Abstract
Neurologic complications of infective endocarditis (IE) are common and frequently life threatening. Neurologic events are not always obvious. The prediction and management of neurologic complications of IE are not easily approached algorithmically, and the impact they have on timing and ability to surgically repair or replace the affected valve often requires a painstaking evaluation and joint effort across multiple medical disciplines in order to achieve the best possible outcome. Although specific recommendations are always tailored to the individual patient, there are some guiding principles that can be used to help direct the decision-making process. Herein, we review the pathophysiology, epidemiology, manifestations, and diagnosis of neurological complications of IE and further consider the impact they have on clinical decision making.
Keywords: infectious disease, bacterial endocarditis, mycotic aneurysm, stroke, intracerebral hemorrhage, meningitis, brain abscess
Introduction
In developed countries, the incidence of infective endocarditis (IE) ranges from 3 to 9 cases per 100 000 per year, and it is twice as common in men.1–3 Staphylococcus aureus is the most common organism. Up to 30% of those having bacteremia with staphylococcus will develop endocarditis.4 Patients with valvular abnormalities, such as prostheses, mitral regurgitation (MR), or aortic stenosis or with endocardial damage from circulating particulate matter as from intravenous (IV) drug use, are at the greatest risk of contracting endocarditis. Indeed, some 75% of patients with IE have structurally abnormal hearts.4
In addition to host factors, IE development also overwhelmingly involves microbes that have certain characteristics, such as platelet aggregating capabilities. Staphylococcus and Streptococcus species are the most common etiologic agents of IE, owing in part to this capacity.4 Ability of the microorganism to activate platelets creates an environment conducive to vegetation formation and theoretically supports the use of antiplatelet agents in the management of disease. However, studies of antiplatelet use in neurological complications of endocarditis have had neutral or negative results.5,6
Neurologic sequelae are the most frequent extra cardiac complications of IE, occurring in anywhere from 25% to 70% of cases.1,7,8 Mortality is higher in those with neurological complications than in those without.9 These complications can be the presenting symptoms of infection, most commonly in the form of embolic stroke. However, clinical manifestations of neurologic disease are protean and include ischemic or hemorrhagic stroke, infected intracranial aneurysm, meningitis, brain abscess, spinal epidural abscess, encephalopathy, mononeuropathy, and seizure. Conversely, complications may be completely silent, and clinically inevident neurologic disease has been shown to occur in 30% of cases with IE by imaging evaluation.8 The risk of developing neurologic complication from IE depends principally on characteristics of the vegetation and duration of antibiotic treatment. Larger, left-sided lesions on the mitral valve are more likely to embolize, and this is more likely to occur before antibiotics are started or within the first week after antibiotic initiation. Anticoagulation use at the time of presentation is a risk factor for hemorrhagic complications. The presence of neurologic complications and their type and severity impact the management of valvular disease, but cases must be considered individually, as severity of cardiac disease, comorbid conditions, and patient preferences may affect decisions regarding valvular surgery. In some instances, neurosurgical intervention is also warranted. If so, this may impact timing of valve repair. No properly controlled trials have been undertaken to address such interventions. There are, however, many small studies that can be used to help the neurologist make these important and difficult clinical decisions. A summary of the recommendations outlined in this article can be found in Table 1.
Table 1.
Summary of Author Recommendations.
| Neurologic Complication | Epidemiology | Clinical Manifestations in IE | Management | Implications for Cardiac Surgery if Indicated |
|---|---|---|---|---|
| Ischemic stroke | Clinically present in 20% to 40% of patients with IE Asymptomatic ischemia can be found in an additional 30% to 40% of patients with IE | Focal deficits, encephalopathy, and seizure | Avoid IV tPA, antiplatelet agents, and anticoagulation | Clinically silent/small infarcts should not delay cardiac surgery Larger infarcts may warrant delaying surgical intervention for up to 4 weeks |
| Intracerebral hemorrhage | Present in 4% to 27% of patients with IE Microhemorrhage is present in up to 57% of patients with IE | Focal deficits, headache, encephalopathy, and seizure | NVE: avoid all antiplatelets and anticoagulants PVE: prophylactically, convert oral anticoagulants to IV heparin and should hemorrhage develop stop anticoagulation for 10 to 14 days | Postpone cardiac surgery for 4 weeks following clinically significant hemorrhage |
| Infectious intracranial aneurysms | Present in at least 2% to 4% of patients with IE | Headache, seizures, focal deficits, encephalopathy, ophthalmoplegia, and rarely proptosis | Antibiotics and serial imaging for stable, small, unruptured aneurysms. Endovascular repair of large or enlarging unruptured aneurysms if amenable. Open surgical clipping for large or enlarging unruptured aneurysms not amenable to endovascular techniques or in eloquent areas where surgical anastamoses may spare function | Postpone cardiac surgery for 1 to 2 weeks following aneurysmal repair |
| Cerebral abscess | Present in 1% to 7% of patients with IE | Focal deficits, headache, encephalopathy, persistent fever, and seizure | Antibiotics alone for small or multifocal abscesses. Surgical drainage for abscesses that are large or do not respond to antibiotics. Neurosurgical intervention as appropriate for hydrocephalus or significant mass effect | Typically will not interfere with surgical planning. Prioritize neurosurgical intervention in the setting of hydrocephalus or significant mass effect. If hemorrhage accompanies, manages as mentioned earlier |
| Meningitis | Present in 1% to 20% of patients with IE | Headache, encephalopathy, seizure, neck/back pain, nuchal rigidity, and photophobia | At least 4 weeks of antibiotics | Typically will not interfere with surgical planning |
Abbreviations: IE, infective endocarditis; IV, intravenous; tPA, tissue plasminogen activator; PVE, prosthetic valve endocarditis; NVE, native valve endocarditis.
