Abstract
Significant advances in the diagnosis and management of bacterial brain abscess over the past several decades have improved the expected outcome of a disease once regarded as invariably fatal. Despite this, intraparenchymal abscess continues to present a serious and potentially life-threatening condition. Brain abscess may result from traumatic brain injury, prior neurosurgical procedure, contiguous spread from a local source, or hematogenous spread of a systemic infection. In a significant proportion of cases, an etiology cannot be identified. Clinical presentation is highly variable and routine laboratory testing lacks sensitivity. As such, a high degree of clinical suspicion is necessary for prompt diagnosis and intervention. Computed tomography and magnetic resonance imaging offer a timely and sensitive method of assessing for abscess. Appearance of abscess on routine imaging lacks specificity and will not spare biopsy in cases where the clinical context does not unequivocally indicate infectious etiology. Current work with advanced imaging modalities may yield more accurate methods of differentiation of mass lesions in the brain. Management of abscess demands a multimodal approach. Surgical intervention and medical therapy are necessary in most cases. Prognosis of brain abscess has improved significantly in the recent decades although close follow-up is required, given the potential for long-term sequelae and a risk of recurrence.
Keywords: abscess, bacteria, fungi, imaging, infection, brain
Introduction
Brain abscess is a serious and life-threatening clinical entity. Pyogenic infection of brain parenchyma begins with a localized area of inflammatory change referred to as cerebritis. This early stage of infection is characterized by increased blood vessel permeability without angiogenesis. When unrecognized, this process will progress to an immature capsular stage and then to brain abscess, a condition defined by an area of parenchymal infection containing pus encapsulated by a vascularized membrane.
Intraparenchymal abscess is the result of spread from a contiguous focus of infection, hematogenous seeding from a distant source, the sequelae of head trauma or neurosurgical procedure. In some instances, an etiology cannot be identified. Diagnosis can be challenging, as abscess presentation is highly variable and routine studies frequently lack specificity. High clinical suspicion is necessary for early recognition and initiation of appropriate treatment. In recent years, there have been significant refinements of neurosurgical technique and advances in the development and use of new antibiotics. Despite this, there is controversy regarding the role of surgical management and the optimal use of medications. The outcomes associated with brain abscess are guarded but have improved remarkably over the prior several decades. We present a review of the literature of the clinically relevant epidemiology, diagnosis, treatment, sequelae, and ultimate outcome of this uncommon condition.
Epidemiology
The incidence of brain abscess has decreased in the United States through the 20th century. A single-center study from Olmstead County Minnesota showed a change in incidence of 2.7 cases per 100 000 in 1935 to 1944 to 0.9 in 1965 to 1981.1 More recent investigations of incidence of abscess are lacking. Brain abscess may occur at any age, but the majority of cases occur between the third and fifth decades of life. Recent case series demonstrate a significant variance in mean age at presentation ranging from 24 to 57 years.2–12 Mean age of onset is higher in series from centers in developed nations than in developing nations. This relationship has not been formally investigated but may reflect the greater proportion of older immunosuppressed individuals, greater life expectancy, and lower rates of traumatic brain injury in younger individuals in developed areas. There is a significant male predominance reported consistently through the literature which is independent of geography2,4–6,8–16 with male to female ratios ranging from 1.5:1 to 4.5:1.2,4,5,8–15
Etiology
Pathogenesis
Etiology can be identified in many cases, but the source of infection remains unclear in a significant proportion of those with abscess even after a thorough investigation. In recent case series, cryptogenic abscess occurs between 4.6% and 43.4% of cases.2,3,6–8,11,13,14,16,17 Brain abscesses are often attributed to hematogenous spread, contiguous spread, recent neurosurgical procedure, or penetrating head trauma. Endocarditis or pulmonary infections (pneumonia, empyema, and abscess) are the most common sources of hematogenous spread 2,6–11,13,14 Cyanotic heart disease and pulmonary arteriovenous malformation are consistently reported in association with brain abscess.6–8,10,11,15,16 The pulmonary circulation represents a potential filtering apparatus for systemic bacterial pathogens. In patients with right to left cardiac shunt bypass, this mechanism and seeding to the central nervous system (CNS) is thought to occur more readily. Further some authors hypothesize that ischemic injury from hypoxemia and polycythemia may act as a nidus for infection.18 Infection from any systemic source may lead to bacteremia and subsequent spread to brain parenchyma even in the absence of cyanotic heart disease. Rarely, authors report bacterial meningitis as a source of hematogenous spread.3,7 Hematogenous spread accounts for 9% to 43% of brain abscesses.2,6,7,9–12,14 Contiguous infection may result from primary dental, sinus, ear infections, or mastoiditis 2,3,6–16 and represents 14% to 58% proportion of brain abscess. Invasive neurosurgical procedures are a known risk factor for development of brain abscess. They account for 3% to 18% of brain abscess.2,3,7,8,11–15 A recent series that compiles infectious complications of neurosurgery at a single center demonstrates that surgical risk of abscess is low (.2%).19
