Abstract
Poliomyelitis is a highly infectious disease caused by a virus belonging to the Picornaviridae family. It finds a mention even in ancient Egyptian paintings and carvings. The clinical features are varied ranging from mild cases of respiratory illness, gastroenteritis, and malaise to severe forms of paralysis. These have been categorized into inapparent infection without symptoms, mild illness (abortive poliomyelitis), aseptic meningitis (nonparalytic poliomyelitis), and paralytic poliomyelitis. This disease has been associated with crippling deformities affecting thousands of lives throughout the world. Only due to the perseverance and determination of great scientists in 1900s, the genomic structure of the virus and its pathogenesis could be elucidated. Contribution of Salk and Sabin in the form of vaccines—oral polio vaccine (OPV) and the inactivated polio vaccine—heralded a scientific revolution. In 1994, the World Health Organization (WHO) Region of The Americas was certified polio free followed by the WHO Western Pacific Region in 2000 and the WHO European Region in June 2002 of the 3 types of wild poliovirus (types 1, 2, and 3). In 2013, only 3 countries remained polio endemic—Nigeria, Pakistan, and Afghanistan. Global eradication of polio is imperative else the threat of an outbreak will hover forever. Today, all the governments of the world in collaboration with WHO stand unified in their fight against poliomyelitis and the task when achieved will pave the way for eliminating other infections in future.
Keywords: poliomyelitis, infectious disease medicine, epidemiology
Introduction
Eradication of polio is a success story for medicine and public health and teaches us much about how to combat infectious diseases. Poliovirus has been used as a model virus because a large body of research data exists on the physical, chemical, and biological properties of the virus, vaccination is available, and it is easy to culture as compared to other viruses. The word poliomyelitis originates from the Greek word “polio” meaning “grey” and “myelon” meaning “marrow.” It is an infectious disease caused by the poliovirus, a member of the genus Enterovirus, belonging to the Picornaviridae family.1 Poliomyelitis is an exclusive human disease transmitted from a patient or a symptom-free carrier through the fecal-oral route. Manifestations are varied ranging from asymptomatic (most common) to the most severe forms of debilitating paralysis. Historians have laid proof of the existence of poliomyelitis in ancient times. Egyptian paintings from the period 1403 to 1365 BC depict children with deformed limbs, walking with sticks. In 1789, an English physician Michael Underwood gave the first clinical description where he referred to polio as “debility of the lower extremities.” Polio was known as Heine-Medin disease due to the contributions of physicians Jakob Heine and Karl Oskar Medin in 1840.2,3
In the United States, localized paralytic polio epidemics began to appear around 1900. In June 1916, a directive was issued by the US public health authorities in Brooklyn, New York, regarding the existence of an epidemic of polio. More than 27 000 patients were reported, and fatality was more than 6000 in the country. There were more than 2000 deaths in New York City alone.4Authorities realized that they were dealing with an uncontrollable problem when a polio epidemic began to appear each summer. The crescendo came in 1940 to 1950 when “polio” came to be associated as the “wrath of God.” Quarantine of exposed children led to widespread panic and anxiety among the parents.4,5
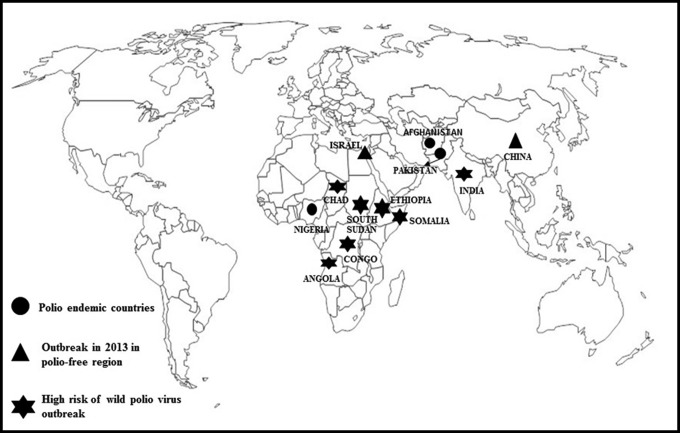
An urgent medical revolution came in the form of polio vaccines introduced in 1950 by Salk and Sabin. The number of cases of polio decreased from nearly 58 000 to just 5600 in a year’s time. Further decline in number of cases was seen after the second wave of mass immunization. By 1961, only 161 cases were recorded. The last case of paralytic polio through endemic transmission was recorded in 1979 in the midwest United States. In 1988, World Health Organization (WHO) passed a resolution to free the world from wild-type polio by 2000 in the form of Global Polio Eradication Initiative. In 1994, the WHO Region of The Americas was certified polio free followed by the WHO Western Pacific Region in 2000. World Health Organization European Region was declared polio free of the 3 types of wild poliovirus (types 1, 2, and 3) in June 2002. A world map depicting polio-endemic countries, regions of recent outbreak of poliovirus, and countries at high risk of poliovirus outbreak is depicted as Figure 1.