Neurologic Complications and Their Management
Encephalopathy
Encephalopathy is a common complication of IE that should prompt further workup. Encephalopathy may be secondary to systemic insults such as fever, azotemia, electrolyte disturbances, or hypercarbia or point to underlying central nervous system involvement in the form of ischemic stroke, hemorrhage, cerebral abscess, or meningitis as discussed subsequently.
Stroke
Acute ischemic stroke is the most common neurological complication of IE, manifesting clinically in 20% to 40% of patients with IE.8,10,11 Asymptomatic ischemia recognized by neuroimaging studies occurs in another 30% to 40% of patients.7,8 Thus, ischemic stroke may be more likely than not in patients with IE.
There are some scenarios in which the risk of cerebral ischemia is more likely. First, anterior mitral valve leaflet endocarditis confers the highest risk.12 Second, left-sided endocarditis is associated with a much higher risk of stroke than right-sided IE. Third, S aureus infection, before or less than 1 week after initiation of antibiotics, increases the likelihood of stroke.
The mechanism of acute cerebral ischemia in IE is very likely embolic. Ischemic strokes in IE most commonly occur in the middle cerebral artery territory12 (Figure 1), likely as a result of the high percentage of blood volume in these territories. However, multifocal infarction is also common and frequently involves the end arterial territories of cerebral vessels (Figure 2B and C).7
Figure 1.
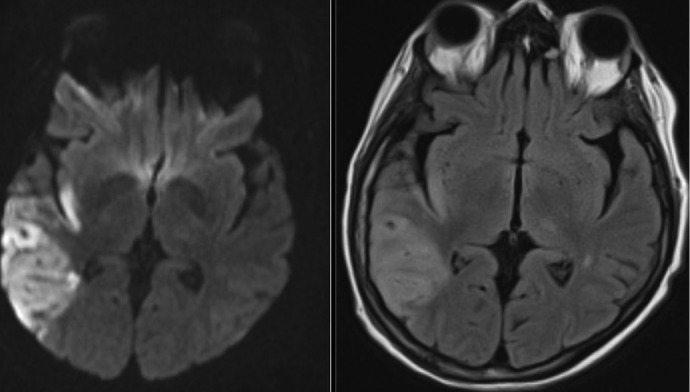
Stroke complicating endocarditis. Axial diffusion-weighted imaging (left) and T2 fluid-attenuated inversion recovery (FLAIR) imaging (right) of a 64-year-old female with a history of severe mitral regurgitation who presented with confusion 2 weeks after a dental procedure. Imaging shows a large right middle cerebral artery territory embolic infarct. The patient was found to have Streptococcus mitis bacteremia and mitral valve endocarditis. Vessel imaging was patent, and she underwent successful valve repair 2 weeks after antibiotics were started.
Figure 2.
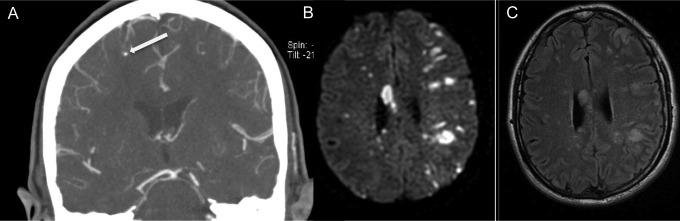
Infectious intracranial aneurysm and multifocal stroke in endocarditis. Coronal computed tomography angiography (CTA) of intracranial circulation (A), axial diffusion-weighted (DWI) magnetic resonance imaging (B), and axial T2 fluid-attenuated inversion recovery (FLAIR) imaging (C) from a 54-year-old right-handed female who was found down by her family. CTA shows infective intracranial aneurysm (arrow) in the right middle cerebral artery territory, and DWI and FLAIR demonstrate innumerable bilateral embolic infarcts. The patient was found to have methicillin-sensitive Staphylococcus aureus bacteremia and endocarditis of the mitral valve, septic shock, and multiorgan failure. Despite antibiotics, the patient remained too unstable for surgical intervention and died 20 days after presentation.
Management of stroke in the setting of IE differs from that of stroke due to noninfective mechanisms insofar as anticoagulation and antiplatelet agents are contraindicated, at least acutely.5,13 Evaluation of cardiac function may reveal an indication for valvular surgical intervention, and stroke complicating IE can impact the timing of this, as discussed further on.
Thrombolytic therapy with recombinant tissue plasminogen activator (r-tPA) or related agents has not been well studied in IE-related stroke. In the largest case series,14 4 patients having IE presented with ischemic stroke and were treated with thrombolysis. All 4 developed hemorrhage after IV r-tPA, 3 of whom died. For this reason and due to poor outcomes with acute anticoagulation, r-tPA may increase the risk of bleeding in these patients. The management differences to other types of ischemic stroke pose a potential clinical conundrum in the scenario whereby stroke is the presenting symptom of IE. If a patient appears with a stroke that is amenable to thrombolytic treatment, antiplatelet agents, or anticoagulation, one could inadvertently use a thrombolytic drug in a patient with occult endocarditis, thereby precipitating devastating results. This has not been studied thoroughly enough for a uniform recommendation to be established, but if there is a suspicion that IE could be present, caution should be exercised regarding the use of thrombolysis, acute anticoagulation, or antiplatelet therapy.