Abscess occurs most commonly in the frontal lobe but may occur in any location.2,4,7,10–15 Location is closely associated with source. Otogenic abscess occurs almost exclusively in the temporal lobe and cerebellum, while abscess associated with sinus infection is predominantly frontal.2,3,8,12,16,20 Abscess has been found to consistently occur more often on the left than on the right.7,12 Several authors have hypothesized that this is the result of the observed greater relative incidence of left-sided penetrating trauma which in turn has been attributed to the handedness of attackers. A significant proportion of individuals develop multiple abscesses (9.3%-28%).3,4,6,8–14,16
Immunocompromise raises risk of CNS infection. Solid organ transplantation leads to an increased incidence in fungal brain abscess mostly related to Aspergillus although other species of fungi including Candida, dematiaceous fungi, or phaeohyphomyctes may produce localized infection as well. Nocardia-related infection is not uncommon in solid organ transplant and bone marrow transplant recipients and appears most often after cardiac transplant with incidence reported as high as 37.5%. Central nervous system spread resulting from secondary dissemination from a primary pulmonary infection may produce single or multiple abscesses.21 Tuberculous abscess is uncommon but will occur in about 1% of solid organ transplant recipients.22 Other bacterial pathogens are uncommon causes of abscess formation in transplant recipients accounting for less than 1% of abscess in liver transplant and bone marrow transplant recipients.21 Abscesses must be distinguished from toxoplasma encephalitis which is the most common multifocal infectious process encountered in advanced HIV. Individuals with AIDS having brain abscess are more likely to have multiple abscesses and tuberculous abscess.8 This population is also more susceptible to intracranial infection from Listeria, Cryptococcus, and Nocardia.23
Microbiology
A single organism is isolated in the majority of bacterial brain abscess. However, isolation of multiple pathogens from abscess materials is not uncommon (4%-23%).6–11,14,16,24,25 Cultures are negative in 14%-34% of samples.9–11,14,24,25 Administration of antibiotics prior to collection of abscess material is often cited as the explanation for sterile culture. The authors of 1 case series note that abscesses drained within 3 days of antibiotic administration had much greater yield than otherwise (84% and 32%, respectively).5 All individuals with positive cultures after this period had large abscesses. Aerobic organisms are more commonly identified than anaerobes. Streptococci are most often identified among aerobic pathogens. Bacteroides fragilis and Peptostreptococcus spp are the most common anaerobic organisms isolated.6,8,16,24 Organisms vary significantly with the etiology of abscess (Table 1). Otogenic abscess is most often associated with Proteus, Streptococcus milleri group organisms, and Streptococcus pneumoniae.6,7,20 Mixed and negative cultures are more common in otogenic abscess.12,20 Anaerobic organisms are isolated more frequently in this population.16 Paranasal sinusitis is most often associated with intracranial complications from Streptococcus sp, Staphylococcus sp, and less commonly by the Enterobacteriaceae.7,26 Staphylococcus aureus, Staphylococcus epidermidis, and Enterobacteriaceae (with Pseudomonas aeruginosa being the most common pathogen in this group) are the most common organisms found in abscess related to traumatic brain injury. Similarly, S aureus, S epidermidis, P aeruginosa, and Propionibacterium acnes are associated with abscess related to neurosurgical procedure.8,12,13 Staphylococcus aureus, Streptococcus viridans, and Klebsiella pneumonaie are the most common organisms isolated in abscess attributed to hematogenous seeding.6,8
Table 1.
Spectrum of organisms differs by anatomic source.
| Source | Most Commonly Cultured Organisms |
|---|---|
| Paranasal sinus infection | Streptococcus spp |
| Staphylococcus spp | |
| Enterobacteriaceae (especially Hemophilus spp, Pseudomonas aerugonisa) | |
| Otogenic infection | Proteus mirabilis |
| Streptococcus milleri group organisms | |
| Streptococcus pneumoniae | |
| Staphylococcus aureus | |
| Dental infection | Streptococcus spp |
| Bacteroides fragilis | |
| Traumatic brain injury | Staphylococcus aureus |
| Staphylococcus epidermidis | |
| Enterobacteriaceae (most commonly P aerugonisa, Enterobacter spp) | |
| Neurosurgical procedure | Staphylococcus aureus |
| Staphylococcus epidermidis | |
| Pseudomonas aeruginosa | |
| Propionibacterium acnes | |
| Streptococcus spp. | |
| Hematogenous spread | Staphylococcus aureus |
| Streptococcus viridans | |
| Klebsiella pneumoniae |
Diagnosis
Clinical presentation
The presenting signs of brain abscess are variable and nonspecific. Patients most commonly present with headache (49%-93%), fever (14%-88%), altered mental status (33%-70%), focal neurologic symptoms (29%-71%), and nausea and vomiting (26%-71%).2–7,9–16,24 Seizures are less common (2%-49%) and may have either focal or generalized presentation. Rarely, brain abscess will present with status epilepticus (0.3%).7 Neck stiffness and meningismus have been reported (4%-23%).2,3,6,7,11,13–16 These symptoms may suggest a temporal or cerebellar location6 but often occur in the context of a concomitant meningitis or prior intraventricular rupture. Papilledema should be sought on physical examination but is an insensitive sign (1%-19%).3,7,14,16 The classic clinical triad of fever, headache, and focal neurologic deficit is suggestive of abscess, but recent reports indicate that this constellation occurs in a minority of cases (2%-34%).6,10,11,13,14,16
Since most patients with brain abscess present with nonspecific and unclear symptoms, high clinical suspicion is necessary for prompt diagnosis. The diagnosis should be considered in all patients with new progressive headache, signs of increased intracranial pressure, or gradual onset focal neurologic deficit. Higher clinical suspicion may be necessary in immunosuppressed individuals, as they are at greater risk and their limited ability to mount an immune response may shroud typically associated infectious signs.