Figure 1.
World map showing polio-endemic countries' outbreak of wild poliovirus in polio-free regions and countries at higher propensity of wild poliovirus outbreak in the year 2013.
Pathogenesis
Poliovirus has a diameter of 25 to 30 nm. Its outer coat or capsid is composed of 60 protomers each made of 4 virion proteins VP1, VP2, VP3, and VP4 arranged in icosahedral symmetry. All the 4 virions are made of 8 strands of protein arranged in β sheet array forming a β barrel. Due to the intermingling of various proteins, loops are created, which serve as antigenic sites for combination with corresponding antibodies. Three serotypes of poliovirus have been recognized as types 1, 2, and 3. The prototype strains are Brunhilde and Mahoney strains for type 1, Lansing and MEFI for type 2, and Leon and Saukett for type 3. Each of the viruses has been crystallized and studied in detail.1,6
The poliovirus enters the oropharynx and multiplies locally in the tonsils, lymph nodes of the neck, and then subsequently in Peyer patches and small intestine. The incubation period ranges from 2 to 35 days. There is also a hypothesis to suggest that sometimes, virus enters the blood stream and then secondarily invades the tonsils.6 After 3 to 5 days, the virus is shed in stool and also can be recovered from the throat swabs of exposed patients. This period may be entirely asymptomatic or mild viremic symptoms may be seen. Self-limiting episodes of gastroenteritis, respiratory tract infection, and influenza-like illness can occur. The viremia may subside due to the appearance of antibodies or spread to the central nervous system (CNS) via bloodstream. Published literature also points to spread via the afferent nerve pathway in the brain as virus has special affinity for cellular receptor CD155 that helps in cell attachment and entry.7 The virus primarily causes destruction owing to its cytopathic nature. There is extensive damage to the anterior horn cells of the spinal cord. This causes limb paralysis. The virus may spread to the posterior horn cells, motor neuron of the thalamus, and hypothalamus. In bulbar form of poliomyelitis, there is involvement of brain stem, which may be fatal. Histological appearance of the affected brain cells shows vacuolation and infiltration. There is accumulation of plasma cells, polymorphonuclear neutrophils, and microglia. Infected cells get phagocytosed by macrophages causing degeneration of axons. Widespread muscular atrophy occurs leading to flaccid paralysis. Death usually occurs due to respiratory paralysis in extreme cases. Postpolio syndrome (PPS) can occur 25 to 30 years after the initial paralytic attack.8 In PPS, progressive muscle atrophy is seen probably due to ongoing motor neuron deterioration. Another hypothesis suggests abnormal presence of cytokines possibly due to the persisting poliovirus in the brain and spinal cord.6
Modes of Transmission
The spread of the disease is through the fecal-oral route. The dissemination of the virus in the feces is the reason of it being a highly communicable disease. Maximum excretion of the virus is seen in 2 to 3 days prior and 1 week after appearance of symptoms.