Cerebral Hemorrhage and Microhemorrhage
Hemorrhage in the brain in the setting of IE usually presents in the parenchyma or subarachnoid space. Parenchymal hemorrhage can be caused by hemorrhagic conversion of a prior ischemic infarct, microhemorrhage with or without progression to clinical hemorrhage due to vascular friability, or rupture of an infectious aneurysm. Quoted frequencies of cerebral hemorrhage in the literature may vary based on inclusion criteria of the aforementioned mechanisms. A series of 198 patients who defined cerebral hemorrhage as primary intracerebral hemorrhage, hemorrhagic conversion of a prior ischemic infarct, or rupture of infectious aneurysm found hemorrhage in 27% of patients.10 Another study of 113 patients with a similar definition found the rate of cerebral hemorrhage to be only ˜4%.15 The risk of hemorrhage may be higher in those who are on anticoagulant drugs at the time of presentation13 or who are treated with anticoagulation or antiplatelet agents early after diagnosis. Infection as well as concomitant medications may prolong the international normalized ratio (INR), and not surprisingly patients with a supratherapeutic INR seem to be at particularly high risk of fulminant hemorrhage.16 Nonetheless, the decision to continue anticoagulation in prosthetic valve endocarditis remains controversial. At least one series13 showed a higher mortality in those maintained on anticoagulation, several other studies report contradictory findings. For instance, one series of 50 cases of prosthetic valve endocarditis found an increased rate of neurological complications in patients with prosthetic valve endocarditis who were not adequately anticoagulated.17 The series was too small to note any significant difference in mortality. A previous study of 52 cases of prosthetic valve endocarditis18 reported a benefit in both mortality and neurologic complications in patients with prosthetic valve endocarditis who were kept on adequate anticoagulation. In that series, the onset of stroke in those patients in whom anticoagulation was discontinued occurred between 7 and 23 days with a mean of 17 days from the time of diagnosis. A series including patients with both native and prosthetic valve endocarditis found that while the incidence of neurologic complications was increased overall in anticoagulated patients, it was not increased in the subgroup of patients with prosthetic valves.19 Furthermore, this series failed to find a mortality difference in either the larger group of all patients with IE or the subgroup of patients with prosthetic valve endocarditis.
Cerebral microhemorrhage is increasingly acknowledged as a silent complication of endocarditis7,20 and recently has been implicated in predicting overt hemorrhage.21 Cerebral microhemorrhage has been detected in 57% of cases with IE, usually located cortically and with an average of about 8 microbleeds per patient.7 The proposed mechanism is that of infective vasculitis22 although this is speculative. Additionally, although there is no data linking the presence of microhemorrhage to later overt hemorrhage when antiplatelet agents or anticoagulants are used, there could be an increased risk in this setting.
In our practice, we do not recommend acutely treating patients with native valve IE and ischemic stroke or microhemorrhage with anticoagulation. Should they develop another indication for anticoagulation such as atrial fibrillation, deep venous thrombosis (DVT), or pulmonary embolism, the decision must be individualized. In general, we favor delaying anticoagulation for at least 14 days following treatment initiation in ischemic stroke. An inferior vena cava filter may act as a temporizing measure in patients with DVT. In prosthetic valve endocarditis, we usually convert patients from oral anticoagulants to IV heparin immediately with lower intensity than in patients without IE. In patients with prosthetic valve endocarditis and moderate to large ischemic strokes, we usually recommend discontinuing anticoagulation for at least 10 to 14 days. For smaller strokes in patients with prosthetic valve endocarditis, such as asymptomatic punctuate infarcts seen only on magnetic resonance imaging (MRI), we may continue anticoagulation using heparin with serial surveillance imaging. As anticoagulation is often required during or after a surgical procedure, neurologic deterioration is much more likely when surgery for valve replacement or repair is undertaken too soon in the setting of intracranial blood as is described further on.
Infectious Intracranial Aneurysm
Although most intracranial hemorrhages (ICHs) in patients with IE are caused by septic erosion of the arterial wall, a smaller number are caused by infectious intracranial, also known as mycotic, aneurysms. Although the term “mycotic aneurysm” has historically been used to describe septic aneurysms from any microorganism, the word “mycotic” connotes a fungal etiology. Since fungi can produce intracranial aneurysms as well as bacteria, “infectious intracranial aneurysm” (IIA) is emerging as the preferred term.23 Infectious intracranial aneurysms are relatively rare complications of infectious endocarditis, found in only 2% to 4% of patients23 with IE and accounting for 5% to 12% of patients having IE with neurological manifestations.24 However, the actual incidence is probably higher, as they can be clinically silent and subsequently resolve with antibiotic therapy. Therefore, if imaging is not obtained, IIAs can go undetected. Infectious intracranial aneurysms are more common than infected aneurysms in other locations in the body. They are thought to arise from emboli of cardiac valvular vegetations to the vasa vasorum of the cerebral vessels or from emboli directly to the distal vasculature.
Infectious intracranial aneurysms in the setting of bacterial endocarditis are most frequently found in the very distal branches of the cerebral circulation and usually occur in the middle cerebral artery territories (Figure 1), whereas fungal aneurysms may have a predilection for proximal vessels such as the internal carotid and basilar arteries.24 The mechanism is likely destruction of the vessel wall through interaction of organisms with the immune inflammatory response of host.25,26
Management of IIAs depends on several factors, including size, location, expertise of the managing clinicians, and whether there has been rupture. It is likely that the most important feature in IIAs is whether they have ruptured. This is based on the high mortality in patients with ruptured aneurysms—up to 80% in one study27 versus 30% mortality in those with unruptured aneurysms. It is unknown whether the increased mortality is due to rebleeding or to the initial hemorrhage itself. Ruptured IIAs are managed by either open or endovascular means, following which a 2- to 3-week delay is recommended prior to cardiac valve replacement.28 Clipping, the surgical procedure favored in noninfectious aneurysms, may be technically difficult, as IIAs tend to be fusiform with poorly defined necks and friable walls. Proximal ligation is therefore often necessary. Anastomotic procedures that can spare distal vessels in eloquent areas are sometimes possible. Endovascular therapies are less invasive alternatives that may be more appropriate in patients who are unfit for surgery due to cardiac disease. Detachable coils are preferred for proximal aneurysms, while distal aneurysms that are not accessible to microcatheters can be managed with acrylic glue or autologous clot injections. It is possible that valve surgery may be safe after a shorter time period, but data are lacking.