Mean times from symptom onset to presentation at a medical center range from 7 to 25 days. Most reports indicate that the time to presentation in individual cases is quite variable and may occur hours to more than 60 days from symptom onset.3,4,6,7,10,11,14
Laboratory data
Laboratory data are of limited utility in diagnosis. Leukocytosis and elevation in erythrocyte sedimentation rate are common, but absence of these laboratory abnormalities will not exclude the diagnosis.3,4,6,11,14,15
A significant minority of patients present with leukopenia. Blood cultures should be performed early in all patients with suspected abscess, given their relative ease in collection. Their reported yield is modest (14%-50%), but the potential value for identification of the organism is substantial in circumstances where collection of abscess material cannot be performed promptly or is not advisable due to the associated risks.4,6,9,10,13,14
Cerebrospinal fluid (CSF) analysis may reveal pleocytosis, elevated protein, and decreased glucose but will be normal in a significant proportion of individuals.3,6,7,10,11,13,14
Cerebrospinal fluid culture is infrequently positive (0%-43%). Lumbar puncture may be complicated by rapid neurologic deterioration typically due to downward brain herniation in as many as 20% of patients with brain abscess.7,9 Routine CSF collection is often discouraged in circumstances where abscess is suspected because of the perception of low yield and significant risk. This is a controversial topic, however, and lumbar puncture may be considered in instances where there is limited mass effect, and the organism cannot be identified from an alternative source.
Imaging
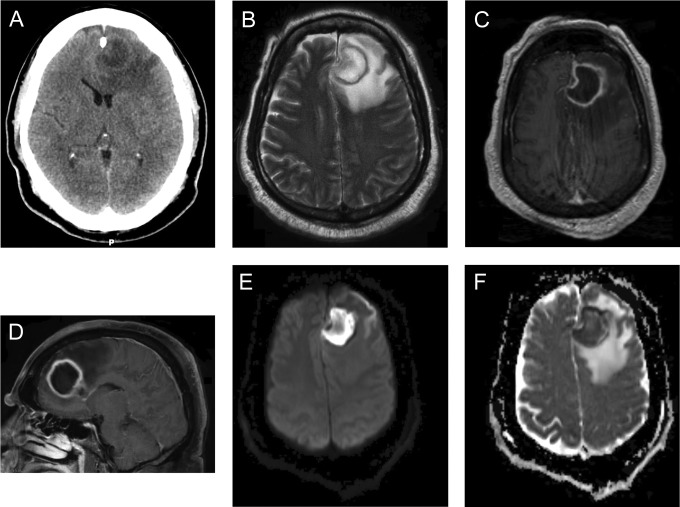
Brain imaging is critical to diagnosis and management and is critical to improving outlook for brain abscess. Typical characteristics of imaging studies are illustrated in Figure 1. The early stages of cerebritis are characterized on noncontrast computed tomography (CT) by localized hypoattenuation. Contrast enhancement is variable in this stage and when present may demonstrate a nodular or ring-like pattern.27 This pattern remains unchanged or progresses on delayed images performed 30 to 60 minutes after contrast bolus administration. In the late cerebritis phase, noncontrast CT again demonstrates an area of hypoattentuation but contrasted images demonstrate a thick ring-like or nodular enhancement that persists on delayed images. As a capsule begins to form, a round or ovoid area of hypoattenuation will present with ring enhancement that dissipates on delayed scans.
Figure 1.
A 56-year-old gentleman with a history of type 2 diabetes mellitus presented with a 5-day history of progressive fatigue, malaise, and subjective fever. On the day prior to presentation, he developed severe headache and had significant change in mental status. His examination was notable for confusion and mild right arm weakness. Noncontrast CT (A) demonstrates a left frontal mass at the gray-white junction with surrounding vasogenic edema. On magnetic resonance imaging (MRI), there is subfalcine herniation. T2 (B) and T1 postcontrast (C and D) maps demonstrate a heterogeneous ring enhancing, T1-hypointense, T2-hyperintense fluid collection. There is thinning of the periventricular rim. The central nonenhancing portion demonstrates restricted diffusion (E) and is hypointense on apparent diffusion coefficient (ADC) images (F).