The spread is rapid in areas with poor sanitation, especially among the nonimmune population. The propagation of the virus is mainly seen in summer months in temperate regions. Tropical regions have no such distinction.
Poliomyelitis has been present endemically through infection among susceptible infants. Mainly due to the presence of antibodies to all the 3 serotypes of the virus (types 1, 2, and 3) in women of childbearing age and also due to the protective effect of maternal antibody, infants can be infected and protected simultaneously without any residual effects. The disease changed its form from endemic to causing various outbreaks of paralysis only in the late 19th century. Improper sanitation facilities and lack of personal hygiene were found to be the most important contributory factors, which led to infants getting exposed to the virus at an age beyond the protection of maternal antibodies.4,9
Clinical Presentation
Clinical features have been classified according to the severity of symptoms. The majority of exposed patients (around 95%) are asymptomatic. During this period, there is shedding of the virus in stool and it can be isolated from throat swabs also. The ratio of asymptomatic to paralytic cases ranges from 50:1 to 1000:1.10 Abortive poliomyelitis, which is a mild viremic form, accounts for around 4% to 8% of infections. There may be gastroenteritis, influenza-like illness, and mild respiratory tract infections, which usually subside within 1 week. Around 1% of the clinical cases present as aseptic meningitis.9 There can be severe muscle spasm of the neck, back, and lower limbs, which follows a brief prodrome like the one in abortive poliomyelitis. Complete recovery usually takes place within 10 days. The most severe form, paralytic poliomyelitis, which is seen in less than 1% of patients, presents as excruciating episodes of pain in back and lower limbs. In children, the disease may present in biphasic form—a period of prodrome followed by a brief symptom-free period of 7 to 10 days and then appearance of asymmetrical paralysis of limbs. Flaccid paralysis is the hallmark with loss of deep tendon reflexes eventually.9
Recovery may be complete in some patients but if loss of motor functions persists beyond 12 months, lifelong disability ensues. The 3 forms of paralytic poliomyelitis are spinal poliomyelitis, which is most common, bulbar poliomyelitis (2%), and a combination of above 2, bulbospinal poliomyelitis (around 19%)4 Bulbar poliomyelitis has the maximum fatality as the brain stem neurons are involved. In PPS, there is progressive muscular weakness, joint deterioration, and increasing skeletal deformities. Fatigue, following even minimal physical activity may lead to severe handicap of the day-to-day functioning.
Laboratory Diagnosis
The current method of diagnosis is polymerase chain reaction (PCR) for detection of poliovirus, which can be isolated from samples of stool, throat swabs, blood, and cerebrospinal fluid (CSF). Stool samples of the infected person are the primary sample source. The virus is excreted intermittently for a long period of 1 to 2 months after infection. In all, 80% of exposed people excrete the virus in the first 2 weeks, which declines to around 25% in the third week. Therefore, 2 samples of stool must be collected ideally at an interval of 24 hours within 2 weeks’ time for maximizing the chances of isolation of virus.11 Presence of the virus in the oropharynx is usually early in the infection. The virus can rarely be isolated from CSF in cases of aseptic meningitis. During first phase of viremia (3-5 days after infection), virus can be isolated from blood, but it is not of diagnostic importance.
Cell Culture
Initially the virus was cultivated in Rhesus and Cyanomolgus monkey cell lines, but these methods are not preferred now. At present, human cell lines like human amnion cell line and human embryo cell line are generally preferred methods. In India, polio laboratories work on RD cell line ,which is derived from human rhabdomyosarcoma and L20B cell line, which are very specific for poliovirus.11 Virus growth is determined by its cytopathic effect on the cell lines. This usually occurs within 7 days of inoculation. If the cytopathic changes are seen only in RD cell line, inoculation is done in L20B cell line to confirm poliovirus. The isolate is then subjected to neutralization tests using specific antisera for serotyping. Tests are also done to confirm whether the isolate is a wild strain or a vaccine-derived one. These tests are called intratypic differentiation tests.11 These are either based on the principle of enzyme-linked immunosorbent assay or based on the hybridization techniques.