There is more disagreement regarding unruptured aneurysms. As in other cerebrovascular complications of IE, the data here are limited by small sample sizes, lack of randomization, selection biases, and failure to include measures of morbidity in addition to mortality outcomes. There are little data to guide management in the scenario of a patient with an unruptured aneurysm and an emergent or urgent indication for cardiac valve replacement. A 2002 study29 reported on 5 patients who had endovascular repair for unruptured aneurysms and underwent valve repair within a week without complication. A more recent review30 suggests the use of antibiotics and serial imaging for stable, small, unruptured aneurysms or antibiotics and endovascular treatment for large, enlarging, or symptomatic unruptured aneurysms. If endovascular intervention is unfeasible, clip reconstruction or proximal vascular occlusion with or without bypass is recommended. Anticoagulation, antiplatelet, and thrombolytic therapy should not be used in the setting of a known IIA, as there would be very few scenarios in which the risk of aneurysm rupture is outweighed by the need for acute anticoagulation.
Bacterial Brain Abscess
Bacterial brain abscess is a rare complication of endocarditis,11 affecting between 1% and 7% of patients with IE.10,31 They are most commonly seen in the setting of methicillin-resistant S aureus (MRSA) IE (Figure 3).24 By MRI, these are usually seen as multiple rim-enhancing lesions at the gray–white junction, which can be the cause of significant edema, hemorrhage, and/or mass effect. Given the potential for co-occurrence and potential implications on surgery, we recommend intracranial vessel imaging to evaluate for IIAs in the setting of abscess, although no strict guidelines exist. Although these can be solitary and the evaluation of a single brain abscess should include evaluation for IE, most solitary brain abscesses are not the presenting manifestation of IE.24
Figure 3.
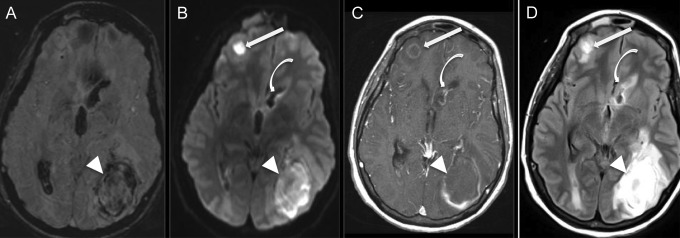
Multiple abscesses and ventriculitis complicating endocarditis. Axial susceptibility-weighted imaging (SWI, A), diffusion-weighted imaging (DWI, B), T1-weighted imaging after administration of gadolinium (C), and T2 fluid-attenuated inversion recovery (FLAIR) imaging (D) of a 36-year-old, right-handed man with a history of intravenous drug use who presented with malaise and multiple sites of pus-expression on the skin and in the left orbit, subsequently found to have methicillin-resistant Staphylococcus aureus bacteremia and aortic valve endocarditis. Magnetic resonance imaging shows 2 space-occupying lesions (arrow and arrow head) with internal restricted diffusion (B) and rim enhancement (C), and 1 with areas of susceptibility artifact, consistent with blood (A). Additionally, the lining of the frontal horn of the lateral ventricle demonstrates enhancement postcontrast (curved arrow, C), some diffusion restriction (B), and T2 hyperintensity (D) on FLAIR, consistent with ventriculitis. The patient underwent intensive antibiotic treatment, parapharyngeal abscess debridement, and left parietal craniectomy and lesionectomy with right frontal extraventricular drain placement with subsequent removal, and at follow up 6 months later had only residual right homonymous hemianopia and a seizure disorder. Native valve intervention was not required.
The treatment for brain abscess is first and foremost with antibiotics to include MRSA coverage if the organism is not known. As they are most commonly multifocal, surgical resection may not be feasible. However, when significant hydrocephalus or impending herniation is present, supportive surgical interventions may be necessary. In general, following the guidelines for non-IE-associated bacterial brain abscesses, we recommend at least 4 weeks of IV antibiotics for those who have been surgically managed and 6 to 8 weeks for those medically managed,32 monitored by serial imaging to assess the size and diffusion restriction of the abscess. A longer period of treatment can be necessary to achieve resolution.
Meningitis
Meningitis is a relatively rare complication of IE. Meningitis may occur during bacterial endocarditis caused by pyogenic organisms such as S aureus and enterococci. In more chronic bacterial endocarditis (formerly known as subacute bacterial endocarditis), sterile emboli to the brain may lead to signs of meningeal irritation and cerebrospinal fluid pleocytosis. A history of cardiac valve disease, dental manipulation, and recurrent fever preceding the meningeal signs should be sought. Careful examination for heart murmurs and peripheral stigmata of endocarditis (eg, Janeway lesions, Roth spots, and Osler nodes) are key components of the evaluation. The reverse may also occur. That is, in patients with pneumococcal meningitis and concomitant bacteremia and pneumonia, acute endocarditis may develop as a complication, most commonly manifested with vegetations on the aortic valve. A new cardiac murmur that appears after the meningitis has been diagnosed should suggest this possibility.
Recent large case series suggest meningitis can be associated with 1% to 20% of cases with endocarditis.10,31,33 In a French multicenter study of 198 patients with left-sided endocarditis, 108 (55%) patients had at least 1 neurological complication. Meningitis or meningeal reactions were present in 41 (20.7%) patients. of these 41 patients, 39 had fever, nuchal rigidity, and/or altered mental status, rather than only cerebrospinal fluid (CSF) abnormalities. Cerebrospinal fluid revealed elevated cell counts (32-600 cells/L) with an average of 90% (86%-97%) polymorphonuclear leukocytes and elevated protein levels (0.63-1.57g/L). Cultures of CSF were positive in 16 patients, but organisms found were not reported.10
In a multicenter cohort of 1345 consecutive episodes of left-sided IE, 340 (25%) patients experienced neurological complications. Encephalopathy occurred in 69 (5%) patients, while meningitis occurred in 17 (1.2%) and brain abscesses in 2 (0.1%). Staphylococcus aureus was the most commonly implicated bacteria, present in the cultures of 33 (48%) patients with encephalopathy and in 8 (47%) patients with meningitis.9
In another cohort study, endocarditis was identified in 24 (2%) of 1025 episodes of adults with community-acquired bacterial meningitis. An immunocompromised state was identified in 8 patients, alcohol abuse in 4 patients, and heart valve disease in 3 patients. One patient had an intracardiac device. Five (21%) patients presented with Osler triad of meningitis, endocarditis, and pneumonia caused by Staphylococcus pneumoniae. Cultures yielded S pneumoniae in 13 patients, S aureus in 8 patients, and Staphylococcus agalactiae, Staphylococcus pyogenes, and Staphylococcus salivarius in 1 patient each. Of the 24 patients, 7 (29%) with meningitis and endocarditis died.33
Choice of treatment for IE-related meningitis should be guided by standard principles of bacterial meningitis management; however, the course of antibiotic therapy should last 4 to 6 weeks, which is significantly longer than the duration for patients treated for meningitis without endocarditis (10-14 days).11
Issues Regarding Surgical Intervention
No large, prospective, controlled studies have directly addressed the issues of whether or when to undertake valve repair in patients with IE. More recent studies have incorporated propensity analyses in order to adjust for the inherent biases of treatment selection. Nonetheless, such analyses have yielded inconsistent results likely due to variation in methodology.34 As such, the data are conflicting, and even guidelines issued by the major European and American cardiac associations differ. Although the European guidelines offer guidance regarding the timing of surgery, the American guidelines do not, see Table 2 for a summary of these guidelines.