Magnetic resonance (MR) imaging has greater sensitivity and specificity than CT in identifying pyogenic infection (Figure 1). Early cerebritis is characterized by poorly defined hyperintensity on T2-weighted sequences and hypointensity on T1-weighted sequences. As the infection matures, localized collections of fluid will be apparent on imaging. On T1-weighted imaging, these areas will be hyperintense relative to CSF and hypointense relative to the surrounding white matter. Fluid appears isointense to hyperintense to CSF on T2-weighted images. Vasogenic edema appears hypointense on T1 images and hyperintense on T2 sequences. A smooth contrast-enhancing capsule which is isointense to hyperintense to white matter on T1 images and isointense to hypointense on T2 images will be evident. Imaging in immunocompromised patients may lack ring-enhancing regions and vasogenic edema may be less prominent than in immunocompetent patients.
Although characteristic of abscess, the MR constellation of a ring-enhancing lesion with surrounding evidence of vasogenic edema is nonspecific. Diffusion-weighted imaging (DWI) may aid in diagnosis. The central nonenhancing portion of an abscess has diffusion restriction, appearing hyperintense on diffusion weighted sequences and hypointense on apparent diffusion coefficient maps. Infrequently, diffusion restriction may not be present in previously treated abscess. Diffusion restriction is an uncommon finding in ring-enhancing neoplasms (Table 2).28,29
Table 2.
Magnetic Resonance Imaging Features Differentiating Abscess From Neoplasm.
| Postcontrast T1 Imaging | T2 Imaging | Diffusion Imaging | ||
|---|---|---|---|---|
| Abscess | Ring enhancement | Hyperintense center | Hyperintense on DWI | |
| Thinning of internal border of capsule | Hypointense border | Hypointense on ADC | ||
| Low-grade glioma | Variable solid enhancement | Hyperintense | Hypointense on DWI | |
| Hyperintense on ADC | ||||
| High-grade glioma | Nonnecrotic lesions | Hyperintense | Nonnecrotic lesions | |
| Solid enhancement in most | Nonnecrotic lesions may appear isointense | Isointense to hyperintense on DWI | ||
| A minority will not enhance | Hypointense to isointense on ADC | |||
| Necrotic lesions | ||||
| Necrotic lesions | Hypointense on DWI | |||
| Hyperintense on ADC | ||||
| Very rarely necrotic lesions are hyperintense on DWI, hypointense on ADC | ||||
| Ring enhancement | ||||
| Lymphoma | Solid enhancement | Isointense to hyperintense | Typically hyperintense on DWI | |
| Hypointense on ADC | ||||
| Metastases | Ring or solid enhancement | Variable | Typically hypointense on DWI | |
| Hyperintense on ADC |
Abbreviations: ADC, apparent diffusion coefficient; DWI, diffusion weighted imaging.
Recently, 1H nuclear MR spectroscopy has been shown to be of value in the differentiation of abscess from cystic tumor. Spectroscopy allows for the detection of products of bacterial metabolism (lactate, acetate, and succinate) and neutrophil proteolysis (cytosolic amino acids). A recent study investigated the added value of spectroscopy and diffusion weighted imaging (DWI) to MR imaging in the diagnosis of intracranial cystic lesions in 50 patients.30 Spectroscopy and DWI raised sensitivity and specificity of MR for abscess from 61.9% and 60.9% to 95.2% and 100%, respectively.
Positron emission tomography (PET) investigations using the radiotracers 18F-fluoro-2-deoxyglucose and [methyl-11C]-L-methionine consistently show increased tracer accumulation in brain abscess but may show increased uptake in neoplastic lesions as well. A new radiotracer, O-(2-18Ffluoroethyl)-L-tyrosine, has significant diagnostic value in the evaluation of glioma but will show uptake in some cases of abscess.31 As such currently PET imaging is of limited utility in differentiating the underlying etiology of a ring-enhancing mass and will not spare biopsy.
Management
The appropriate management of brain abscess typically necessitates a multimodal approach including both medical and surgical therapies. All patients should receive prompt empiric antibiotic coverage. In most cases, surgical drainage of purulent material is necessary for abscess resolution. Stereotactic drainage by CT guidance is typically considered the intervention of choice, but en bloc excision may be considered as initial therapy in particular circumstances. The choice of surgery remains a matter of significant debate.
Although most patients will require surgical intervention, in a carefully selected subset of patients, a purely medical approach may be attempted. Patients with intact mental status and without signs of increased intracranial pressure in whom there is little doubt of diagnosis32 may be considered. This strategy is rarely effective for abscesses larger than 3 cm in diameter.33 Most authors advocate a more conservative threshold of 2.5 cm.34 Further, this approach should not be considered in high-risk patients such as those in which structural factors make prompt evacuation necessary such as infratentorial abscess, developing hydrocephalus, or significant mass effect.
A surgical procedure may be considered when an organism cannot be identified from an alternative source even in the absence of other indications for intervention.10,32 As abscess material culture is frequently unrevealing, and it is rare that culture leads to the discovery of a resistant organism, these decisions should take into account surgical risk and be made on a case-by-case basis.