Serology
A 4-fold rise in antibody titer is essential for confirmation of the infection. Neutralizing antibodies appear very early in the disease process and persist for life.
Molecular Methods
Samples like CSF and serum give a poor yield of virus in culture. In addition, cell culture is technically laborious and time consuming. These challenges have been addressed by the addition of PCR in the armamentarium of diagnostic tests. It has revolutionized the isolation of poliovirus. Polio-specific PCR primers have been designed, which help in the isolation of the virus.12
Genetic sequencing of the virus is essential to determine its origin and mode of transmission in cases of outbreak. When the world today stands on the brink of polio eradication, immediate identification of the genome of the outbreak isolate is imperative. Whether it is a recirculating strain or an imported one, the knowledge will help in curbing transmission.
Management
In the earlier times, when the epidemics of polio were frequent, there was absolute lack of knowledge regarding the management aspects of this crippling disease. Acute cases required immediate relief from pain, and rehabilitation was a challenge for chronic cases with deformities. Various strategies to manage these cases were in vogue at that time. A lot of experimentation was also involved. One of the earliest descriptions regarding management strategies of polio is the heroic work of Sister Elizabeth Kenny (an Australian nurse). She used hot packs to relieve muscle spasms in early stages of the disease13 and discouraged the practice of prolonged immobilization of affected limbs. A large number of patients were benefited.
The first modern rehabilitation center dedicated to patients with polio was set in 1926 by President Franklin Theodore Roosevelt in the United States.14
Later, new inventions were introduced to offer relief to the sick patients. One such instrument was the Iron Lung Machine.15 It was used in patients with respiratory paralysis to prolong their lives by assisted respiration. The drawbacks were the mammoth size, technical adjustments, and cost factor.
Modern medicine has contributed tremendously to the management of polio. In the recovery stage, remedial exercises are prescribed to assist the paralyzed muscles. Appropriate orthotic devices have been designed to prevent deformities due to muscle imbalance16,17 Various sessions of intense physiotherapy are necessary for rehabilitation and recovery. Surgical management includes tendon transplant, contracture relieving surgeries, and joint replacement surgeries.18 Illizarov technique, an orthopedic technique used to stabilize and rehabilitate the limb has also been now increasingly used for correction of deformities.19
Prevention
Salk and Sabin conducted numerous trials on their own blood relations, pets, and school children, which ultimately led to their moment of victory. It is a little known fact that more than 100 000 monkeys were killed for the benefit of humanity during development of the polio vaccine.20 The history of vaccines would be incomplete without mentioning the contribution of Dr David Bodian.21 He described the pathogenesis of the disease and the 3 antigenic types of poliovirus along with his team.22
Herd Immunity
Herd immunity supplements to polio vaccination. Among those individuals who receive oral polio vaccine (OPV), only 95% develop immunity. It is necessary to understand that the population in whom the vaccine fails are still protected by the immunity of those around them.
Numerous hurdles and setbacks were encountered before routine vaccination became reality for polio. In 1 incident, around 200 people became sick and 11 died after vaccination. This led to apprehension and anxiety regarding the safety of immunization. Later investigation found out that an inferior quality batch of inactivated polio vaccine produced by a particular drug company was behind this mishap.23 This laid to rest the anxiety and apprehension regarding polio vaccination.
Sabin continued to work on a live weakened strain that provided gut immunity but did not invade the CNS. He got the vaccine OPV licensed in 1962. Details about the constitution, mode of delivery, efficacy, and safety profile about the vaccines are described in Table 1.23,24
Table 1.