Table 2.
Comparison of Neurologically Relevant Recommendations for Surgical Intervention by American College of Cardiology (2006) and European Society of Cardiology Recommendations (2009).a
| ACC | ESC | |
|---|---|---|
| IE with persistent emboli despite appropriate antibiotic therapy | Surgery is indicated (class IIa) | Urgent surgery is indicated (class I) |
| IE with large left-sided vegetations | Surgery may be considered in NVE with mobile vegetations > 10 mm (class IIb) | Class I indication for urgent surgery with vegetations > 10 mm plus other predictors of complicate course such as HF, persistent infection, abscess (class I). Urgent surgery should be considered for isolated vegetations > 15 mm (class IIb) |
| After silent cerebral embolism or TIA | No recommendation | Surgery should proceed without delay if an indication remains (class I) |
| After intracranial hemorrhage | No recommendation | Surgery must be postponed for at least 1 month (class I) |
| After clinically relevant stroke | No recommendation | Surgery for HF, uncontrolled infection, abscess, or persistent high embolic risk should not be delayed. Surgery should be considered in absence of coma and CT evidence of hemorrhage (class IIa) |
Abbreviations: IE, infective endocarditis; ACC, American College of Cardiology; ESC, European Society of Cardiology Recommendations; NVE, native valve endocarditis; HF, heart failure; TIA, transient ischemia attack; CT, computed tomography.
a Per ESC guidelines, “urgent” surgery should be performed “within a few days”. Class I: evidence and/or general agreement that a given treatment or procedure is beneficial, useful, and effective. Class II: conflicting evidence and/or a divergence of opinion about the usefulness/efficacy of the given treatment or procedure. Class IIa: weight of evidence/opinion is in favor of usefulness/efficacy. Class IIb: usefulness/efficacy is less well established by evidence/opinion. Class III: evidence or general agreement that the given treatment or procedure is not useful/effective and in some cases may be harmful.
Both the American College of Cardiology (ACC 2006)35 and the European Society of Cardiology guidelines (ESC 2009)36 give class I recommendations for surgery for native valve endocarditis in the setting of lesions that result in heart failure or in cases of aortic regurgitation (AR) or MR with hemodynamic evidence of elevated left ventricular end-diastolic or left atrial pressures or greater than moderate pulmonary hypertension. In one propensity analysis,37 there was a significant mortality benefit to surgery even after adjusting for baseline variables associated with mortality. This benefit was most striking in those patients with moderate to severe heart failure in whom mortality decreased from 51% to 14% with surgery. Surgery is recommended on an urgent basis in less severe but persisting heart failure or with echocardiographic evidence of hemodynamic intolerance. The ESC offers a class IIa recommendation that surgery can be postponed on an elective basis in cases of severe AR or MR in the absence of heart failure or in the setting of prosthetic valve dehiscence.36
Both ACC and ESC guidelines recommend surgery in the setting of uncontrolled infection. Uncontrolled infection can manifest locally (with abscess, false aneurysm, fistula, or enlarging vegetation) or systemically (with persisting fever or positive blood cultures after 7 days of appropriate antibiotic therapy). The ACC and ESC guidelines agree that surgical intervention is indicated in locally uncontrolled infections (class I). Both ACC and ESC recommend surgery in the setting of systemically uncontrolled infection (ACC class IIa and ESC class I). Both organizations list infection caused by fungal or multidrug-resistant organisms or prosthetic valve endocarditis caused by staphylococci or gram-negative bacteria as a class I indication for urgent or elective surgery. Class I (ESC) or class IIa (ACC) recommendations exist for urgent surgical intervention in both native and prosthetic valve endocarditis with recurrent emboli and persistent vegetations, despite appropriate antibiotic therapy.
In mobile lesions that are larger than 1 cm, the ACC proposes (class IIb) that it is reasonable to consider surgical intervention, as these lesions are at significantly higher risk of embolization.38 The ESC provides a class I recommendation for urgent surgery for lesions larger than 1 cm following embolic infarct, despite appropriate antibiotic therapy or in the presence of other predictors of a complicated course. The ESC guidelines state that isolated lesions larger than 1.5 cm are a strong predictor of mortality and as such may be considered for operative intervention in the absence of further complications (class IIb).39
In the absence of cerebrovascular pathology, the urgency of surgical intervention should be considered in the context of the above-mentioned scenarios. Of course, the risk of intervention varies widely depending upon the presence of the cerebrovascular complications of endocarditis. The majority of patients with cerebrovascular complications have at least 1 indication for surgical intervention, and prognosis is poorer in these patients if not operated upon.40
Neurologists are often concerned that relative hypotension and full anticoagulation with heparin during cardiopulmonary bypass may exacerbate neurologic injury either by infarction extension or by hemorrhagic conversion. These effects are probably determined by the size of the infarct and its clinical relevance. Several series have shown that it is safe to operate early after silent infarctions.8,40,41 Early surgical intervention after a clinical stroke of small size, defined as less than 15 mm in diameter,42 also appears to be safe. Hence, the presence of silent infarctions on imaging or clinical ischemic strokes of small size should not delay surgical intervention.