Computed tomography should be repeated on a weekly basis in medically managed patients for at least 2 weeks and more often if there is clinical deterioration. Active growth of the abscess, clinical deterioration, or a lack of improvement in size on imaging at 3 to 4 weeks signify medical failure. Surgical management should not be further delayed in these instances.17,35
Surgical management
The ideal method of surgical intervention in brain abscess remains a matter of controversy. En bloc abscess excision had historically been the approach of choice, but in the preceding several decades, CT-guided aspiration has become the more commonly practiced initial intervention. The improved outcome and prognosis of brain abscess in the CT era is largely credited to the feasibility and availability of stereotactic drainage. Despite this, excision has several advantages and a minority of authors still prefer this method at the outset.
Computed tomography-guided aspiration is simpler than open excision and spares the patient the morbidity associated with the complications of extensive surgical trauma.32 It is favored in abscess in eloquent locations or in deep-seated abscess.32,35 It is preferred also in patients with multiple abscesses necessitating drainage. In cases where there is significant uncertainty in diagnosis, abscess wall biopsy is possible through a limited approach and may yield important information.35 Abscess recurrence or failure to improve is not uncommon after aspiration; this constitutes the major limitation to this approach. To ensure abscess resolution, weekly imaging is recommended after aspiration. Patients failing to improve after initial aspiration may require repeat procedures or may ultimately demand excision. Inadequate aspiration, chronic immunosuppression, and inadequate antibiotic therapy are the clinical factors most commonly associated with failure.35
Open craniotomy with excision is associated with a lower rate of recurrence and reaccumulation.32,36 In situations where a structural abnormality underlies the development of abscess, open craniotomy is required for definitive treatment. For instance, this applies in cases in which the abscess has resulted from a contiguous primary source (eg, osteomyelitis from sinus infection), a fistulous connection, or retained foreign bodies following trauma.17,32,36 Similarly, aspiration is often insufficient in the treatment of multiloculated abscess.35,36 Abscess in high-risk locations such as the posterior fossa or with significant clinical consequence from mass effect benefits from open procedure as well.32,35,36 Certain microbiological factors may necessitate an open procedure. Gas-containing abscesses, actinomycotic abscesses, nocardial abscesses, and fungal abscesses respond less well to aspiration and require open intervention.17,34,36–38
Even in the absence of these factors, some authors favor open excision to aspiration. Excision allows for the thorough irrigation and removal of purulent material as well as the subsequent verification of complete evacuation with ultrasound. As such, it is thought to provide a definitive therapy. It abbreviates the length of hospitalization and lowers the rate of reoperation.32,36 It may shorten the necessary duration of antimicrobial treatment and as such may be preferable in situations where outpatient compliance with oral therapy is a concern.36
Medical management
Classically, penicillin G and chloramphenicol were the antimicrobials of choice in the treatment of abscess. The emergence of antibiotic resistance and the development of agents with improved tolerability have led to a shift in preferred agents over the past several decades. A recent retrospective analysis based on a prospectively designed antibiotic treatment protocol concluded that the combination of cefotaxmine and metronidazole may be a safe and effective regimen for empiric coverage.5 Antibiotic adjustment based on antimicrobial resistance was necessary in 2 patients, one of whom was discovered to have a methicillin-resistant Staphylococcus epidermidis species. Methicillin resistance has become more widespread since the publication of that analysis. Currently, most authors recommend the routine addition of vancomycin to a third-generation cephalosporin and metronidazole. In patients with risk factors for pseudomonal infection such as abscess associated with recent neurosurgical procedure, a cephalosporin with the appropriate coverage such as ceftazidime or cefepime or alternatively a carbapenem with pseudomonal coverage such as meropenem is recommended. Empiric treatment of the patient with known immunocompromise must take into account the particular immunodeficiency. Patients with HIV are commonly started on pyrimethamine and sulfadiazine or alternatively clindamycin for Toxoplasma coverage.39 Patients with neutropenia are at significant risk of infection from fungal agents. Some practitioners recommend the routine addition of empiric amphotericin in this population.
Parenteral antibiotics should be continued for a minimum of 6 to 8 weeks. A shorter course of parenteral therapy, potentially as short as 2 weeks, has been suggested; the data for this approach are limited.5 A 2- to 3-month course of oral antibiotics should follow the termination of intravenous therapy.39 Duration of antimicrobial therapy in the immunocompromised patients should be extended, although there is little agreement regarding the optimal duration of treatment. Recommended length of treatment of parenteral antibiotics ranges from 12 weeks to 1 year.14,40 Careful clinical and imaging follow-up are indicated, given the uncertainty of required duration of therapy in any particular case.