Comparison of Oral Polio Vaccine (OPV) and Inactivated Polio Vaccine (IPV).
| Property of Vaccine | Oral Polio Vaccine | Inactivated Polio Vaccine |
|---|---|---|
| Preparation | Live attenuated poliovirus serotypes (Sabin types 1, 2, and 3), in a 10:1:3 ratio, respectively. | Strain of each of the 3 serotypes that have been inactivated (killed) with formalin, adsorbed onto adjuvants. The final vaccine mixture contains 40, 8, and 32 d-antigen units of serotypes 1, 2, and 3, respectively. |
| Valency | Trivalent OPV (tOPV) and monovalent, against type 1 (mOPV1) and against type 3 (mOPV3) bivalent (type 1 and type 3) OPVs (bOPVs) | Only 1-type trivalent |
| Storage | +2°C to +8°C. Should be protected from light. Any vaccine showing particulate matter, turbidity, or change in color should be discarded | +2°C to +8°C. Should be protected from light. Any vaccine showing particulate matter, turbidity, or change in color should be discarded |
| Pathogenesis | Produce a local immune response in the intestines. Mucosal immunity decreases the replication and shedding of the virus | Antibodies are produced against the polio virus which provide humoral immunity, thus decreasing the replication of the virus. |
| Administration | Through mouth as drops | Intramuscularly into the upper arm or anterolateral thigh, can be administered alone or in combination with other vaccines |
| Recommended dosage | No longer in use in polio-free countries like United States and United Kingdom. Used routinely only in polio campaigns in high risk and endemic areas | A total of 5 doses of vaccine at the appropriate intervals |
| Vaccine efficacy | Immunity from oral poliovirus vaccine is probably lifelong. OPV produces excellent gut immunity | The duration of immunity with IPV is not known with certainty. Highly effective in producing immunity to poliovirus and protection from paralytic poliomyelitis. No gut immunity |
| Adverse effects | Vaccine-associated paralytic poliomyelitis (VAPP) outbreaks due to circulating vaccine-derived poliovirus (cVDPV) | Adverse events following administration of IPV are very mild and transient |
Current Status in India
The National Polio Surveillance Project launched in 1988 in collaboration with WHO had the sole objective of making India polio free.11 The initiative in India came in the form of “Pulse Polio” in 1995 to 1996, an immunization campaign launched by the Government of India. Under this extensive drive, 2 drops of OPV was given to all children younger than 5 years of age. Due to the dedicated active surveillance of acute flaccid paralysis cases, the results were impressive. As compared to 24 000 cases in 1988, the number of cases reported in 2003 were only 134. At the end of 2003, Nigeria, India, Pakistan, Niger, Afghanistan, and Egypt25 were the only countries in the world that remained polio endemic. For India to obtain global polio eradication certificate, it was crucial that all laboratory sources of wild polioviruses are destroyed. For this, a National Task Force on Laboratory Containment of Wild Polio Virus was constituted.26 This task force was led by The Director General of Indian Council of Medical Research. It was following the guidelines of the Global Action Plan, which emphasized the formulation of a national inventory of all the wild polioviruses and potentially poliovirus-infected materials. In India, the last reported case was in the state of West Bengal on January 13, 2011. On February 25, 2012, WHO directed that India be struck off from the list of polio-endemic countries. In 2013, only 3 countries remain polio endemic—Nigeria, Pakistan, and Afghanista.27,28 India has recently completed the 2-year milestone of becoming polio free and is expected to receive a global polio eradication certificate in 2014.29
Current Challenges in the World