The timing of surgery in the presence of larger infarctions is more controversial. A series from 2001 showed43 that patients with ischemic embolic infarcts who undergo surgery prior to 72 hours fared better than those treated medically and that those treated more than 8 days after stroke had poorer prognoses. Other case series support the safety of early cardiac surgery.44 In contrast, perhaps the largest study to evaluate timing of surgery in the setting of embolic infarction45 strongly favored delayed surgery, with neurologic complication rates of 45.5% for cardiac surgery within 24 hours, 43.8% within 2 to 7 days, 16.7% within 8 to 14 days, 10.0% within 15 to 21 days, 10.5% within 22 to 28 days, and 2.3% after 28 days. Other large series29,46 are in accord with the recommendation for delayed operation. Disparate results are likely due to heterogeneity of populations, small sample sizes, an overreliance on retrospective data collection, and nonrandomized trial designs rife with treatment biases. A recent attempt to correct for some of these biases was completed.47 The investigators performed a risk adjustment analysis for differences in patient characteristics and compared mortality in early versus late surgical intervention. After adjustment, early surgery was not significantly associated with decreased survival when compared to late surgery. Unfortunately, they did not collect data reflecting clinical neurologic status.
In the setting of intracerebral hemorrhage, there are even less data to guide the timing of surgical intervention. Intracerebral hemorrhage can be due to hemorrhagic conversion of ischemic infarct, rupture of septic material through the arterial wall, or rupture of IIAs. There are very few reported cases of surgical intervention prior to 4 weeks following hemorrhage. In a retrospective review of several series of patients with neurologic complications who underwent valve replacement, 41 patients were found with intracerebral hemorrhage. In those 41 patients, the rate of neurologic deterioration was 20% for those operated upon between 3 and 4 weeks, and 15% for those operated upon after 4 weeks. There are, however, limited case reports of patients with small hemorrhages tolerating valve replacement without further neurological insult.45,48 The ESC has recommended a moratorium on surgery for at least 4 weeks following ICH.
We consider all patients individually in terms of timing of surgery. Small or silent strokes are generally not viewed as an impediment when the need for valve replacement is great. Furthermore, as the risk of continued stroke drops dramatically with initiation of antibiotic therapy from 4.82/1000 patient-days in the first week to 1.71/1000 patient-days in the second week,49 if one were to recommend surgery, it makes sense to perform it early when there is more to gain. The decision to proceed to early surgery in larger infarctions must be weighed in light of the surgical indications, perioperative risk factors, and likelihood of additional embolic events that would further compromise both neurologic function and surgical safety. In general, after a large ischemic stroke, we prefer to postpone intervention to 4 weeks if safe to do so, but at the same time we monitor closely for changes in severity of the clinical scenario. In the setting of intracerebral hemorrhage, we tend to be more conservative and agree with the recommendation of delaying valve replacement by at least 4 weeks, except perhaps in the setting of minor petechial hemorrhage for which we may be more aggressive. The decision of how best to manage infected intracranial aneurysms should be evaluated on a case-by-case approach with the aid of neurosurgery, interventional neuroradiology, and cardiac surgery as mentioned earlier.
Data on implications of bacterial brain abscesses and meningitis on surgical intervention are very limited; in general, their presence does not seem to affect timing so long as they are adequately addressed with antibiotics.48 We have found this true for the majority of the cases. However, in the setting of multiple brain abscesses, excess mass effect or hydrocephalus must be addressed prior to cardiac surgery. Additionally, in hemorrhagic lesions, caution is advised to proceed as in intracerebral hemorrhage, detailed earlier.
Conclusion
Infective endocarditis is an important and serious disorder that is frequently complicated by both occult and overt neurologic disease including ischemic and hemorrhagic stroke, IIA, brain abscess, and/or meningitis. The discovery of such complications demands careful consideration that may alter decision making regarding surgical management of the underlying disease. Interpretation of the literature governing management of neurologic complications of IE remains challenging, given the paucity of prospective, controlled studies. A multidisciplinary collaborative approach is critical in order to optimize outcomes.
Footnotes
Declaration of Conflicting Interests: The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The authors received no financial support for the research, authorship, and/or publication of this article.