Corticosteroids are not routinely used in brain abscess and should be reserved for cases in which abscess-related edema is severe and has led to significant clinical mass effect. In early animal experiments, antibiotic concentration within abscess artificially produced by inoculation of brain tissue was found to be significantly and consistently lower in those animals receiving corticosteroids.41,42 As such corticosteroid administration is thought to lead to a reduction in the penetration of antimicrobials into the abscess.36 Although some retrospective case series show a relationship between steroid administration and poor outcome,3 this is not a consistent finding.43 Further as steroids are typically given only to those with clinically significant mass effect, the consequence of the underlying illness cannot be reliably separated from the effect of corticosteroid administration. In abscesses adjacent to a ventricular wall, it is thought that steroid administration may increase the risk of intraventricular rupture although this is of unclear basis.36
Common complications
Intraventricular rupture represents a potentially preventable complication of deep-seated abscess. Rupture clinically manifests as sudden-onset headache, meningeal irritation, and an abrupt deterioration in mental status. Imaging is notable for hydrocephalus, ependymal enhancement, septation of the ventricle, meningeal enhancement, or the presence of ventricular debris. Short distance between the ventricle and the abscess walls (<1mm) and multiloculated abscess are associated with rupture.44 Some report hematogenous source of abscess as a potential risk factor, but this is an inconsistent finding.40,44 Small abscesses abutting the ventricular space are no less likely to rupture than larger abscesses and should be approached with similar caution.44 There is no standardized treatment protocol for rupture, as prospective data are lacking. Rupture is approached often by urgent surgical evacuation of the abscess by open craniotomy or aspiration. This is often accompanied by lavage and ventriculostomy for drainage and antibiotic administration. It is unclear whether these measures are of benefit and patients often progress poorly despite these interventions. As such, patients with significant structural risk factors for rupture should be treated aggressively at the outset.
An obstructive hydrocephalus may result from occlusion of the ventricular system from mass effect. As of any space-occupying lesion of the posterior fossa, this is not an uncommon complication of brain abscess and represents a source of significant morbidity. A significant proportion of the morbidity associated with posterior fossa abscess is related to complications related to hydrocephalus. Urgent CSF diversion with ventriculostomy is an uncontroversial intervention in the symptomatic patients. The literature suggests that intervening on asymptomatic hydrocephalus noted on imaging with urgent drainage may be of benefit. A South African center, noting poor outcomes in cerebellar abscess associated with hydrocephalus and also unrecognized hydrocephalus discovered on postmortem examination, instituted a policy mandating urgent CSF diversion in all with cerebellar abscess and imaging signs of hydrocephalus, regardless of clinical presentation. They observed a significant reduction in both morbidity and mortality.7
Outcome and Prognosis
The prognosis of brain abscess has improved considerably since the advent of CT. All-cause mortality in patients hospitalized with abscess varies from 5% to 32%.2,3,5–11,13–16,24,25
Degree of compromise in neurologic conditions on initial evaluation, in particular alteration in mental status, is consistently found to be predictive of ultimate prognosis.3,9,10,14–16,25,43
Patients with rapid neurologic decline and those with shorter disease duration prior to hospitalization have worse outcome.3,6,14,40 Other early clinical factors potentially indicative of poor outcome include meningismus, leukocytosis, fever, or sepsis at presentation.3,10,12 The physical characteristics of the abscess may be predictive of outcome. Several series report deep location as a poor prognostic marker.4,11,40 This may be for a variety of reasons including a potential predisposition of these lesions to result in complications as well as limitations in terms of ease of surgical accessibility. Intraventricular rupture is consistently reported to portend poor outcome.3,4,6,7,9,11,40 It results in significant morbidity in up to 50% of patients and commonly heralds fatal outcome. Preoperative hydrocephalus may also result from deep abscess and has been associated with morbidity.14 Other structural factors affecting outcome include multiloculated abscess11 and number of lesions.7 Outcome in the immunocompetent patients is typically unrelated to the responsible microbe. Abscess from organisms, such as Listeria, Nocardia, and Actinomyces, affecting the immunocompromised patients presents a greater challenge in management and indicates poorer prognosis. An immunocompromised host is found to be an independent factor of worse outcome.10,11
A significant proportion of patients with appropriately treated abscess recover completely and can survive without significant neurologic sequelae.5,24 Symptomatic epilepsy from abscess is the most common persistent deficit.15,25 Focal neurologic symptoms such as hemiparesis, loss of vision, and dysphagia3,5,15,24 are not uncommon as are global cognitive deficits.5 Routine follow-up of patients with abscess is advisable, as abscess recurrence may occur many months to years after the initial event.5,6,15,25 One series reports a case of abscess recurrence 7 years after initial treatment.25 Often recurrence is related to inadequate duration or choice of antimicrobial agent, but in other cases no explanation is found.
Appendix.
| Summarizing points | |
|---|---|
| Epidemiology |
|
| Etiology |
|
| Diagnosis |
|
| Management |
|
| Outcome and Prognosis |
|
Footnotes
Declaration of Conflicting Interests: The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The authors received no financial support for the research, authorship, and/or publication of this article.