The last defined case of natural polio in the United Kingdom was in the year 1984. Between 1985 and 2002, 40 cases of paralytic polio were reported.24 Of these, 30 were vaccine-associated paralytic polio. In 6 patients, the infection was contracted overseas, and in 5 patients, the source of infection was unknown. The wild virus was not detected. In 2000, an outbreak occurred in Hispaniola.7 The causative isolate was a recombinant of the vaccine strain and an unknown virus. Vaccine-associated paralytic poliomyelitis will continue to be a serious issue as long as OPV is being used. Scientific data prove beyond doubt that polio eradication will also require the eventual disuse of OPV, otherwise there will be resurgence in a polio-free world due to vaccine-associated paralytic polio and polio outbreaks due to circulating vaccine-derived polioviruses. Polio-free countries will be under a constant threat of importing a vaccine-derived poliovirus from places where OPV is used. But the greatest challenge is to stop the transmission of the wild strain circulation. In 2012, WHO declared completion of polio eradication a Programmatic Emergency. The Stop Transmission of Polio (STOP) program was launched to strengthen surveillance and promote maximum vaccination campaigns.30 Many factors pose challenges to complete eradication. Geographically difficult terrains make vaccine delivery difficult. There are serious sanitation issues. In some areas, cooperation from the local administration is lacking. Some religious factions have been campaigning against the vaccine drive.31 The crisis is not contained within the political boundaries of countries but spill over in the form of wild strains being exported to polio-free areas. One such recent outbreak occurred in Xinjiang province of China in 2011.32 This region had been certified polio free for the past 10 years. Investigation traced the origin to a wild polio strain from Pakistan. Outbreak management on a colossal scale was launched to contain this outbreak. Oral polio vaccine of 43.7 million doses was delivered in 5 rounds of vaccination. This report reiterates the already known fact that until the time polio is exterminated from all the countries, polio-free countries will be under threat. The efforts of thousands of people working religiously for decades should not go waste.
Footnotes
Declaration of Conflicting Interests: The authors declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: Dr Prachi Mehndiratta did 3 months voluntary service in polio eradication program during her medical graduation.
Funding: The authors received no financial support for the research, authorship, and/or publication of this article.
References
- 1. Bodian D, Horstmann DM. Polioviruses. In: Horsfall FL, Tamm I, eds. Viral and Rickettsial infections of Man. 4th ed. Philadelphia, PA: JB Lippincott Co;1965: 430–473 [Google Scholar]
- 2. Daniel TM, Robbins FC, eds. A history of poliomyelitis. Polio. Rochester, New York: University of Rochester Press; 1997:5–22 [Google Scholar]
- 3. Poliomyelitis. http://www.newworldencyclopedia.org/entry/Poliomyelitis. Accessed November 12, 2013
- 4. Melnick JL. Current status of poliovirus infections. Clin Microbiol Rev. 1996;9(3):293–300 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Trevelyan B, Smallman-Raynor M, Cliff A. The spatial dynamics of poliomyelitis in the United States: from epidemic emergence to vaccine-induced retreat, 1910–1971. Ann Assoc Am Geogr. 2005;95(2):269–293 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Schaulies JS, Schaulies SS, Meulen VT. Infections of the central nervous system. In: Topley and Wilson’s Microbiology and Microbial Infections, Virology. Vol. 2. 10th ed. Wiley-Blackwell; 2005:1401–1498 [Google Scholar]