References
- 1. Hoen B, Duval X. Infective endocarditis. N Engl J Med. 2013;369 (8):785. [DOI] [PubMed] [Google Scholar]
- 2. Hoen B, Duval X. Clinical practice. Infective endocarditis. N Engl J Med. 2013;368 (15):1425–1433 [DOI] [PubMed] [Google Scholar]
- 3. Sy RW, Kritharides L. Health care exposure and age in infective endocarditis: results of a contemporary population-based profile of 1536 patients in Australia. Eur Heart J. 2010;31 (15):1890–1897 [DOI] [PubMed] [Google Scholar]
- 4. Keynan Y, Rubinstein E. Pathophysiology of infective endocarditis. Curr Infect Dis Rep. 2013;15 (4):342–346 [DOI] [PubMed] [Google Scholar]
- 5. Chan KL, Dumesnil JG, Cujec B, et al. A randomized trial of aspirin on the risk of embolic events in patients with infective endocarditis. J Am Coll Cardiol. 2003;42 (5):775–780 [DOI] [PubMed] [Google Scholar]
- 6. Chan KL, Tam J, Dumesnil JG, et al. Effect of long-term aspirin use on embolic events in infective endocarditis. Clin Infect Dis. 2008;46 (1):37–41 [DOI] [PubMed] [Google Scholar]
- 7. Hess A, Klein I, Iung B, et al. Brain MRI findings in neurologically asymptomatic patients with infective endocarditis. AJNR Am J Neuroradiol. 2013;34 (8):1579–1584 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Snygg-Martin U, Gustafsson L, Rosengren L, et al. Cerebrovascular complications in patients with left-sided infective endocarditis are common: a prospective study using magnetic resonance imaging and neurochemical brain damage markers. Clin Infect Dis. 2008;47 (1):23–30 [DOI] [PubMed] [Google Scholar]
- 9. García-Cabrera E, Fernández-Hidalgo N, Almirante B, et al. Neurological complications of infective endocarditis: risk factors, outcome, and impact of cardiac surgery: a multicenter observational study. Circulation. 2013;127 (23):2272–2284 [DOI] [PubMed] [Google Scholar]
- 10. Sonneville R, Mirabel M, Hajage D, et al. Neurologic complications and outcomes of infective endocarditis in critically ill patients: the ENDOcardite en REAnimation prospective multicenter study. Crit Care Med. 2011;39 (6):1474–1481 [DOI] [PubMed] [Google Scholar]
- 11. Novy E, Sonneville R, Mazighi M, et al. Neurological complications of infective endocarditis: New breakthroughs in diagnosis and management. Med Mal Infect. 2013;43 (11-12):443–450 [DOI] [PubMed] [Google Scholar]
- 12. Derex L, Bonnefoy E, Delahaye F. Impact of stroke on therapeutic decision making in infective endocarditis. J Neurol. 2010;257 (3):315–321 [DOI] [PubMed] [Google Scholar]
- 13. Tornos P, Almirante B, Mirabet S, Permanyer G, Pahissa A, Soler-Soler J. Infective endocarditis due to Staphylococcus aureus: deleterious effect of anticoagulant therapy. Arch Intern Med. 1999;159 (5):473–475 [DOI] [PubMed] [Google Scholar]
- 14. Walker KA, Sampson JB, Skalabrin EJ, Majersik JJ. Clinical characteristics and thrombolytic outcomes of infective endocarditis-associated stroke. Neurohospitalist. 2012;2 (3):87–91 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Salgado AV, Furlan AJ, Keys TF, Nichols TR, Beck GJ. Neurologic complications of endocarditis: a 12-year experience. Neurology. 1989;39 (2 pt 1):173–178 [DOI] [PubMed] [Google Scholar]
- 16. Karchmer AW, Dismukes WE, Buckley MJ, Austen WG. Late prosthetic valve endocarditis: clinical features influencing therapy. Am J Med. 1978;64 (2):199–206 [DOI] [PubMed] [Google Scholar]
- 17. Leport C, Vilde JL, Bricaire F, et al. Fifty cases of late prosthetic valve endocarditis: improvement in prognosis over a 15 year period. Br Heart J. 1987;58 (1):66–71 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Wilson WR, Geraci JE, Danielson GK, et al. Anticoagulant therapy and central nervous system complications in patients with prosthetic valve endocarditis. Circulation. 1978;57 (5):1004–1007 [DOI] [PubMed] [Google Scholar]
- 19. Delahaye JP, Poncet P, Malquarti V, Beaune J, Gare JP, Mann JM. Cerebrovascular accidents in infective endocarditis: role of anticoagulation. Eur Heart J. 1990;11 (12):1074–1078 [DOI] [PubMed] [Google Scholar]
- 20. Klein I, Iung B, Labreuche J, et al. Cerebral microbleeds are frequent in infective endocarditis: a case-control study. Stroke. 2009;40 (11):3461–3465 [DOI] [PubMed] [Google Scholar]
- 21. Okazaki S, Sakaguchi M, Hyun B, et al. Cerebral microbleeds predict impending intracranial hemorrhage in infective endocarditis. Cerebrovasc Dis. 2011;32 (5):483–488 [DOI] [PubMed] [Google Scholar]
- 22. Klein I, Iung B, Wolff M, et al. Silent T2* cerebral microbleeds: a potential new imaging clue in infective endocarditis. Neurology. 2007;68 (23):2043. [DOI] [PubMed] [Google Scholar]
- 23. Peters PJ, Harrison T, Lennox JL. A dangerous dilemma: management of infectious intracranial aneurysms complicating endocarditis. Lancet Infect Dis. 2006;6 (11):742–748 [DOI] [PubMed] [Google Scholar]
- 24. Jones HR, Siekert RG. Neurological manifestations of infective endocarditis. Review of clinical and therapeutic challenges. Brain. 1989;112 (pt 5):1295–1315 [DOI] [PubMed] [Google Scholar]
- 25. Buckmaster MJ, Curci JA, Murray PR, et al. Source of elastin-degrading enzymes in mycotic aortic aneurysms: bacteria or host inflammatory response? Cardiovasc Surg. 1999;7 (1):16–26 [DOI] [PubMed] [Google Scholar]
- 26. Tilson MD. Pathogenesis of mycotic aneurysms. Cardiovasc Surg. 1999;7 (1):1–2 [DOI] [PubMed] [Google Scholar]
- 27. Bohmfalk GL, Story JL, Wissinger JP, Brown WE, Jr. Bacterial intracranial aneurysm. J Neurosurg. 1978;48 (3):369–382 [DOI] [PubMed] [Google Scholar]
- 28. Gillinov AM, Shah RV, Curtis WE, et al. Valve replacement in patients with endocarditis and acute neurologic deficit. Ann Thorac Surg. 1996;61(4):1125–1129; discussion 1130 [DOI] [PubMed] [Google Scholar]