References
- 1. Nicolosi A, Hauser WA, Musicco M, Kurland LT. Incidence and prognosis of brain abscess in a defined population: Olmsted County, Minnesota, 1935-1981. Neuroepidemiology. 1991;10(3):122–131 [DOI] [PubMed] [Google Scholar]
- 2. Faraji-Rad M, Samini F. Clinical features and outcome of 83 adult patients with brain abscess. Arch Iran Med. 2007;10(3):379–282 [PubMed] [Google Scholar]
- 3. Hakan T, Ceran N, Erdem I, Berkman MZ, Goktas P. Bacterial brain abscesses: an evaluation of 96 cases. J Infect. 2006;52(5):359–366 [DOI] [PubMed] [Google Scholar]
- 4. Song L, Guo F, Zhang W, et al. Clinical features and outcome analysis of 90 cases with brain abscess in central China. Neurol Sci. 2008;29(6):425–430 [DOI] [PubMed] [Google Scholar]
- 5. Jansson AK, Enblad P, Sjolin J. Efficacy and safety of cefotaxime in combination with metronidazole for empirical treatment of brain abscess in clinical practice: a retrospective study of 66 consecutive cases. Eur J Clin Microbiol Infect Dis. 2004;23(1):7–14 [DOI] [PubMed] [Google Scholar]
- 6. Kao PT, Tseng HK, Liu CP, Su SC, Lee CM. Brain abscess: clinical analysis of 53 cases. J Microbiol Immunol Infect. 2003;36(2):129–136 [PubMed] [Google Scholar]
- 7. Nathoo N, Nadvi SS, Narotam PK, van Dellen JR. Brain abscess: management and outcome analysis of a computed tomography era experience with 973 patients. World Neurosurg. 2011;75(5-6):716–726; discussion 612-717 [DOI] [PubMed] [Google Scholar]
- 8. Sarmast AH, Showkat HI, Bhat AR, et al. Analysis and management of brain abscess; a ten year hospital based study. Turk Neurosurg. 2012;22(6):682–689 [DOI] [PubMed] [Google Scholar]
- 9. Tattevin P, Bruneel F, Clair B, et al. Bacterial brain abscesses: a retrospective study of 94 patients admitted to an intensive care unit (1980 to 1999). Am J Med. 2003;115(2):143–146 [DOI] [PubMed] [Google Scholar]
- 10. Tseng JH, Tseng MY. Brain abscess in 142 patients: factors influencing outcome and mortality. Surg Neurol. 2006;65(6):557–562; discussion 562 [DOI] [PubMed] [Google Scholar]
- 11. Xiao F, Tseng MY, Teng LJ, Tseng HM, Tsai JC. Brain abscess: clinical experience and analysis of prognostic factors. Surg Neurol. 2005;63(5):442–449; discussion 449-450 [DOI] [PubMed] [Google Scholar]
- 12. Lu CH, Chang WN, Lin YC, et al. Bacterial brain abscess: microbiological features, epidemiological trends and therapeutic outcomes. QJM. 2002;95(8):501–509 [DOI] [PubMed] [Google Scholar]
- 13. Carpenter J, Stapleton S, Holliman R. Retrospective analysis of 49 cases of brain abscess and review of the literature. Eur J Clin Microbiol Infect Dis. 2007;26(1):1–11 [DOI] [PubMed] [Google Scholar]
- 14. Radoi M, Ciubotaru V, Tataranu L. Brain abscesses: clinical experience and outcome of 52 consecutive cases. Chirurgia. 2013;108(2):215–225 [PubMed] [Google Scholar]
- 15. Manzar N, Manzar B, Kumar R, Bari ME. The study of etiologic and demographic characteristics of intracranial brain abscess: a consecutive case series study from Pakistan. World Neurosurg. 2011;76(1-2):195–200; discussion 179-183 [DOI] [PubMed] [Google Scholar]
- 16. Menon S, Bharadwaj R, Chowdhary A, Kaundinya DV, Palande DA. Current epidemiology of intracranial abscesses: a prospective 5 year study. J Med Microbiol. 2008;57(pt 10):1259–1268 [DOI] [PubMed] [Google Scholar]
- 17. Sharma BS, Gupta SK, Khosla VK. Current concepts in the management of pyogenic brain abscess. Neurol India. 2000;48(2):105–111 [PubMed] [Google Scholar]
- 18. Lumbiganon P, Chaikitpinyo A. Antibiotics for brain abscesses in people with cyanotic congenital heart disease. Cochrane Database Syst Rev. 2013;3:CD004469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. McClelland S, 3rd, Hall WA. Postoperative central nervous system infection: incidence and associated factors in 2111 neurosurgical procedures. Clin Infect Dis. 2007;45(1):55–59 [DOI] [PubMed] [Google Scholar]
- 20. Sennaroglu L, Sozeri B. Otogenic brain abscess: review of 41 cases. Otolaryngol Head Neck Surg. 2000;123(6):751–755 [DOI] [PubMed] [Google Scholar]
- 21. Singh N, Husain S. Infections of the central nervous system in transplant recipients. Transpl Infect Dis. 2000;2(3):101–111 [DOI] [PubMed] [Google Scholar]
- 22. Singh N, Paterson DL. Mycobacterium tuberculosis infection in solid-organ transplant recipients: impact and implications for management. Clin Infect Dis. 1998;27(5):1266–1277 [DOI] [PubMed] [Google Scholar]
