;26(3):305–309 [DOI] [PubMed] [Google Scholar]
- 55. Del Brutto OH, Santibáñez R, Noboa CA, et al. Epilepsy due to neurocysticercosis: analysis of 203 patients. Neurology. 1992;42(2):389–392 [DOI] [PubMed] [Google Scholar]
- 56. Del Brutto OH. Prognostic factors for seizure recurrence after withdrawal of antiepileptic drugs in patients with neurocysticercosis. Neurology. 1994;44(9):1706–1709 [DOI] [PubMed] [Google Scholar]
- 57. Nash TE, Pretell EJ, Lescano AG, et al. Perilesional brain oedema and seizure activity in patients with calcified neurocysticercosis: a prospective cohort and nested case-control study. Lancet Neurol. 2008;7(12):1099–1105 [DOI] [PMC free article] [PubMed] [Google Scholar]