- 29. Chapot R, Houdart E, Saint-Maurice JP, et al. Endovascular treatment of cerebral mycotic aneurysms. Radiology. 2002;222 (2):389–396 [DOI] [PubMed] [Google Scholar]
- 30. Gross BA, Puri AS. Endovascular treatment of infectious intracranial aneurysms. Neurosurg Rev. 2013;36(1):11-19; discussion 19 [DOI] [PubMed] [Google Scholar]
- 31. Garcia-Cabrera E, Fernandez-Hidalgo N, Almirante B, et al. Neurological complications of infective endocarditis: risk factors, outcome, and impact of cardiac surgery: a multicenter observational study. Circulation. 2013;127 (23):2272–2284 [DOI] [PubMed] [Google Scholar]
- 32. Arlotti M, Grossi P, Pea F, et al. Consensus document on controversial issues for the treatment of infections of the central nervous system: bacterial brain abscesses. Int J Infect Dis. 2010;14 (suppl 4):S79–S92 [DOI] [PubMed] [Google Scholar]
- 33. Lucas MJ, Brouwer MC, van der Ende A, van de Beek D. Endocarditis in adults with bacterial meningitis. Circulation. 2013;127 (20):2056–2062 [DOI] [PubMed] [Google Scholar]
- 34. Bannay A, Hoen B, Duval X, et al. The impact of valve surgery on short- and long-term mortality in left-sided infective endocarditis: do differences in methodological approaches explain previous conflicting results? Eur Heart J. 2011;32 (16):2003–2015 [DOI] [PubMed] [Google Scholar]
- 35. Bonow RO, Carabello BA, Kanu C, et al. ACC/AHA 2006 guidelines for the management of patients with valvular heart disease: a report of the American college of cardiology/American heart association task force on practice guidelines (writing committee to revise the 1998 Guidelines for the management of patients with valvular heart disease): developed in collaboration with the society of cardiovascular anesthesiologists: endorsed by the society for cardiovascular Angiography and interventions and the society of thoracic surgeons. Circulation. 2006;114 (5):e84–e231 [DOI] [PubMed] [Google Scholar]
- 36. Habib G, Hoen B, Tornos P, et al. Guidelines on the prevention, diagnosis, and treatment of infective endocarditis (new version 2009): the task force on the prevention, diagnosis, and treatment of infective endocarditis of the European society of cardiology (ESC). Endorsed by the European society of clinical microbiology and infectious diseases (ESCMID) and the international society of chemotherapy (ISC) for infection and cancer. Eur Heart J. 2009;30 (19):2369–2413 [DOI] [PubMed] [Google Scholar]
- 37. Vikram HR, Buenconsejo J, Hasbun R, Quagliarello VJ. Impact of valve surgery on 6-month mortality in adults with complicated, left-sided native valve endocarditis: a propensity analysis. JAMA. 2003;290 (24):3207–3214 [DOI] [PubMed] [Google Scholar]
- 38. Mugge A, Daniel WG, Frank G, Lichtlen PR. Echocardiography in infective endocarditis: reassessment of prognostic implications of vegetation size determined by the transthoracic and the transesophageal approach. J Am Coll Cardiol. 1989;14 (3):631–638 [DOI] [PubMed] [Google Scholar]
- 39. Thuny F, Di Salvo G, Disalvo G, et al. Risk of embolism and death in infective endocarditis: prognostic value of echocardiography: a prospective multicenter study. Circulation. 2005;112 (1):69–75 [DOI] [PubMed] [Google Scholar]
- 40. Thuny F, Avierinos JF, Tribouilloy C, et al. Impact of cerebrovascular complications on mortality and neurologic outcome during infective endocarditis: a prospective multicentre study. Eur Heart J. 2007;28 (9):1155–1161 [DOI] [PubMed] [Google Scholar]
- 41. Cooper HA, Thompson EC, Laureno R, et al. Subclinical brain embolization in left-sided infective endocarditis: results from the evaluation by MRI of the brains of patients with left-sided intracardiac solid masses (EMBOLISM) pilot study. Circulation. 2009;120 (7):585–591 [DOI] [PubMed] [Google Scholar]
- 42. Hosono M, Sasaki Y, Hirai H, et al. Considerations in timing of surgical intervention for infective endocarditis with cerebrovascular complications. J Heart Valve Dis. 2010;19 (3):321–325 [PubMed] [Google Scholar]
- 43. Piper C, Wiemer M, Schulte HD, Horstkotte D. Stroke is not a contraindication for urgent valve replacement in acute infective endocarditis. J Heart Valve Dis. 2001;10 (6):703–711 [PubMed] [Google Scholar]
- 44. Thuny F, Beurtheret S, Gariboldi V, et al. Outcome after surgical treatment performed within the first week of antimicrobial therapy during infective endocarditis: a prospective study. Arch Cardiovasc Dis. 2008;101 (11-12):687–695 [DOI] [PubMed] [Google Scholar]
- 45. Eishi K, Kawazoe K, Kuriyama Y, Kitoh Y, Kawashima Y, Omae T. Surgical management of infective endocarditis associated with cerebral complications. Multi-center retrospective study in Japan. J Thorac Cardiovasc Surg. 1995;110 (6):1745–1755 [DOI] [PubMed] [Google Scholar]
- 46. Matsushita K, Kuriyama Y, Sawada T, et al. Hemorrhagic and ischemic cerebrovascular complications of active infective endocarditis of native valve. Eur Neurol. 1993;33 (3):267–274 [DOI] [PubMed] [Google Scholar]
- 47. Barsic B, Dickerman S, Krajinovic V, et al. Influence of the timing of cardiac surgery on the outcome of patients with infective endocarditis and stroke. Clin Infect Dis. 2013;56 (2):209–217 [DOI] [PubMed] [Google Scholar]
- 48. Angstwurm K, Borges AC, Halle E, Schielke E, Einhaupl KM, Weber JR. Timing the valve replacement in infective endocarditis involving the brain. J Neurol. 2004;251 (10):1220–1226 [DOI] [PubMed] [Google Scholar]
- 49. Dickerman SA, Abrutyn E, Barsic B, et al. The relationship between the initiation of antimicrobial therapy and the incidence of stroke in infective endocarditis: an analysis from the ICE Prospective Cohort Study (ICE-PCS). Am Heart J. 2007;154 (6):1086–1094 [DOI] [PubMed] [Google Scholar]