- 23. Cunha BA. Central nervous system infections in the compromised host: a diagnostic approach. Infect Dis Clin North Am. 2001;15(2):567–590 [DOI] [PubMed] [Google Scholar]
- 24. Roche M, Humphreys H, Smyth E, et al. A twelve-year review of central nervous system bacterial abscesses; presentation and aetiology. Clin Microbiol Infect. 2003;9(8):803–809 [DOI] [PubMed] [Google Scholar]
- 25. Sharma R, Mohandas K, Cooke RP. Intracranial abscesses: changes in epidemiology and management over five decades in Merseyside. Infection. 2009;37(1):39–43 [DOI] [PubMed] [Google Scholar]
- 26. Germiller JA, Monin DL, Sparano AM, Tom LW. Intracranial complications of sinusitis in children and adolescents and their outcomes. Arch Otolaryngol Head Neck Surg. 2006;132(9):969–976 [DOI] [PubMed] [Google Scholar]
- 27. Rath TJ, Hughes M, Arabi M, Shah GV. Imaging of cerebritis, encephalitis, and brain abscess. Neuroimaging Clin N Am. 2012;22(4):585–607 [DOI] [PubMed] [Google Scholar]
- 28. Chang SC, Lai PH, Chen WL, et al. Diffusion-weighted MRI features of brain abscess and cystic or necrotic brain tumors: comparison with conventional MRI. Clin Imaging. 2002;26(4):227–236 [DOI] [PubMed] [Google Scholar]
- 29. Faehndrich J, Weidauer S, Pilatus U, Oszvald A, Zanella FE, Hattingen E. Neuroradiological viewpoint on the diagnostics of space-occupying brain lesions. Clin Neuroradiol. 2011;21(3):123–139 [DOI] [PubMed] [Google Scholar]
- 30. Lai PH, Hsu SS, Ding SW, et al. Proton magnetic resonance spectroscopy and diffusion-weighted imaging in intracranial cystic mass lesions. Surg Neurol. 2007;68(suppl 1):S25–S36 [DOI] [PubMed] [Google Scholar]
- 31. Floeth FW, Pauleit D, Sabel M, et al. 18F-FET PET differentiation of ring-enhancing brain lesions. J Nuclear Med. 2006;47(5):776–782 [PubMed] [Google Scholar]
- 32. Cavusoglu H, Kaya RA, Turkmenoglu ON, Colak I, Aydin Y. Brain abscess: analysis of results in a series of 51 patients with a combined surgical and medical approach during an 11-year period. Neurosurg Focus. 2008;24(6):E9. [DOI] [PubMed] [Google Scholar]
- 33. Rosenblum ML, Hoff JT, Norman D, Edwards MS, Berg BO. Nonoperative treatment of brain abscesses in selected high-risk patients. J Neurosurg. 1980;52(2):217–225 [DOI] [PubMed] [Google Scholar]
- 34. Mamelak AN, Obana WG, Flaherty JF, Rosenblum ML. Nocardial brain abscess: treatment strategies and factors influencing outcome. Neurosurgery. 1994;35(4):622–631 [DOI] [PubMed] [Google Scholar]
- 35. Moorthy RK, Rajshekhar V. Management of brain abscess: an overview. Neurosurg Focus. 2008;24(6):E3. [DOI] [PubMed] [Google Scholar]
- 36. Gadgil N, Patel AJ, Gopinath SP. Open craniotomy for brain abscess: a forgotten experience? Surg Neurol Int. 2013;4:34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Valarezo J, Cohen JE, Valarezo L, et al. Nocardial cerebral abscess: report of three cases and review of the current neurosurgical management. Neurol Res. 2003;25(1):27–30 [DOI] [PubMed] [Google Scholar]
- 38. Adeyemi OA, Gottardi-Littell N, Muro K, Kane K, Flaherty JP. Multiple brain abscesses due to Actinomyces species. Clin Neurol Neurosurg. 2008;110(8):847–849 [DOI] [PubMed] [Google Scholar]
- 39. Bernardini GL. Diagnosis and management of brain abscess and subdural empyema. Curr Neurol Neurosci Rep. 2004;4(6):448–456 [DOI] [PubMed] [Google Scholar]
- 40. Takeshita M, Kagawa M, Izawa M, Takakura K. Current treatment strategies and factors influencing outcome in patients with bacterial brain abscess. Acta Neurochirurgica. 1998;140(12):1263–1270 [DOI] [PubMed] [Google Scholar]
- 41. Quartey GR, Johnston JA, Rozdilsky B. Decadron in the treatment of cerebral abscess. An experimental study. J Neurosurg. 1976;45(3):301–310 [DOI] [PubMed] [Google Scholar]
- 42. Kourtopoulos H, Holm SE, Norrby SR. The influence of steroids on the penetration of antibiotics into brain tissue and brain abscesses. An experimental study in rats. J Antimicrob Chemother. 1983;11(3):245–249 [DOI] [PubMed] [Google Scholar]
- 43. Seydoux C, Francioli P. Bacterial brain abscesses: factors influencing mortality and sequelae. Clin Infect Dis. 1992;15(3):394–401 [DOI] [PubMed] [Google Scholar]
- 44. Lee TH, Chang WN, Su TM, et al. Clinical features and predictive factors of intraventricular rupture in patients who have bacterial brain abscesses. J Neurol Neurosurg Psychiatry. 2007;78(3):303–309 [DOI] [PMC free article] [PubMed] [Google Scholar]