- 7. He Y, Mueller S, Chipman PR, et al. Complexes of poliovirus serotypes with their common cellular receptor, CD155. J Virol. 2003;77(8):4827–4835 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Gonzalez H, Khademi M, Borg K, Olssonet T. Intravenous immunoglobulin treatment of the post-polio syndrome: sustained effects on quality of life variables and cytokine expression after one year follow up. J Neuroinflammation. 2012;9:167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. McQuillen D, McQuillen M. Poliomyelitis. In: Jones Royden H, ed. Netter’s Neurology. Philadelphia, PA; Elsevier Saunders; 2005:597–601 [Google Scholar]
- 10. Symptoms of polio. http://www.cdc.gov/vaccines/pubs/pinkbook/downloads/polio.pdf. Accessed November 20, 2013
- 11. Field guide surveillance of Acute Flaccid Paralysis. Ministry of Health and Family Welfare, New Delhi. http://www.searo.who.int/india/topics/poliomyelitis/Field_guide_for_Surveillance_of_Acute_Flaccid_Paralysis_3rd_edition.pdf. Accessed November 24, 2013
- 12. Chezzi C. Rapid diagnosis of poliovirus infection by PCR amplification. J Clin Microbiol. 1996;34(7):1722–1725 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Oppewal SR. Sister Elizabeth Kenny, an Australian nurse, and treatment of poliomyelitis victims. Image J Nurs Sch. 1997;29(1):83–88 [DOI] [PubMed] [Google Scholar]
- 14. A History of March of Dimes. http://www.marchofdimes.com/mission/history/aspx. Accessed November 12, 2013
- 15. Wilson D. Braces, wheelchairs, and iron lungs: the paralyzed body and the machinery of rehabilitation in the polio epidemics. J Med Humanit. 2005;26(2-3):173–190 [DOI] [PubMed] [Google Scholar]
- 16. Jubelt B. Post-polio syndrome. Curr Treat Options Neurol. 2004;6(2):87–93 [DOI] [PubMed] [Google Scholar]
- 17. Brehm A, Beelen A, Doorenbosch CA, Harlaar J, Nollet F. Effect of carbon composite knee–ankle–foot orthoses on walking efficiency and gait in former polio patients. J Rehabil Med. 2007;39(8):651–657 [DOI] [PubMed] [Google Scholar]
- 18. Poliomyelitis Treatment. http://emedicine.medscape.com/article/1259213. Accessed November 20, 2013
- 19. Kocaoglu M, Eralp L, Atalar AC, Bilen FE. Correction of complex foot deformities using the Ilizarov external fixator. J Foot Ankle Surg. 2002;41(1):30–39 [DOI] [PubMed] [Google Scholar]
- 20. National Museum of American History. Polio: two vaccines. http://amhistory.si.edu/polio/virusvaccine/vacraces2.html. Accessed November 24, 2013 [Google Scholar]
- 21. Nathanson N. David Bodian’s contribution to the development of poliovirus vaccine. Am J Epidemiol. 2005;161(3):207–212 [DOI] [PubMed] [Google Scholar]
- 22. Bodian D. Pathogenesis of poliomyelitis. Am J Public Health Nations Health. 1952;42(11):1388–1402 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Polio Vaccines. http://www.historyofvaccines.org/content/timelines/polio. Accessed November 24, 2013
- 24. Polio: the green book, Chapter 26; January 18, 2013. https://www.gov.uk/government/publications/polio-the-green-book-chapter-26. Accessed November 24, 2013
- 25. Polio Endemic Countries. http://www.who.int/mediacentre/factsheets/fs114/en/index.html. Accessed November 24, 2013
- 26. National Task Force for Containment of Wild Polio Virus. http://www.npspindia.org/. Accessed December 4, 2013
- 27. Pulse Polio Immunization, India. http://indiagovernance.gov.in/files/pulse_polio_immunization_india.pdf. Accessed November 24, 2013
- 28. Global health—polio. http://www.cdc.gov/polio/progress/. Accessed December 10, 2013
- 29. India hails polio-free ‘milestone’. http://www.bbc.co.uk/news/world-asia-india-25708715. Accessed March 7, 2014
- 30. CDC. The global polio eradication initiative stop transmission of polio (STOP) program—1999-2013. MMWR Morb Mortal Wkly Rep. 2013;62(24):501–503 [PMC free article] [PubMed] [Google Scholar]
- 31. Bhaumik S. Polio eradication: current status and challenges. J Fam Med Primary Care. 2012;1(2):84–85 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Luo HM, Zhang Y, Wang XQ. Identification and control of a poliomyelitis outbreak in Xinjiang, China. N Engl J Med. 2013;369(21):1981–1990 [DOI] [PubMed] [Google Scholar]